Introduction
Transient ischemic attack (TIA) is an acute episode of reversible temporary neurologic dysfunction caused by transient ischemia of the focal brain and can be resolved in 24 h []. Despite the transient episodes of clinical symptoms, patients with TIA may exhibit different degrees of cognitive impairment []. Moreover, TIA is an important risk factor for eventual stroke or silent stroke []. Therefore, detection of brain abnormality resulted from TIA is important for early diagnosis and intervention of stroke.
Brain imaging is increasingly used to objectively identify TIA that is presumed to have a vascular etiology []. Advanced noninvasive magnetic resonance imaging (MRI) techniques can provide promising avenues to detect structural and functional brain alterations in TIA [, ]. Resting-state functional MRI (rs-fMRI) measures the blood oxygenation level-dependent signal, the low frequency fluctuations of which reflect spontaneous neuronal activities of the brain [, ]. Rs-fMRI methods have been increasingly applied to investigate the pathological alterations in patients with TIA [, ]. Functional connectivity (FC) is the most commonly used method for rs-fMRI data analysis that could provide information about how regions are related to each other within brain functional networks [].
The default mode network (DMN), the most important subnetwork among brain functional networks, is characterized by deactivation during goal-directed cognitive performance and increased activity in resting state [, ]. The DMN is composed by a set of brain regions, including medial prefrontal cortex (mPFC), posterior cingulate cortex/precuneus cortex (PCC/Pcu), angular gyrus (AG), medial/inferior/middle temporal gyrus (MTG), lateral/inferior parietal cortex, hippocampus, medial dorsal thalamus, bilateral posterior cerebellum, and occipital gyrus [-]. There is a growing body of evidence supporting that the DMN is associated with cognitive functions, such as emotional processing [], self-referential mental activity [], and recollection of prior experiences []. It has been proved that the FC alterations within DMN are of great significance for exploring the neuropathological mechanism of various brain disorders [, ], especially for patients after stroke onset [, ]. For example, Tuladhar et al. [] observed decreased interregional FC in right middle temporal cortex and right superior frontal cortex and left middle temporal cortex and right superior frontal cortex within DMN in stroke patients. Jiang et al. [] detected decreased FC in the right mPFC and right Pcu within DMN in stoke patients. However, how the FC altered within DMN in patients with TIA was still unclear.
In the present study, we aimed to explore the FC abnormalities within DMN in patients with TIA. Specially, we sought to determine whether and how TIA disrupts the FC within DMN and whether associations exist between these FC alterations and clinical characteristics of the TIA patients. Further, we examined whether these FC abnormalities could predict follow-up ischemic attacks of the patients during the 1-year follow-up.
Materials and Methods
Participants
From April 2015 to June 2016, 51 suspected TIA patients who had transient neurologic symptoms (i.e., <24 h and evaluated by experienced clinical neurologists) were recruited from Department of Neurology, Anshan Changda Hospital. Patients with leukoaraiosis, hemorrhage, migraine, epilepsy, or psychiatric diseases history were excluded from this study. For each patient, we recorded basic information as follows: (1) history of TIA and stroke; (2) potential risk factors such as hypertension, diabetes mellitus, coronary artery disease, current smoking, and drinking; (3) medications used before the MRI scanning; (4) in-hospital evaluation of arterial stenosis (carotid duplex ultrasound and MR angiography), atrial fibrillation (electrocardiogram), and brain infarcts (diffusion-weighted imaging and T2-FLAIR); and (5) 1-year telephone follow-up of stroke and/or TIA attack. Based on the methods described by Johnston et al. [], an ABCD2 score, which considered age, blood pressure, clinical features, duration of symptoms, and history of diabetes, was generated for each patient to evaluate the risk for subsequent stroke. The patients were accompanied with several symptoms including movement difficulties on left side of the body (21 patients, 43.8%); movement difficulties on right side of the body (18 patients, 37.5%); numbness on left side of the body (3 patients, 6.3%); sudden dimming or loss of vision (6 patients, 12.5%); mouth droop (1 patient, 2.1%); and difficulty speaking (21 patients, 43.8%).
Forty-one age- and sex-matched healthy controls (HCs) with no physical diseases or history of psychiatric or neurologic disorders from local community were also recruited in this study through advertising. Three patients were excluded on account of inadequate image quality of multimodal MRI data (incomplete coverage of the whole brain for rs-fMRI scan or the loss of 3D T1 image), leaving 48 TIA patients (age = 57.6 ± 9.8 years; 37 males) and 41 HCs (age = 55.0 ± 8.0 years; 30 males) in the final analysis.
Physiological and Biochemical Tests
All patients and 35 HCs (the indices from 6 HCs were missing) completed a series of physiological/biochemical tests within 24 h before the MRI data acquisition, including blood systolic pressure, blood diastolic pressure, blood sugar level, total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Additionally, all participants underwent the mini-mental state examination to evaluate global cognition [].
MR Data Acquisition
MR data were acquired using a GE MR-750 3.0 T scanner (GE Medical Systems, Waukesha, WI, USA) at Anshan Changda Hospital, China. The time interval between the latest TIA attack and subsequent MRI scanning was 0.25–16 days for the patients. During the data acquisition, participants were instructed to keep awake, relax with their eyes closed, and remain still as much as possible.
Resting-State fMRI Data
Resting-state fMRI data were obtained using an echo-planar imaging sequence with following protocols: 43 axial slices, TR = 2,000 ms, TE = 30 ms, flip angle = 60°, matrix = 64 × 64, in-plane resolution of 3.44 × 3.44 mm2, thickness/gap = 3.2/0 mm, 240 contiguous EPI functional volumes, 8 min.
Structural MRI Data
3D high-resolution T1-weighted anatomical images were acquired using a 3D-MPRAGE sequence: 176 sagittal slice, TR = 8,100 ms, TE = 3.1 ms, matrix = 256 × 256, voxel size: 1 × 1 × 1 mm3, thickness/gap = 1/0 mm. This session lasted for about 5 min.
Data Preprocessing
Rs-fMRI data were processed using Data Processing and Analysis for Brain Imaging [] including: (1) removing first 10 time points to make the longitudinal magnetization reach steady state and to let the participant get used to the scanning environment; (2) slice-timing to correct the differences in image acquisition time between slices; (3) head motion correction; (4) spatial normalization to the Montreal Neurological Institute space via the deformation fields derived from tissue segmentation of structural images (resampling voxel size = 3 × 3 × 3 mm3); (5) spatial smoothing with an isotropic Gaussian kernel with a full-width at half-maximum of 6 mm; (6) removing linear trend; (7) regressing out the head motion effect (using Friston 24 parameter) [], white matter signals and cerebrospinal fluid signals; and (8) band-pass filtering (0.01–0.08 Hz). No participants were excluded due to large head motion (>3.0 mm of maximal translation in any direction of x, y, or z or 3.0° of maximal rotation throughout the course of scanning). We did not regress out the global mean signals since this was a controversial step for rs-fMRI data preprocessing []. Then, FC was further calculated based on the preprocessed fMRI data.
FC Calculation
In our study, voxel-wise FC and pairwise FC were performed to investigate the abnormal FC within DMN.
Voxel-Wise FC Analysis
The DMN was proved to be composed of 2 subnetworks: anterior DMN and posterior DMN [, ]. mPFC and PCC/Pcu were 2 main hubs of anterior DMN and posterior DMN, respectively [, ]. In the current study, 2 spherical regions of interest (ROIs; radius = 5 mm) centered at PCC/Pcu (0, –52, 27) and mPFC (–1, 54, 27), which were taken from previous study, were generated to calculate voxel-wise FC (mPFC-seeded FC and PCC/Pcu-seeded FC) []. For each ROI, the correlation analysis was performed between the averaged time course of the ROI and the time courses of all other voxels in the brain. The resultant correlation coefficients were transformed to z-value using Fisher’s r-to-z transformation.
Pairwise FC analysis
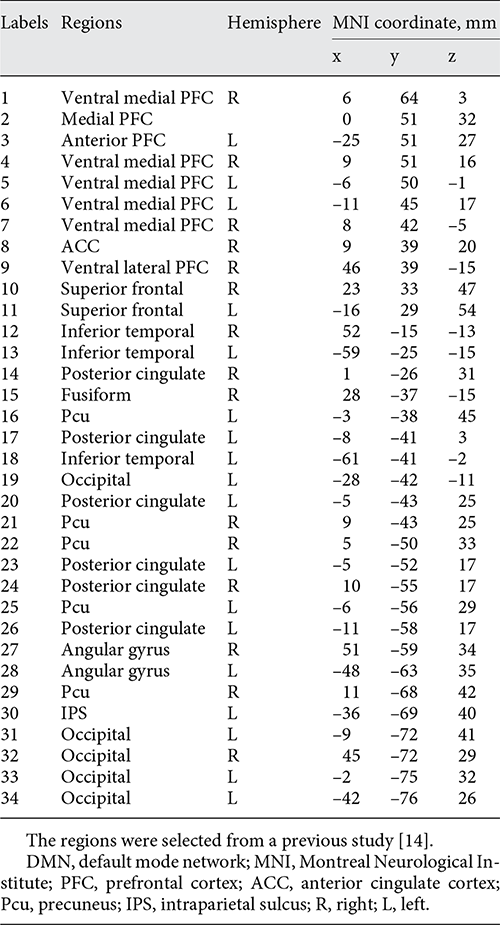
We further calculate pairwise FC to investigate the interregional FC changes within DMN. Thirty-four ROIs (Table 1, Fig. 1), which were taken from previous study with 5 mm radius, were used to calculate the interregional FC within DMN []. The averaged time course of each ROI was first extracted, and temporal correlations were then calculated for each pair of averaged time courses (a total of 561 pairwise FC). The resultant correlation coefficients were transformed to z-value using Fisher’s r-to-z transformation.
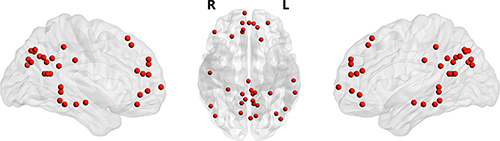
Fig. 1
The distribution of 34 ROIs within DMN (Table 1) was visualized in surface space using the BrainNet viewer []. R, right; L, left.
Statistical Analysis
Between-Group Differences in Demographic and Clinical Characteristics
We tested between-group differences in age, clinical/physiological/biochemical variables with the Statistical Package for the Social Sciences (SPSS; SPSS Inc., Chicago, IL, USA). Between-group differences in age and clinical/physiological/biochemical characteristics were obtained with two-sample t tests. Sex difference was obtained with the Pearson’s chi-square test.
Between-Group Differences in FC within DMN
One-sample t test was performed on PCC/Pcu-seeded FC maps of 41 HCs (p < 0.01, Bonferroni correction, cluster size >10 voxels) to obtain the DMN mask. Between-group differences in voxel-wise FC were inferred within DMN mask using two-sample t tests. The resultant T-map was threshold with voxel p < 0.001, cluster p < 0.05, Gaussian Random Field theory correction for multiple comparisons.
Between-group differences of 561 pairwise FCs were inferred using two-sample t tests. A false discovery rate procedure was used to correct for multiple comparisons (p < 0.05).
Relationship between FC Alterations and Clinical Variables in TIA
For any TIA-related FC alterations, a Pearson’s correlation analysis was used to assess its associations with clinical, physiological, and biochemical characteristics of the patients, including ABCD2 scores, blood systolic pressure, blood diastolic pressure, blood sugar level, total cholesterol, triglycerides, HDL-C and LDL-C. These correlation analyses were threshold with p < 0.05 (two-tailed).
Prediction of Follow-Up Ischemic Attacks
To examine whether TIA-related FC alterations within DMN could predict the follow-up attacks in patients (including TIA recurrence and stroke attack) in 1 year, 44 patients (4 patients dropped out in 1-year follow-up study) were divided into 2 subgroups: patients with (n = 12, 10 patients had stroke) and without (n = 32) follow-up ischemic attacks. For FC showing significant between-group differences, two-sample t tests were performed to compare the FC differences between these 2 subgroups. The analyses were performed on SPSS software.
Results
Participants’ Demographic and Clinical Characteristics
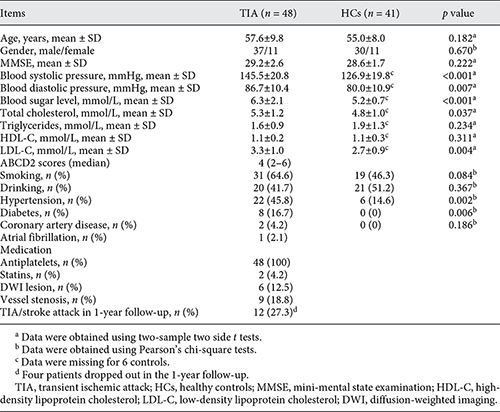
Demographic and clinical information for the final 48 patients with TIA and 41 HCs was summarized in Table 2. The TIA patients and HCs were matched in age (p = 0.182) and sex (p = 0.670). Compared with HCs, TIA patients showed significantly higher systolic pressure (p < 0.001), diastolic pressure (p = 0.007), blood sugar level (p < 0.001), total cholesterol (p = 0.037), and LDL-C (p = 0.004). The median ABCD2 score for the TIA patients was 4 (2–6).
Out of the 48 patients, 4 (8.3%) experienced a history of stroke, 25 (52.1%) had a history of TIA before they were recruited in the current study, 6 (12.5%) had white matter lesion in diffusion-weighted images, and 9 (18.8%) had intracranial large-vessel stenosis or carotid artery stenosis.
Disrupted FC within DMN
Voxel-Wise FC Alterations
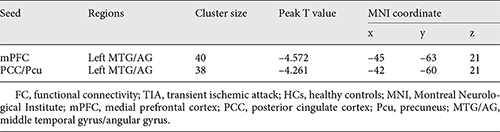
Compared with HCs, TIA patients exhibited significantly decreased PCC/Pcu-seeded FC and mPFC-seeded FC both with left MTG/AG (voxel p < 0.001, cluster p < 0.05, Gaussian Random Field correction, cluster size >38 voxels for mPFC-seeded FC and cluster size >32 voxels for PCC/Pcu-seeded FC; Table 3; Fig. 2).
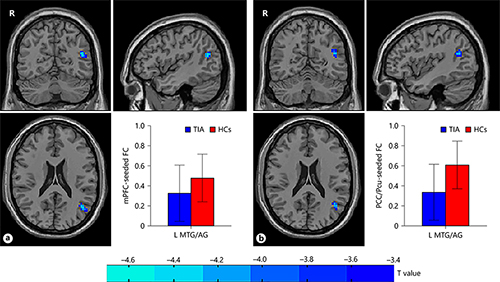
Fig. 2
The group differences of voxel-wise FC between TIA patients and HCs. The left MTG/AG showed decreased FC both with mPFC (a) and PCC/Pcu (b) in patients with TIA as compared with HCs (p < 0.001, GRF correction). mPFC, medial prefrontal cortex; FC, functional connectivity; TIA, transient ischemic attack; HCs, healthy controls; MTG/AG, middle temporal gyrus/angular gyrus; PCC/Pcu, posterior cingulate cortex/precuneus; GRF correction, Gaussian Random Field correction; R, right; L, left.
Pairwise FC Alterations
TIA patients exhibited significantly decreased pairwise FC between mPFC and right Pcu, mPFC and left PCC, mPFC and left AG, right Pcu and left PCC as compared with HCs (p < 0.05, false discovery rates correction; Fig. 3).
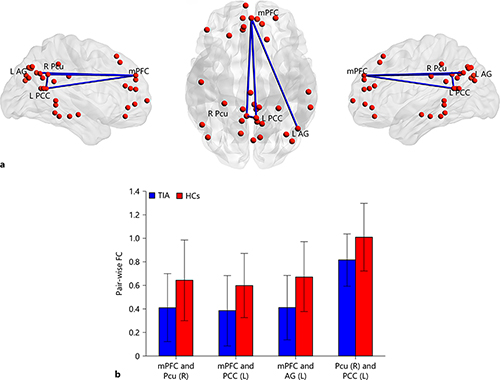
Fig. 3
The group differences of pairwise FC between TIA patients and HCs. a Four pairs of significantly decreased FC in patients with TIA were depicted with blue lines. b The mean and SD of FC for each pair of regions in 2 groups (p < 0.05, FDR correction). mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; FC, functional connectivity; TIA, transient ischemic attack; HCs, healthy controls; Pcu, precuneus; AG, angular gyrus; R, right; L, left.
Relationship between FC and Clinical/Physiological/Biochemical Characteristics
No significant correlations were observed between these FC alterations and clinical/physiological/biochemical characteristics in TIA patients (p > 0.05).
Prediction of Follow-Up Ischemic Attacks
In comparison with the patients without follow-up ischemic attacks, those suffering from ischemic attacks 1 year after the MR scanning showed significantly lower FC between mPFC and left PCC (p < 0.05; with follow-up ischemic attacks: 0.22 ± 0.25; without follow-up ischemic attacks: 0.45 ± 0.28).
Discussion
In present study, we applied voxel-wise FC and pairwise FC to investigate FC alterations within DMN in patients with TIA and examined the associations of these connectivity alterations with clinical risk factors and further explored the abilities of aberrant FC to predict follow-up ischemic attacks. Decreased FC were observed between several regions within DMN: mPFC and left MTG/AG, PCC/Pcu and left MTG/AG, mPFC and right Pcu, mPFC and left PCC, right Pcu and left PCC. Moreover, the FC between mPFC and left PCC could predict future ischemic attacks in patients. These findings provided evidence for the existence of impaired FC within DMN in TIA, which may help to understand the pathophysiological underpinnings in patients with TIA.
The left MTG/AG showed decreased FC both with mPFC and PCC/Pcu in patients with TIA as compared with HCs. The findings were consistent with previous study that observed decreased FC in left MTG within DMN in TIA using independent component analysis []. It was worth noting that lower amplitude of low-frequency fluctuation in left MTG/AG was also observed in our previous study for TIA patients []. Our results implied that impaired function in left MTG/AG may be a reliable marker in TIA. The MTG was involved in in several cognitive processes, including language and semantic memory processing, as well as visual perception [, ]. AG was considered to be essential for semantic processing, episodic stimulation, and episodic memory [, ]. Thus, we conjectured that FC alterations in left MTG/AG may at least partly contribute to cognitive impairments, such as language, visual, or memory function in TIA. The speculation could be examined in future studies.
TIA-related FC decreases were observed among each pair of mPFC, left PCC and right Pcu, which were the hubs of subnetworks in DMN and played vital roles in self-related activities [, ]. Specifically, mPFC was involved in self-referential judgments and emotional processing [, , ]; Pcu was associated with self-processing operations, episodic memory retrieval, and visuospatial imagery [, ]; and PCC was essential for supporting internally directed cognition, retrieving autobiographical memories and planning for the future and regulating the focus of attention [, ]. It should be noted that aberrant FC between these hubs has also been reported in patients with stroke [, ]. Zhang et al. [] found decreased FC between bilateral Pcu and bilateral PCC in post-stroke depression patients as compared with HCs. Jiang et al. [] reported decreased FC between right mPFC and right Pcu in acute brainstem ischemic stroke patients when compared with HCs. Our results indicated that impaired FC between these hubs within DMN was already detectable in TIA, an important risk for stroke. Notably, FC between mPFC and left PCC could predict follow-up ischemic attacks for the patients: the patients with follow-up brain ischemic attacks showed decreased FC when compared with those without ischemic attacks. These findings together suggested the important role of FC between hubs within DMN in brain ischemia. Given that many TIA patients would experience a completed ischemic stroke in the near future [, ], it would be interesting for future follow-up studies to explore how the TIA-related FC impairments within DMN alters when TIA progress to stroke.
The present study has some limitations. First, this study lacked cognitive/emotional data, thus it failed to explore the associations between these FC alterations and cognitive/emotional dysfunction in the TIA. A comprehensive cognitive and emotional assessments, including attention, memory, reasoning, judgment, emotion, and other related information may need be involved in further TIA studies. Second, we did not collect MRI data during the follow-up study, and thus failed to examine how functional brain networks reorganize as TIA continues to develop. Future longitudinal studies should be performed to examine whether the current approach could be applied to monitor disease progression of TIA. Third, 25 (52.1%) of 48 patients had a history of TIA before they were recruited in the current study. However, we failed to collect detailed history of TIA episodes for those patients, and thus cannot evaluate TIA history effect on our findings. Future studies enrolling first-episode patients can help validate our results.
Conclusion
In conclusion, we found disrupted FC within DMN in patients with TIA using voxel-wise FC and pairwise FC. Moreover, the aberrant FC between mPFC and left PCC could predict follow-up ischemic attacks. These FC alterations within DMN may contribute to further understanding of the underlying pathological mechanism in TIA and guide for imaging diagnosis and early intervention of TIA.
Acknowledgments
We thank all the patients and volunteers for participating in this study.
Statement of Ethics
The study was approved by the Ethics Committee of the Center for Cognition and Brain Disorders, Hangzhou Normal University. Written informed consent was obtained from all participants.
Disclosure Statement
The authors declare that they have no conflict of interest.
Funding Sources
This work was supported by grants from National Key R&D Program of China (No. 2017YFC1310000), National Natural Science Foundation of China (Nos. 81771911, 81301210).
Author Contributions
Y.L. and X.H. designed the study. Y.S., Y.H., C.Z., D.Z., F.Z., Q.X., J.L., L.Z., and C.Z. performed the experiments and collected the data. T.Z. and L.L. analyzed and interpreted the data, draft the manuscript. Y.L. also revised the manuscript critically for important intellectual content.
References
- 1. Albers GW, Caplan LR, Easton JD, Fayad PB, Mohr JP, Saver JL, et al.; TIA Working Group. Transient ischemic attack—proposal for a new definition. <X00_Journal>N Engl J Med</X00_Journal>. 2002 Nov;347(21):1713–6.
- 2. Bakker FC, Klijn CJ, Jennekens-Schinkel A, Kappelle LJ. Cognitive disorders in patients with occlusive disease of the carotid artery: a systematic review of the literature. <X00_Journal>J Neurol</X00_Journal>. 2000 Sep;247(9):669–76.
- 3. Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. <X00_Journal>Lancet Neurol</X00_Journal>. 2007 Dec;6(12):1063–72.
- 4. Ay H, Arsava EM, Johnston SC, Vangel M, Schwamm LH, Furie KL, et al. Clinical- and imaging-based prediction of stroke risk after transient ischemic attack: the CIP model. <X00_Journal>Stroke</X00_Journal>. 2009 Jan;40(1):181–6.
- 5. Li R, Guo J, Ma X, Wang S, Zhang J, He L, et al. Alterations in the gray matter volume in transient ischemic attack: a voxel-based morphometry study. <X00_Journal>Neurol Res</X00_Journal>. 2015 Jan;37(1):43–9.
- 6. Qiao XJ, Salamon N, Wang DJ, He R, Linetsky M, Ellingson BM, et al. Perfusion deficits detected by arterial spin-labeling in patients with TIA with negative diffusion and vascular imaging. <X00_Journal>AJNR Am J Neuroradiol</X00_Journal>. 2013 Nov-Dec;34(11):2125–30.
- 7. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. <X00_Journal>Magn Reson Med</X00_Journal>. 1995 Oct;34(4):537–41.
- 8. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. <X00_Journal>Nat Rev Neurosci</X00_Journal>. 2007 Sep;8(9):700–11.
- 9. Li R, Wang S, Zhu L, Guo J, Zeng L, Gong Q, et al. Aberrant functional connectivity of resting state networks in transient ischemic attack. <X00_Journal>PLoS One</X00_Journal>. 2013 Aug;8(8):e71009.
- 10. Guo J, Chen N, Li R, Wu Q, Chen H, Gong Q, et al. Regional homogeneity abnormalities in patients with transient ischaemic attack: a resting-state fMRI study. <X00_Journal>Clin Neurophysiol</X00_Journal>. 2014 Mar;125(3):520–5.
- 11. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. <X00_Journal>Ann N Y Acad Sci</X00_Journal>. 2008 Mar;1124(1):1–38.
- 12. Raichle ME. The brain’s default mode network. <X00_Journal>Annu Rev Neurosci</X00_Journal>. 2015 Jul;38(1):433–47.
- 13. Raichle ME. The restless brain. <X00_Journal>Brain Connect</X00_Journal>. 2011;1(1):3–12.
- 14. Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. Prediction of individual brain maturity using fMRI. <X00_Journal>Science</X00_Journal>. 2010 Sep;329(5997):1358–61.
- 15. Simpson JR Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. <X00_Journal>Proc Natl Acad Sci USA</X00_Journal>. 2001 Jan;98(2):688–93.
- 16. Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. <X00_Journal>Proc Natl Acad Sci USA</X00_Journal>. 2001 Mar;98(7):4259–64.
- 17. Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. <X00_Journal>J Neurophysiol</X00_Journal>. 2006 Dec;96(6):3517–31.
- 18. Wang C, Qin W, Zhang J, Tian T, Li Y, Meng L, et al. Altered functional organization within and between resting-state networks in chronic subcortical infarction. <X00_Journal>J Cereb Blood Flow Metab</X00_Journal>. 2014 Apr;34(4):597–605.
- 19. Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: A review. <X00_Journal>Neurosci Biobehav Rev</X00_Journal>. 2015 Sep;56:330–44.
- 20. Tuladhar AM, Snaphaan L, Shumskaya E, Rijpkema M, Fernandez G, Norris DG, et al. Default Mode Network Connectivity in Stroke Patients. <X00_Journal>PLoS One</X00_Journal>. 2013 Jun;8(6):e66556.
- 21. Jiang L, Geng W, Chen H, Zhang H, Bo F, Mao CN, et al. Decreased functional connectivity within the default-mode network in acute brainstem ischemic stroke. <X00_Journal>Eur J Radiol</X00_Journal>. 2018 Aug;105:221–6.
- 22. Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. <X00_Journal>Lancet</X00_Journal>. 2007 Jan;369(9558):283–92.
- 23. Schultz-Larsen K, Lomholt RK, Kreiner S. Mini-Mental Status Examination: a short form of MMSE was as accurate as the original MMSE in predicting dementia. <X00_Journal>J Clin Epidemiol</X00_Journal>. 2007 Mar;60(3):260–7.
- 24. Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. <X00_Journal>Neuroinformatics</X00_Journal>. 2016 Jul;14(3):339–51.
- 25. Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. <X00_Journal>Magn Reson Med</X00_Journal>. 1996 Mar;35(3):346–55.
- 26. Murphy K, Fox MD. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. <X00_Journal>Neuroimage</X00_Journal>. 2017 Jul;154:169–73.
- 27. Kim DY, Lee JH. Are posterior default-mode networks more robust than anterior default-mode networks? Evidence from resting-state fMRI data analysis. <X00_Journal>Neurosci Lett</X00_Journal>. 2011 Jul;498(1):57–62.
- 28. Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. <X00_Journal>Biol Psychiatry</X00_Journal>. 2007 Sep;62(5):429–37.
- 29. De Simoni S, Grover PJ, Jenkins PO, Honeyfield L, Quest RA, Ross E, et al. Disconnection between the default mode network and medial temporal lobes in post-traumatic amnesia. <X00_Journal>Brain</X00_Journal>. 2016 Dec;139(Pt 12):3137–50.
- 30. Lv Y, Li L, Song Y, Han Y, Zhou C, Zhou D, et al. The Local Brain Abnormalities in Patients With Transient Ischemic Attack: A Resting-State fMRI Study. <X00_Journal>Front Neurosci</X00_Journal>. 2019 Jan;13:24.
- 31. Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. <X00_Journal>J Cogn Neurosci</X00_Journal>. 2000 Jan;12(1):1–47.
- 32. Bonilha L, Hillis AE, Hickok G, den Ouden DB, Rorden C, Fridriksson J. Temporal lobe networks supporting the comprehension of spoken words. <X00_Journal>Brain</X00_Journal>. 2017 Sep;140(9):2370–80.
- 33. Thakral PP, Madore KP, Schacter DL. A Role for the Left Angular Gyrus in Episodic Simulation and Memory. <X00_Journal>J Neurosci</X00_Journal>. 2017 Aug;37(34):8142–9.
- 34. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. <X00_Journal>Brain</X00_Journal>. 2006 Mar;129(Pt 3):564–83.
- 35. Zhang P, Wang J, Xu Q, Song Z, Dai J, Wang J. Altered functional connectivity in post-ischemic stroke depression: A resting-state functional magnetic resonance imaging study. <X00_Journal>Eur J Radiol</X00_Journal>. 2018 Mar;100:156–65.
- 36. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. <X00_Journal>JAMA</X00_Journal>. 2000 Dec;284(22):2901–6.
- 37. Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. <X00_Journal>PLoS One</X00_Journal>. 2013 Jul;8(7):e68910.
T.Z. and L.L.: contributed equally to this study. They shared first authorship.