Case presentation
A 5-year-old male with past medical history of surgical repair of cleft lip and palate but no known cardiac history presented to the emergency department with persistent fevers for 9 days duration and worsening respiratory distress despite completion of cefdinir and azithromycin courses for presumed pneumonia. His cardiac exam was normal with no murmur auscultated. Chest X-ray demonstrated focal consolidation consistent with pneumonia and new finding of mild cardiomegaly that was not present on prior chest X-ray performed 5 days prior. A screening transthoracic echocardiogram performed to evaluate the cardiomegaly demonstrated normal size of all cardiac structures and identified a small, haemodynamically insignificant patent ductus arteriosus with an echo-bright mobile mass, concerning for vegetation at the pulmonary artery end (Figure 1b). Chest CT scan was performed to further evaluate the lung fields, which demonstrated scattered lesions in all lobes concerning for septic pulmonary emboli (Figure 1c). Brain MRI and ophthalmologic evaluation were normal. He had multiple blood cultures throughout his hospitalisation that remained without bacterial growth, possibly due to pre-treatment with several antibiotic courses. Of note, the patient had not undergone recent dental work in the preceding weeks.
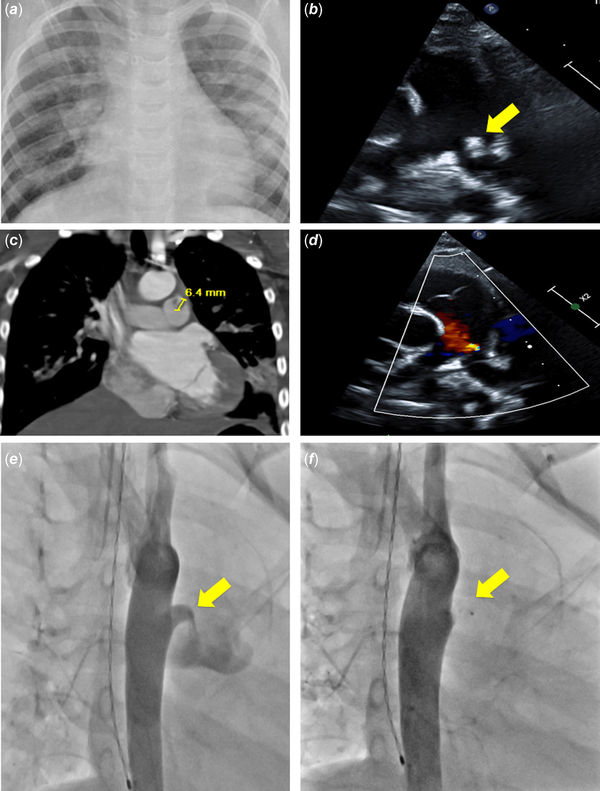
Figure 1
Imaging studies throughout clinical course. (a) Initial chest X-ray demonstrating cardiomegaly and pulmonary infiltrates; (b ) echocardiogram demonstrating vegetation associated with patent ductus arteriosus extending into the pulmonary artery (arrow); (c) CT coronal slice demonstrating location and size of vegetation as well as septic emboli; (d) echocardiogram following antibiotic course demonstrating no residual vegetation; (e) angiography demonstrating patent ductus arteriosus (arrow); (f) angiography post-device placement demonstrating successful closure (arrow).
After multi-disciplinary discussion between cardiology and infectious disease teams, the patient received a 6-week course of intravenous ceftriaxone therapy due to streptococcus and staphylococcus species being the most common causes of endarteritis in children. Repeat echocardiogram and CT following treatment demonstrated resolution of the vegetation (Figure 1d), while blood cultures and Karius Test (blood test that measures non-human deoxyribonucleic acid in the blood by next-generation sequencing) remained negative. He was placed on amoxicillin at 15 mg/kg three times a day until undergoing successful transcatheter device occlusion using a 6 mm KA Micro Plug (Merit Medical, South Jordan, UT, USA) (Figure 1e, f), after which amoxicillin was continued for an additional 6 weeks. Following current American Heart Association guidelines, subacute bacterial endocarditis prophylaxis was recommended for indicated procedures for 6 months following device occlusion. Although not standard management given removal of the nidus for infection, after shared decision-making with the family, antibiotic prophylaxis was extended to the foreseeable future given the high morbidity and mortality associated with infective endarteritis. He has remained without recurrence for 28 months since initial diagnosis and 26 months since device implantation.
Discussion
This case highlights two important points for discussion: 1) Patients with small, silent, and haemodynamically insignificant patent ductus arteriosus can still develop infective endarteritis, necessitating caution when interpreting the most recent guidelines, and 2) this set of patients can be treated successfully with antibiotics and transcatheter device occlusion without the need for surgery.
The ductus arteriosus is essential for fetal life, providing a connection from the pulmonary artery to the aorta. While it usually undergoes spontaneous closure shortly after birth, it can remain patent in up to 0.4% of term infants. Patent ductus arteriosus can be grouped by their ability to be auscultated on exam (audible versus silent) or by haemodynamic significance. Whether audible or silent, hemodynamically significant patent ductus arteriosus causing congestive heart failure, pulmonary over circulation, failure to thrive, or left heart enlargement meet Class I indication for transcatheter or surgical closure via the most recent American Heart Association guidelines. While there is a Class IIb recommendation to consider closure for a small, inaudible ductus without left heart enlargement, there remains significant discrepancy in management strategy as some clinicians routinely recommend closure and others discharge these patients from cardiology care without intervention or plans for further follow-up. – .
We report successful transcatheter device occlusion in a paediatric patient after antibiotic clearance of infective endarteritis in a silent ductus. This case is notable as most previously documented cases have been performed in adults, patients with an audible ductus, or required surgical closure. – . Per the most recent guidelines for subacute bacterial endocarditis prophylaxis in patients with CHD, antibiotics are not recommended for patients with left to right shunting lesions such as patent ductus arteriosus and ventricular septal defects. While not currently supported by the guidelines, transcatheter occlusion can be considered for those patients with a “silent” ductus at increased risk for bacteraemia and subsequent endarteritis including poor dental hygiene, “short gut” syndrome or other risk factors for bacterial translocation from the gastrointestinal tract, immunodeficiency, or long-term indwelling catheters. Despite the decrease in use of antibiotics after the American Heart Association guidelines, there has not been a subsequent increase in reported cases of infective endocarditis. However, given the high morbidity and mortality associated with infective endocarditis, a high index of suspicion is required to make the diagnosis, particularly in those patients without high-risk lesions and utilisation of advanced imaging modalities such as CT may be helpful. Endocarditis is also a rare entity in children and the incidence is estimated to be 0.43–0.69 cases per 100,000 children/year; however, they may present with nonspecific symptoms that can be confused for more common conditions. Multi-disciplinary approach with infectious disease and cardiology was utilised for our patient to tailor his treatment plan given the lack of clear published guidelines.
Conclusion
This case highlights two important points. Silent patent ductus arteriosus poses a small but real risk of endarteritis with the potential for severe morbidity and requires a high index of suspicion to diagnose correctly in a timely manner. Additionally, in patients with persistent fevers despite appropriate antibiotic management, further work-up and advanced imaging should be considered to rule out infective endocarditis/endarteritis.
References
- 1. Vicent L, Luna R, Martinez-Selles M. Pediatric infective endocarditis: a literature review. J Clin Med 2022; 11: 3217.
- 2. Fortescue EB, Lock JE, Galvin T, McElhinney DB. To close or not to close: the very small patent ductus arteriosus. Congenit Heart Dis 2010; 5: 354–365.
- 3. Feltes TF, Bacha E, Beekman RH, et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American heart association. Circulation 2011; 123: 2607–2652.
- 4. Bhat YA, Almesned A, Alqwaee A, Al Akhfash A. Catheter closure of clinically silent patent ductus arteriosus using the amplatzer duct occluder II-additional size: a single-center experience. Cureus 2021; 13: e17481.
- 5. Hancock H, Ansong AK, Massarella D, et al. Clinical practice algorithm for the follow-up of unrepaired and repaired patent ductus arteriosus. J Am Coll Cardiol 2022; 31: 1.
- 6. Sattwika PD, Hartopo AB, Anggrahini DW, Mumpuni H, Dinarti LK. Right-sided infective endocarditis in patients with uncorrected ventricular septal defect and patent ductus arteriosus: two case reports. Clin Case Rep 2018; 6: 2168–2173.
- 7. Saucedo-Orozco H, Vargas-Barron J, Vazquez-Antona CA, Castillo-Castellon F. Echocardiographic findings in patent ductus arteriosus-associated infective endarteritis. Anatol J Cardiol 2021; 25: 774–780.
- 8. Grewing A, Furtun B, Webb M. Utility of computed tomography in diagnosis of a patent ductus arteriosus in pulmonary artery endarteritis. JACC Case Rep 2023; 5: 101649.
- 9. Sadiq M, Latif F, ur-Rehman A. Analysis of infective endarteritis in patent ductus arteriosus. Am J Cardiol 2004; 93: 513–515.
- 10. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation 2020; 143: e72–e227.
- 11. Pasquali S, He X, Mohamed Z, et al. Trends in endocarditis hospitalizations at US children’s hospitals: impact of the 2007 American heart association antibiotic prophylaxis guidelines. Am Heart J 2012; 163: 894–899.
