Buruli ulcer (BU) caused by Mycobacterium ulcerans is a devastating skin disease []. Cases are reported from over 30 countries, mostly children in rural West and Central Africa []. Lesions are nonulcerative (papules, nodules, plaques or edema) or ulcerative [], often leading to permanent disability in advanced disease [, ]. The pathology is characterized by coagulative necrosis and lack of inflammatory infiltration attributed to cytotoxic effects of the M. ulcerans derived exotoxine mycolactone [, ]. For decades surgical excision was the standard therapy. Since 2004 rifampicin and streptomycin for 8 weeks is recommended by the World Health Organization (WHO) []. Its efficacy has been demonstrated in various studies including a randomized controlled trial [].
The temperature sensitivity of M. ulcerans has long been recognized [] and the efficacy of local hyperthermia demonstrated in 2 pilot studies with one using commercially available cheap silicone bags filled with sodium acetate trihydrate (phase-change material [PCM]) as easily rechargeable heat source [, ].
The aim of this trial was to confirm in a large patient cohort the efficacy of PCM-based thermotherapy as previously described [, ].
METHODS
Trial Design
This phase II open label single center noncomparative clinical trial (ISRCT 72102977) enrolled patients from February 2009 to November 2012 after informed written consent had been obtained. The trial was conducted under Good Clinical Practice (GCP) standards with external monitoring and data management (Coordinating Centre for Clinical Studies (KKS), Heidelberg University Hospital). The trial was approved by the Ethics Committees of Cameroon and Heidelberg University Hospital, Germany.
Trial Site and Patient Enrolment
The BU treatment center at Ayos district hospital in Cameroon maintains an operation theatre, physiotherapy, and a school for patients. The local medical team is very experienced in the diagnosis and management of BU patients.
Patients with skin lesions clinically diagnosed as BU in accordance with WHO guidelines were eligible for enrolment. Lesions were classified following the WHO classification: Category I: Single lesion (eg, nodule, papule, plaque or ulcer) <5 cm in diameter; Category II: Single lesion (nonulcerative and ulcerative, plaque and edematous forms) 5–15 cm in diameter; Category III: (a) Single lesions >15 cm in diameter, and osteomyelitis, (b) lesion(s) at a critical site (eye, breast, genitalia), and (c) multiple lesions []. Patients with significant other communicable and noncommunicable diseases, ongoing or previous BU chemotherapy, or with lesions not suitable for the heat treatment device, such as facial lesions, were excluded. Only patients with laboratory confirmed lesions were included in the endpoint analysis.
Intervention
Heat was applied through commercially available silicone bags (210 × 155 × 20 mm) filled with approximately 800 g of sodium acetate trihydrate (C2H3NaO2•3H2O) as PCM (Uni-Hot Pack, ITC-Inter Trade Consult, Hoyerswerda, Germany), licensed as medical device. It maintains its melting temperature of 58°C during heat emission. BU lesions and the surrounding skin were covered with sterile pads, tube gauze, elastic bandage and cotton as protection and to lower the PCM melting temperature to the therapeutic range of 39–42°C at skin surface as previously described []. An insulation layer (polyethylene foam or neoprene) reduced ambient heat loss and stabilized the PCM packs.
The daily heat treatment started late afternoon after cleaning and dressing of the lesions. Heat packs exchanged before going to bed stayed overnight. Heat treatment continued for 42 days plus a safety margin of up to 14 days, if ulcer margins had not fully collapsed and/or induration had not fully subsided. Treatment terminated earlier, if a lesion had completely closed.
Adjuvant therapy was not part of the study protocol. Surgical procedures were based on the clinical judgment of the local team. Removal of necrotic tissue and skin grafting were considered part of general wound management without relevant impact on bacterial load []. Patients received nutritional support and physiotherapy. No incentives were offered.
If progression defined as increase in lesion size was observed or new lesions developed, specimens for laboratory investigations were collected and analyzed and the patient was offered WHO recommended chemotherapy [].
To ensure GCP trial standards and standardized wound management the trial was hospital-based.
Outcomes
Endpoints were ‘absence of clinically BU specific features’ according to WHO guidelines [] or ‘wound closure’ within 6 months after completion of heat treatment (“primary cure”) and ‘absence of BU recurrence for 24 months after completion of heat treatment’ (“definite cure”). Secondary outcomes were rates of withdrawal for low compliance, consent withdrawal, and adverse events.
Clinical progress was documented on case record forms and by digital photos. The temperature at the edge of lesions was recorded every 10 minute on data loggers (Testo 175-177-T3, Testo AG, Lenzkirch, Germany).
After clinical BU specific features subsided, patients were followed up monthly until wound closure and all patients at month 6, 12, and 24 after completion of heat treatment including medical history, documentation of primary and new lesions, and digital photos.
Laboratory Investigations
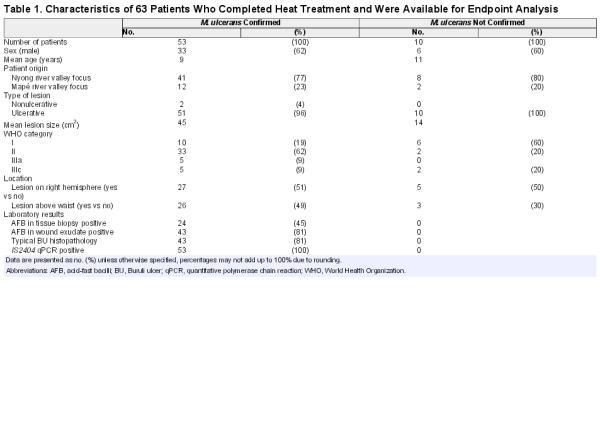
Laboratory confirmation (Table 1) was based on 3 WHO recommended methods, IS2404 quantitative polymerase chain reaction (qPCR), microscopy, and histopathology. In selected cases wound aspirates were cultured.
Wound exsudate collected from undermined edges of ulcers on day 0, 7, 14, 28 after start of treatment and a fine needle aspirate of a nodule on day 0 were immediately transferred onto glass slides, heat inactivated, and stained with Ziehl-Neelsen-methylene blue (ZN) to detect acid-fast bacilli (AFBs) []. IS2404 qPCR was performed as described []. Laboratory confirmation required positive results in at least 2 methods or positive IS2404 qPCR results at 2 different time points (Table 1). M. ulcerans culturing was performed as described [].
For ulcerative lesions 4 mm punch biopsies were taken at days 0 and 21–28, fixed for 24 hours in 10% neutral buffered formalin (4% formaldehyde), transferred to 70% ethanol, embedded into paraffin, cut into 5 µm thin sections, deparaffinized, rehydrated, and stained with Haematoxylin-Eosin (HE) and ZN according to WHO protocols [].
Data Handling and Statistical Analysis
Images were managed with LaTex (MiKTeX version 2.9.5105 and LaTexEditor build 0.536501). Lesion size was measured from images using Image J software []. Picture files in *.NEF format were developed with Adobe Photoshop Lightroom 5 version 5.4 (Adobe Systems Incorporated, San Jose, California). Patient and lesion characteristics were captured in a trial specific data bank using MACRO version 3.0.84 (InferMed, London, UK). For laboratory confirmed patients we calculated the percentage and 95% confidence interval for “primary” and “definite cure.” We used Fisher exact to assess differences between WHO categories. Microsoft Excel version 2013 (Microsoft Corporation, Redmond, Washington) and Stata version 13 (Stata Corporation, College Station, Texas) was used for statistical analysis.
RESULTS
Baseline Data
In total, 157 patients were screened for inclusion (Figure 1); 55 did not fulfill clinical BU criteria, 37 met exclusion criteria or declined consent. Of 65 patients starting thermotherapy, 10 were not laboratory confirmed, 1 was not compliant and 1 died from a cranial injury.
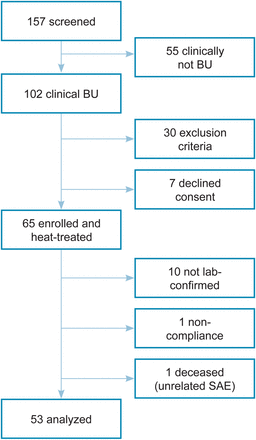
Figure 1
Study cohort. Clinical and laboratory categorization of patients from recruitment to data analysis. Abbreviations: BU, Buruli ulcer; SAE, serious adverse event.
The baseline data of 63 patients who completed heat treatment are summarized in Table 1. The range of disease severity is illustrated in Figure 2.
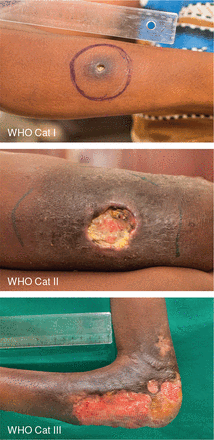
Figure 2
Clinical presentation. The range of disease severity affecting enrolled patients by World Health Organization (WHO) category.
Heat Application
Heat was applied for up to 42 days in 19 patients and extended to a maximum of 56 days in another 34 patients. The time period above 39°C ranged in total from 233 to 731 hours (mean 501) and daily from 4.7 to 13.6 hours (mean 10.7) (Figure 3).
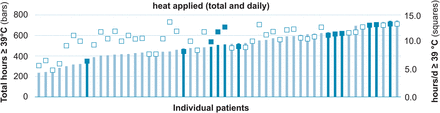
Figure 3
Heat treatment. Total (bars) and average daily (squares) hours of heat ≥39°C applied to 53 Buruli ulcer patients, who entered the analysis. The 12 patients subsequently classified as treatment failures are highlighted.
Outcomes
Primary Cure Within Six Months After Completion of Heat Treatment
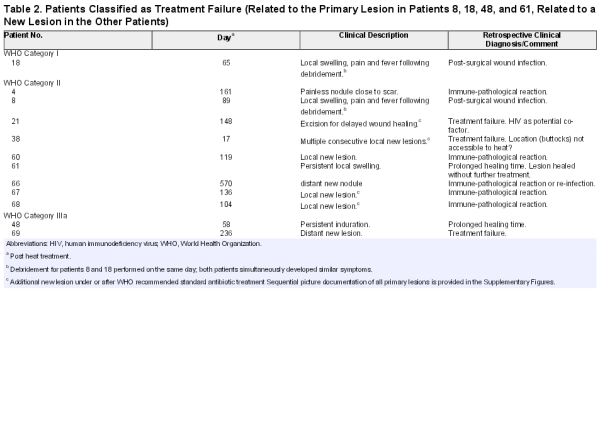
Within 6 months after completion of heat treatment 41 patients had complete closure of lesions (see Figure 4 and Supplementary Data) and 8 absence of clinical BU specific features []. Seven of the latter subsequently healed (Supplementary Figures 1, 12, 14, 20, 50–52), 1 remained with a small non-BU specific skin lesion (Supplementary Figure 29). Four patients were classified as treatment failures based on the clinical criteria and offered WHO recommended chemotherapy (Table 2, Supplementary Figures 8, 17, 43 and 55). One declined antibiotic treatment and healed under wound care alone. Sequential picture documentations of all patients are available in the Supplementary Figures 1–65.
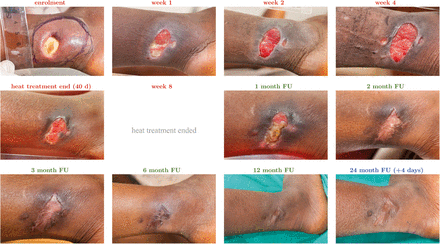
Figure 4
Clinical evolution of patient 14 (laboratory confirmed ulcer of the right lateral ankle) exemplary for all patients. Images of all other patients are available as Supplemental Figures 1–65. Pictures were taken within a range of ±2 days (heat treatment period), ±15 days (follow-up [FU] months 1–3), ±30 days (FU month 6) and ±60 days (FU months 12 + 24 months) from the designated time points. Deviations beyond these ranges are specifically indicated.
“Primary cure” within 6 months was 92.4% (95% CI, 81.8% to 98.0%). Analysis by WHO category did not show an association with outcome (Fisher exact test P-values >.28, data not shown).
In 2 trial patients M. ulcerans positive nodules distant from heat treated primary lesions also healed (Figure 5).
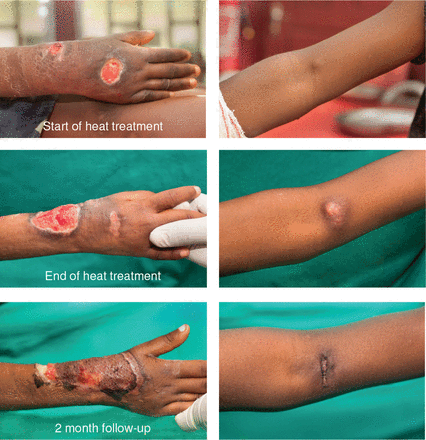
Figure 5
Distant healing. Patient with Buruli ulcer (BU) lesion at the right wrist and laboratory confirmed BU nodule at the left elbow, which healed with heat applied to the right wrist only.
Time to Wound Closure
Time to wound closure ≤1 month after completion of heat treatment in 15, ≤2 months in 5, ≤4 months in 15, and ≤6 months in 6 patients is illustrated in relation to “absence of clinical BU specific features” in Figure 6.
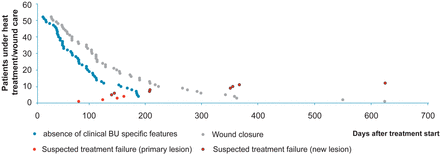
Figure 6
Endpoints. Blue circles illustrate the number of days until clinical Buruli ulcer (BU) specific features were no longer detectable in relation to the number of days to “wound closure” represented by grey circles. The days when patients were categorized as treatment failure are indicated by red circles (if related to the primary lesion) with a black outline (if related to a new lesion). This figure is available in black and white in print and in colour at Clinical Infectious Diseases online.
Absence of Recurrence 24 Months After Completion of Heat Treatment (Definite Cure)
No patient was lost to follow-up. Images of follow-up visits are documented in the Supplementary Figures 1–65. In total, 36 of 49 patients with cured primary lesions remained without new lesion for 24 months (Figure 7). Of 13 patients with new lesions 5 healed under wound care, in 2 combined with minor excisions (Supplementary Figures 16, 21, 24, 28, 34). The remaining 8 patients with new lesions were classified as treatment failure and offered WHO recommended chemotherapy (Supplementary Figures 4, 20, 36, 54, 60–63) resulting in a “definite cure” in 83.7% (95% CI, 70.3% to 92.7%).
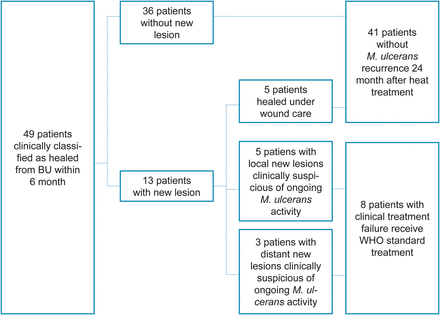
Figure 7
New lesions. Appearance and outcome of new lesions during the 24 month follow-up period in patients, who achieved primary cure of their Buruli ulcer (BU) lesion. Abbreviation: WHO, World Health Organization.
Compliance, Tolerability and Adverse Events
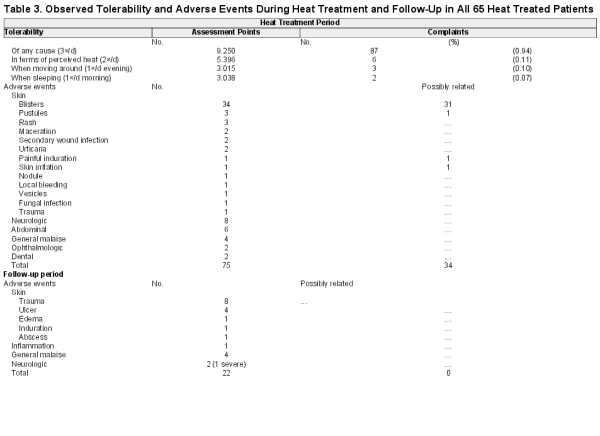
We included all 65 patients, who started heat treatment, in the analysis of compliance, tolerability, and adverse events (AE). One patient dropped out despite remarkable improvement (see Supplementary Figure 46). No patient withdrew consent after starting heat treatment. It was well tolerated (Table 3) with few therapy related complaints resolvable by adjustment of heat pack placement.
During thermotherapy 75 AEs affecting 41/65 (63.1%) patients were recorded; 34 instances affecting 23/65 (35.4%) patients were at least possibly related to heat treatment, all minor skin reactions at the site of heat application, mainly blisters (n = 31) subsiding under skin care within a few days (Table 3 and Supplementary Figure 66 “adverse events”). During follow-up 22 additional AEs, all unrelated to heat treatment, occurred including a death due to a cranial injury.
Patients With Clinical Disease Without Laboratory Confirmation
The demographic profile of the 10 unconfirmed patients corresponded widely with the 53 confirmed patients (Table 1). All lesions healed under heat treatment.
DISCUSSION
Controlled hyperthermia to treat microorganisms and cancers is receiving renewed attention []. It holds promise for BU since its first evaluation in the 1970s and has become practicable with cheap and easily applicable PCM [].
We achieved a primary cure rate of 92.4% (95% CI, 81.8% to 98.0%) defined as “wound closure” or “absence of clinical features of M. ulcerans infection” within 6 months after completion of heat treatment. The latter endpoint is required to differentiate the effect of anti-mycobacterial therapy from the subsequent general wound management (Figure 6). Our result is in the range of 2 chemotherapy trials [, ]. Comparison is limited, however, by differences in enrolment criteria (larger lesions in our study), definition and timing of study endpoints.
When determining the 24 months relapse rate, we observed 13 patients with new lesions appearing as late as 19 months after completion of treatment; 36 patients had uneventful follow-ups. As reported from other clinical trials, new lesions cannot be equated with treatment failure, however [, –]. Five of our 13 patients with new lesions healed under wound care alone. Eight were classified as treatment failure with the uncertainty intrinsic to the clinical diagnosis of BU (Figure 7).
With few exceptions clinical features of BU lesions are nonspecific with a wide range of differential diagnoses, most importantly immune-pathological reactions and non-BU related delayed wound healing [, ]. Currently, available laboratory tests to confirm the clinical suspicion of persisting BU activity or relapse after BU specific treatment are unsatisfactory. Positive PCR and ZN-stain are inconclusive, because mycobacterial DNA and dead bacteria may persist for a long time in affected tissue. Culture is not sensitive and takes too long to be useful for clinical decision making. Even positive M. ulcerans cultures 32 weeks and microscopic detection of AFBs 1.1 years after WHO recommended chemotherapy were compatible with successful treatment [, , ]. There is evidence from the BURULICO trial, other studies, and our own observations (eg, case no. 26) that after controlling the bulk of the mycobacterial burden remaining viable M. ulcerans may be eliminated by the immune system without additional specific treatment. Equally, confirmed BU nodules distant to heat treated primary lesions healed. Protective immune responses may be triggered by thermotherapy and chemotherapy [, , ] but not surgery, where the mycobacterial antigens are largely removed.
With these difficulties and the fact that in case of doubt BU suspicious lesions are declared treatment failure and offered standard treatment, efficacy of BU treatment in clinical trials is, if anything, underestimated.
To estimate the effect of the diagnostic uncertainty on the efficacy estimate, we looked at our “treatment failures” again after the full evidence of all investigations including the evolution of lesions was available. These were 12 cases, 4 related to the primary lesion, 8 to a new lesion. We calculated the heat treatment efficacy for the 6-month endpoint and the relapse rate for the 24-month endpoint for best and worst case scenarios.
For the 6-month endpoint the best case scenario is 100% (95% CI, 93.3% to 100%) with 4 “treatment failures” reclassified as wound infections (n = 2) and as non-BU related delayed healing (n = 2). The worst case scenario is 89.1% (95% CI, 77.8% to 59.9%) counting in addition to the 4 “treatment failures” also the patients as failures, who did not complete heat treatment (n = 1) and who died of unrelated reasons (n = 1). For the 24-month endpoint the best case scenario is 93.9% (95% CI, 84.3% to 98.8%), reclassifying 5 of 8 patients as immune pathological reactions and the worst case scenario 83.7% (95% CI, 70.3% to 92.7%) counting all 8 new clinically BU suspicious lesions as “treatment failures.”
It is worth noting that 4 of 8 patients who developed new lesions after thermotherapy and were declared “treatment failures” developed additional new BU suspicious lesions during (n = 1) or after (n = 3) chemotherapy. All healed without further specific treatment.
Until a point-of-care test with a high predictive value for clinically relevant M. ulcerans persistence or relapse is available the problem with diagnostic uncertainty can be pragmatically compensated by continuing heat treatment under close observation. The clinical evolution of the lesion will provide additional evidence in which direction the wound develops— non-BU related delayed healing (most commonly secondary bacterial infection) or clinically relevant non-thermotherapy-responsive M. ulcerans persistence or relapse.
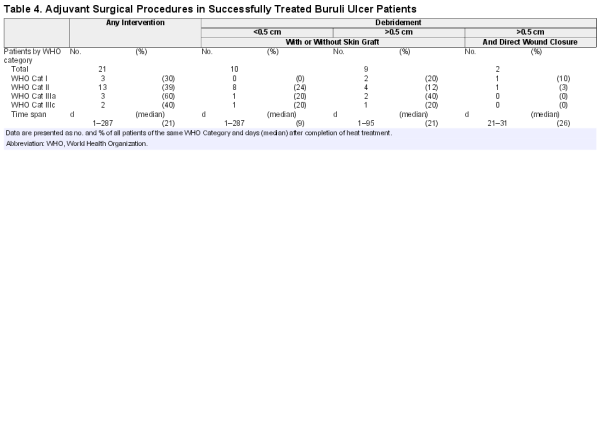
Adjuvant therapy, in particular surgical removal of nonviable tissue, may affect efficacy estimates. In line with Nienhuis et al [] we consider these procedures as normal wound care not triggering the classification treatment failure. For completeness surgical procedures in our patients are documented in Table 4 and the Supplementary Figures.
Osteomyelitis is a much debated problem and has been reported subsequent to WHO recommended chemotherapy []. Uncertainty remains on the cause in a significant proportion of published cases [–]. A recent prospective study could not link M. ulcerans to coexisting osteomyelitis in 22 laboratory confirmed BU patients []. We report 1 patient who developed osteomyelitis 13 months after completion of heat treatment and 2 months after treatment failure had been declared because of a new skin lesion suspicious of BU. The clinical diagnosis of osteomyelitis—at 2 sites unrelated to the locations of the primary and the new BU skin lesions—was subsequently confirmed by X-ray. The patient has received a prolonged antibiotic treatment and remains under follow-up.
Thermotherapy was well tolerated. Daytime activities including schooling were not interrupted.
In addition to its specific effect heat treatment promotes wound healing by increasing blood circulation, reducing edema through gentle compression and protecting the wound and renewal of heat packs is inevitably connected to inspection and care for the wound.
Heat related adverse events where limited to mild, short-lasting local skin reactions. PCM cannot exceed its melting temperature of 58°. Coverage of the wound and surrounding skin with sterile pads and a bandage are further measures to prevent tissue damage. With this set-up thermotherapy appears safe compared to the potentially severe and irreversible adverse effects of systemic chemotherapy including persistent hearing loss in over 25% of the participants in the BURULICO clinical drug trial in Ghana (2006–2008) [].
Cost of heat packs and recharging for 10 minutes in boiling water are thermotherapy-specific costs. Nursing time mainly goes into wound management, which is not different from chemotherapy patients. Positioning of heat packs is straightforward. The nurses were confident in heat pack application after few demonstrations. Cost and operational aspects were not formally assessed, however.
The skin lesions of 10 unconfirmed patients also healed under thermotherapy. This is encouraging for regions with limited access to laboratory confirmation and where quality assurance, in particular of PCR in reference labs, remains a problem. In a multicenter external quality assessment for PCR detection of M. ulcerans thus only 36% of the laboratories in a first round and 31% in a second round had more than 90% concordant results [].
CONCLUSION
Thermotherapy should be considered an alternative to chemotherapy as primary treatment for BU across all age classes for several reasons: it is highly effective, is easy to apply, cheap, well tolerated, free of relevant adverse effects, has nonspecific positive effects on wound healing and does not compromise wound healing in non-BU lesions in cases of misclassification. This is an undebatable advantage in settings where treatment decisions need to rely primarily on clinical diagnosis. Changing of heat packs urges health staff and patients to take notice of the wound and thus increases the probability of regular wound care. Heat therapy has potential as home remedy for BU suspicious lesions, ideally, combined with general wound management—a “package” we want to test at community level in low resource settings.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank all study participants and health-care and supportive staff involved in the trial particularly from the Ayos Regional Hospital, the Hospital of Bankim and FAIRMED Cameroon. We thank Steffen Luntz, Gregor Ottawa and Almaz Desta from the Coordinating Centre for Clinical Studies (KKS), Heidelberg University Hospital, for monitoring and data management. We thank the staff of the Pharmaceutical Medicine Unit, Swiss Tropical and Public Health Institute (TPH), for their advice in the planning of the study and the initiation visit. We thank Arianna Andreoli and Sarah Kerber from the Department of Infection Biology, Swiss TPH, for field support in Bankim and laboratory support in Basel.
Financial support. This study was supported by the Volkswagen Foundation, Germany (grant I/82 232).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.
- 2. Bratschi MW, Bolz M, Minyem JC, et al.. Geographic distribution, age pattern and sites of lesions in a cohort of Buruli ulcer patients from the Mape Basin of Cameroon. PLoS Negl Trop Dis 2013; 7:e2252.
- 3.
- 4. Asiedu K, Etuaful S. Socioeconomic implications of Buruli ulcer in Ghana: a three-year review. Am J Trop Med Hyg 1998; 59:1015–22.
- 5. Agbenorku P, Edusei A, Agbenorku M, et al.. Buruli-ulcer induced disability in Ghana: a study at Apromase in the Ashanti region. Plast Surg Int 2012:752749.
- 6. George KM, Chatterjee D, Gunawardana G, et al.. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 1999; 283:854–7.
- 7. Demangel C, Stinear TP, Cole ST. Buruli ulcer: reductive evolution enhances pathogenicity of Mycobacterium ulcerans. Nat Rev Microbiol 2009; 7:50–60.
- 8.
- 9. Nienhuis WA, Stienstra Y, Thompson WA, et al.. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet 2010; 375:664–72.
- 10.
- 11. Meyers WM, Shelly WM, Connor DH. Heat treatment of Mycobacterium ulcerans infections without surgical excision. Am J Trop Med Hyg 1974; 23:924–9.
- 12. Junghanss T, Um Boock A, Vogel M, Schuette D, Weinlaeder H, Pluschke G. Phase change material for thermotherapy of Buruli ulcer: a prospective observational single centre proof-of-principle trial. PLoS Negl Trop Dis 2009; 3:e380.
- 13. Braxmeier S, Hellmann M, Beck A, et al.. Phase change material for thermotherapy of Buruli ulcer: modelling as an aid to implementation. J Med Eng Technol 2009; 33:559–66.
- 14.
- 15.
- 16. Lavender CJ, Fyfe JA. Direct detection of Mycobacterium ulcerans in clinical specimens and environmental samples. Methods Mol Biol 2013; 943:201–16.
- 17. Bratschi MW, Bolz M, Grize L, et al.. Primary cultivation: factors affecting contamination and Mycobacterium ulcerans growth after long turnover time of clinical specimens. BMC Infect Dis 2014; 14:636.
- 18. Collins TJ. ImageJ for microscopy. Biotechniques 2007; 43(1 suppl):25–30.
- 19. Valencia BM, Miller D, Witzig RS, Boggild AK, Llanos-Cuentas A. Novel low-cost thermotherapy for cutaneous leishmaniasis in Peru. PLoS Negl Trop Dis 2013; 7:e2196.
- 20. Phillips RO, Sarfo FS, Abass MK, et al.. Clinical and bacteriological efficacy of combination of rifampicin and streptomycin for 2 weeks followed by rifampicin and clarithromycin for 6 weeks for treatment of Mycobacterium ulcerans disease. Antimicrob Agents Chemother 2014; 58:1161–6.
- 21. Nienhuis WA, Stienstra Y, Abass KM, et al.. Paradoxical responses after start of antimicrobial treatment in Mycobacterium ulcerans infection. Clin Infect Dis 2012; 54:519–26.
- 22. Ruf MT, Chauty A, Adeye A, et al.. Secondary Buruli ulcer skin lesions emerging several months after completion of chemotherapy: paradoxical reaction or evidence for immune protection? PLoS Negl Trop Dis 2011; 5:e1252.
- 23. Beissner M, Piten E, Maman I, et al.. Spontaneous clearance of a secondary Buruli ulcer lesion emerging ten months after completion of chemotherapy—a case report from Togo. PLoS Negl Trop Dis 2012; 6:e1747.
- 24. Schutte D, Pluschke G. Immunosuppression and treatment-associated inflammatory response in patients with Mycobacterium ulcerans infection (Buruli ulcer). Expert Opin Biol Ther 2009; 9:187–200.
- 25. Kibadi K, Boelaert M, Fraga AG, et al.. Response to treatment in a prospective cohort of patients with large ulcerated lesions suspected to be Buruli Ulcer (Mycobacterium ulcerans disease). PLoS Negl Trop Dis 2010; 4:e736.
- 26. Debacker M, Aguiar J, Steunou C, et al.. Mycobacterium ulcerans disease (Buruli ulcer) in rural hospital, Southern Benin, 1997–2001. Emerg Infect Dis 2004; 10:1391–8.
- 27. Portaels F, Aguiar J, Debacker M, et al.. Mycobacterium bovis BCG vaccination as prophylaxis against Mycobacterium ulcerans osteomyelitis in Buruli ulcer disease. Infect Immun 2004; 72:62–5.
- 28. Phanzu DM, Bafende EA, Dunda BK, et al.. Mycobacterium ulcerans disease (Buruli ulcer) in a rural hospital in Bas-Congo, Democratic Republic of Congo, 2002–2004. Am J Trop Med Hyg 2006; 75:311–4.
- 29. Trellu LT, Nkemenang P, Bastard M, et al.. Differential diagnoses of Buruli ulcer: Data from Akonolinga, Cameroon. In: WHO Meeting on Buruli ulcer, Control and Research 2015. Geneva, Switzerland: WHO Headquarters.
- 30. Klis S, Stienstra Y, Phillips RO, Abass KM, Tuah W, van der Werf TS. Long term streptomycin toxicity in the treatment of Buruli Ulcer: follow-up of participants in the BURULICO drug trial. PLoS Negl Trop Dis 2014; 8:e2739.
- 31. Eddyani M, Lavender C, de Rijk WB, et al.. Multicenter external quality assessment program for PCR detection of Mycobacterium ulcerans in clinical and environmental specimens. PLoS One 2014; 9:e89407.