Introduction
The articular cartilage is a thin connective tissue that lines the epiphysis in synovial joints and is mainly composed of chondrocytes and extracellular matrices. The adult articular cartilage can be divided into four layers based on its histological characteristics: surface, intermediate, deep, and calcified layers, which are connected to the subchondral bone beneath it. This tissue has unique viscoelastic properties, providing a low-friction surface for joint movement and cushioning the underlying bone during loading. However, cartilage can degenerate as consequences of trauma, mechanical stress, and other molecular biological processes. Degenerated cartilage is difficult to repair owing to its avascular nature, which results in limited self-renewal properties. Osteoarthritis (OA) and rheumatoid arthritis are major diseases associated with cartilage degeneration that affect increasing numbers of patients worldwide. Cartilage disorders impair physical function,, contributing to the deterioration of patients’ quality of life.
In OA, which is mainly characterized by cartilage degeneration, the mainstay of therapy is pain management using drugs such as acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase-2 inhibitors, hyaluronic acid (HA), and corticosteroids. HA has been suggested to have a chondroprotective effect on osteoarthritic cartilage,; however, current clinical evidence is limited. Despite recent advances regarding both drug (such as inhibitors of matrix-degrading enzymes, growth factors, Wnt signal inhibitors, senolytic agents, and bone resorption inhibitors) and cell therapies (allogeneic chondrocytes expressing transforming growth factor beta 1 and mesenchymal stem cells) that are expected to have disease-modifying effects on OA,, there is no prospect for their practical application. Given the lack of available disease-modifying drugs for the treatment of cartilage degeneration, a re-evaluation of existing symptom-modifying drugs based on their chondroprotective effects may help optimize the treatment of diseases associated with cartilage degeneration.
Diclofenac etalhyaluronate (DEH; SI-613/ONO-5704) is a derivative of high-molecular-weight fermented HA (600-1,200 kDa), of which glucuronic acid moieties are chemically bound to the NSAID diclofenac (DF) via a 2-aminoethanol linker. DF is gradually released from DEH by the hydrolytic cleavage of the ester linkage, in a pH-dependent manner. Intra-articularly administered DEH remains in the joint for a long period and locally releases DF in a sustained manner. Furthermore, DEH has been shown to exert anti-inflammatory and analgesic effects in experimental animal models; in addition, DEH has been shown to improve knee and hip OA pain in clinical trials.- In Japan, DEH has become clinically available as a drug to improve joint function in OA; however, the effect of DEH on cartilage degeneration remains unclear.
In the present study, we investigated the chondroprotective effect of DEH in vivo in rats with collagen-induced arthritis (CIA) and interleukin-1β (IL-1β)-stimulated human chondrocytes in vitro.
Materials and Methods
Materials
DEH (DF: approximately 11.8% [w/w]) and HA (600-1,200 kDa) were manufactured by Seikagaku Corporation (Tokyo, Japan). DF and 10% neutral-buffered formalin were purchased from Fujifilm Wako Pure Chemical Corporation (Osaka, Japan). Distilled water was purchased from Otsuka Pharmaceutical Factory Inc. (Tokushima, Japan). Incomplete Freund’s adjuvant was purchased from Becton Biosciences (Franklin Lakes, NJ). Bovine type II collagen was purchased from the Collagen Research Center (Tokyo, Japan). Phosphate-buffered saline (PBS), TRIzol reagent, PureLink RNA Mini Kit, High-Capacity cDNA Reverse Transcription Kit with RNase Inhibitor, and Dulbecco’s Modified Eagle’s Medium (DMEM)/F-12 were purchased from Thermo Fisher Scientific (Waltham, MA). Fetal bovine serum (FBS) was purchased from Sigma-Aldrich (St. Louis, MO). TB Green Premix Ex Taq II was purchased from Takara Bio, Inc. (Shiga, Japan). Recombinant human IL-1β and enzyme-linked immunosorbent assay (ELISA) kits for human matrix metalloproteinase (MMP)-3 and MMP-13 were purchased from R&D Systems (Minneapolis, MN).
Animals
Female Dark Agouti rats (9-10 weeks old) were purchased from Japan SLC, Inc. (Shizuoka, Japan). The animals were housed in specific pathogen-free conditions at a room temperature ranging from 20 to 26 °C, humidity of approximately 50%, and a 12-hour light/dark cycle. Animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of Seikagaku Corporation and were performed under an animal husbandry/management system in an appropriate environment for animal welfare.
Induction and Treatment of CIA
The bovine collagen type II solution was emulsified by mixing it with an equal amount of incomplete Freund’s adjuvant. Rats were anesthetized with isoflurane inhalation and immunized by intradermal injection of the emulsion (0.05 mg/100 μL/site) at four sites on the back; this protocol results in an autoimmune reaction to collagen and the induction of arthritis. The normal control rats were non-treated or underwent the same procedures but were intradermally injected with physiological saline. Fourteen days after immunization, 50 μL of PBS, DEH (0.05-0.5 mg for dose-response analysis and 0.5 mg for other experiments), HA (0.5 mg), or a mixture of DF (59 μg, the same amount contained in 0.5 mg DEH) and HA (0.5 mg) was administered into the articular cavity of the knees of both hindlimbs under isoflurane anesthesia. DF was orally administered once daily from 14 to 21 or 28 days after immunization. The DF dose was set at 2 mg/kg, which is the same weight-adjusted dose of DF (maximum dose, 100 mg daily) prescribed for an OA or rheumatoid arthritis patient weighing 50 kg. DEH, HA, and the mixture of DF and HA were dissolved in PBS; DF was prepared in distilled water. Arthritis was induced in 81, 28, and 56 rats for joint swelling, histological, and gene expression analyses, respectively (see Supplementary Table S1-S6 for grouping). Forty-seven rats served as non-arthritic controls.
Histological Assessment
The rats were exsanguinated under isoflurane inhalation anesthesia, and the proximal end of the tibia at the hind limb knee joints was collected. Tissues were collected before immunization; at 7, 14, 21, and 28 days after immunization for a time-course study; and at 21 days after immunization for pharmacological evaluation. In the time-course study, the right knees of three rats were evaluated at each time point (Supplementary Table S1). In the pharmacological study, eight knees (the left and right knees of four CIA rats) per group were evaluated (Supplementary Table S2). In the non-arthritic control group, both knees of two rats were evaluated. Tissues were fixed in 10% neutral-buffered formalin and decalcified in formic acid formalin. The medial tibia was sectioned longitudinally and embedded in paraffin. The sections were stained with safranin O/fast green, and cartilage degeneration severity was scored according to the Mankin scoring system (Table 1), with a slight modification: the “Tidemark integrity” category was scored 1 when the tidemark was destroyed. The effects of the test materials were evaluated in the anterior one-third of the tibial cartilage, where degeneration due to model progression was evident. Scoring was performed in a blinded manner.
Assessment of Joint Swelling
Rats were anesthetized using isoflurane inhalation on the day before immunization and at 7, 13, 14, 15, 16, 17, 19, 21, 23, 24, 26, and 28 days after immunization to measure knee joint width. Nine CIA rats per group were evaluated in both dose-response and pharmacological studies (Supplementary Tables S3 and S4). In the non-arthritic control group, five rats were evaluated. The rats were fixed in a supine position, and the horizontal width (in mm) of the knee joints of the left and right hindlimbs was measured using a digital thickness gauge. Using the knee joint width on the day before collagen sensitization as the baseline, the change in the knee joint width (in mm) of both hindlimbs was calculated, and the mean was defined as knee swelling (in mm) for each animal after sensitization. Measurements after administration of the test materials were performed in a blinded manner.
RNA Extraction and Real-Time Polymerase Chain Reaction
The rats were exsanguinated under isoflurane inhalation anesthesia, and the synovial tissue and cartilage of the hind limb knee joints were collected and rapidly frozen in liquid nitrogen. Synovial tissue or cartilage from both knees of each rat were pooled in tubes to obtain sufficient RNA for real-time polymerase chain reaction (RT-PCR) analysis. Tissue collection was performed before immunization and at 7, 14, and 21 days after immunization for a time-course study, or 21 days after immunization for pharmacological evaluation. Eight rats per group were evaluated in both studies (Supplementary Tables S5 and S6). Tissues were homogenized in TRIzol reagent. Total RNA was extracted using the PureLink RNA Mini Kit according to the manufacturer’s instructions, and RNA concentrations were measured using a microvolume spectrophotometer. The cDNA samples were prepared using a high-capacity cDNA reverse transcription kit with an RNase Inhibitor. The expression levels of Il1b, Mmp3, and Mmp13 were quantified using the CFX384 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA) with TB Green Premix Ex Taq II. The thermal cycle program was one initial cycle at 95 °C for 30 seconds, followed by a 30-cycle sequence of denaturing at 95 °C for 5 seconds and annealing/extension at 60 °C for 30 seconds. The housekeeping gene Hprt1 was used as a reference gene for normalization. The primer sequences were: Mmp3, 5’-ATGATGAACGATGGACAGATGA-3’ (forward) and 5’-CATTGGCTGAGTGAAAGAGACC-3’ (reverse); Mmp13, 5’-TGGAACTAAAGAACATGGTGACTTCTA-3’ (forward) and 5’-CCCCGCCAAGGTTTGG-3’ (reverse). Commercially available primer pairs for Il1b (RA063423) and Hprt1 (RA015379) were purchased from Takara Bio, Inc. The expression level of each gene was quantified using a linearly regressed calibration curve and normalized to the expression level of Hprt1.
Chondrocytes Culture
Human chondrocytes (Lonza [Basel, Switzerland] and Cell Applications Inc. [San Diego, CA]) were cultured in DMEM/F-12 supplemented with 10% FBS at 37 °C in 5% CO2. Cells passed once or twice were seeded in 24-well plates at a starting density of 1.5 × 105 cells/mL per well, 22-24 hours before treatment. The culture medium was replaced with 1 mL of DMEM/F-12 containing 10% FBS and the corresponding materials: HA (2 mg/mL), DEH (0.5-2 mg/mL), or DF (0.03-3 µg/mL). The concentrations of DF were set below the cytotoxic range (>30 µg/mL) according to our preliminary experiments (data not shown). After a 30-minute incubation, IL-1β (0.1 ng/mL) was added and incubated for 16 hours, after which culture supernatants were collected. The concentrations of MMP-3 and MMP-13 in the culture supernatants were measured using an ELISA kit, according to the manufacturer’s instructions. Six wells were treated with each test material, and samples from each well were analyzed separately. Data are representative of several independent experiments.
Statistical Analysis
Statistical analyses were performed using the Statistical Analysis System (SAS; SAS Institute Inc., Cary, NC). In the in vivo study, the histological scores of each group, except for the normal group, were statistically analyzed using the Steel-Dwass test. The mean joint swelling values from days 14 to 28 after immunization were subjected to statistical analysis. The data for each group, except the normal group, were statistically analyzed using Williams’ test (for the dose-response study) or Tukey’s test (for the pharmacological study). Gene expression levels in each group were analyzed using Tukey’s test. In the in vitro study, MMP concentrations were statistically analyzed using Williams’ test or Student’s t test. Statistical significance was set at P < 0.05. Data are presented as mean and standard error of the mean.
Results
Cartilage Degeneration in a Rat CIA Model
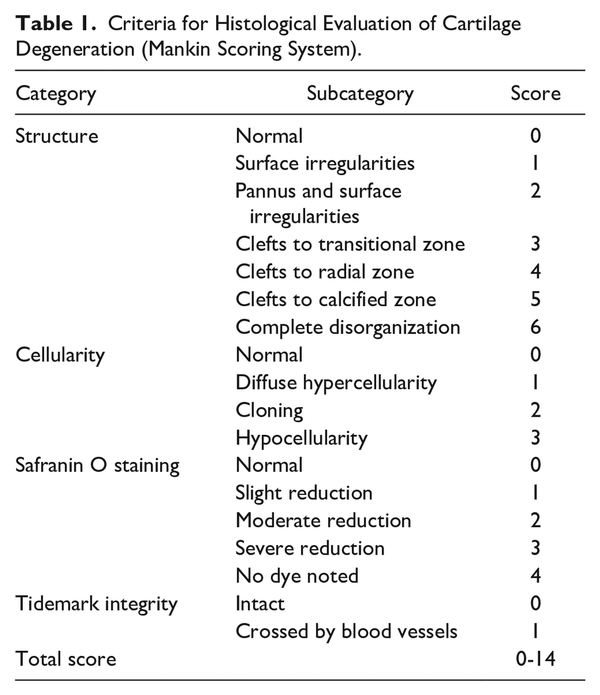
Time-dependent cartilage degeneration in a rat model of CIA was histopathologically evaluated. Before sensitization, the cartilage surface was smooth without roughening (Fig. 1). The extracellular matrix contained proteoglycans, which were stained with safranin O. Cells were present throughout the cartilage, with no clustering. No tidemark irregularities were observed. Cartilage histology 7 and 14 days after immunization was similar to that before sensitization, with no abnormalities in surface structure, cellularity, safranin O staining intensity, or tidemark. From 21 days after immunization onward, safranin O staining was reduced, and progression of cartilage degeneration was observed. No abnormalities in surface structure, cellularity, or tidemarks were observed.
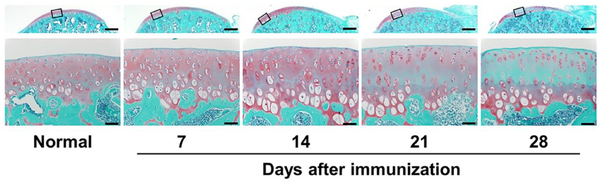
Figure 1
Sections of the tibial cartilage of a knee joint were stained with safranin O and fast green, where proteoglycan in the cartilage is in red and bone is in green. The upper images present an overview of the cartilage; the lower images correspond to the high magnification of the squared area in the upper image. Representative images are shown (n = 3). Scale bar = 500 µm (upper images) and 50 µm (lower images).
Effect of DEH on Joint Swelling, Il1b Transcription, and Cartilage Degeneration in a CIA Model
A dose-dependent inhibition of joint swelling by DEH was observed at 0.05-0.5 mg/joint doses (Fig. 2A). No inhibitory effect on joint swelling was observed with HA or the mixture of DF and HA (Fig. 2B). DEH inhibited joint swelling more potently than oral DF. The expression of Il1b mRNA in the synovial tissue was reduced by DEH and DF administration (Fig. 2C). HA administered in the same manner as DEH resulted in a slightly lower Il1b expression; however, this effect was not statistically significant compared with PBS. Histopathological evaluation demonstrated that DEH inhibited the reduction in safranin O staining elicited by arthritis induction (Fig. 2D). No effects of HA were observed. The inhibitory effect of DEH on Safranin O staining reduction was comparable to that of DF administered orally. Furthermore, administration of DEH and DF resulted in a statistically significant reduction in the total Mankin score compared with PBS (Fig. 2E).
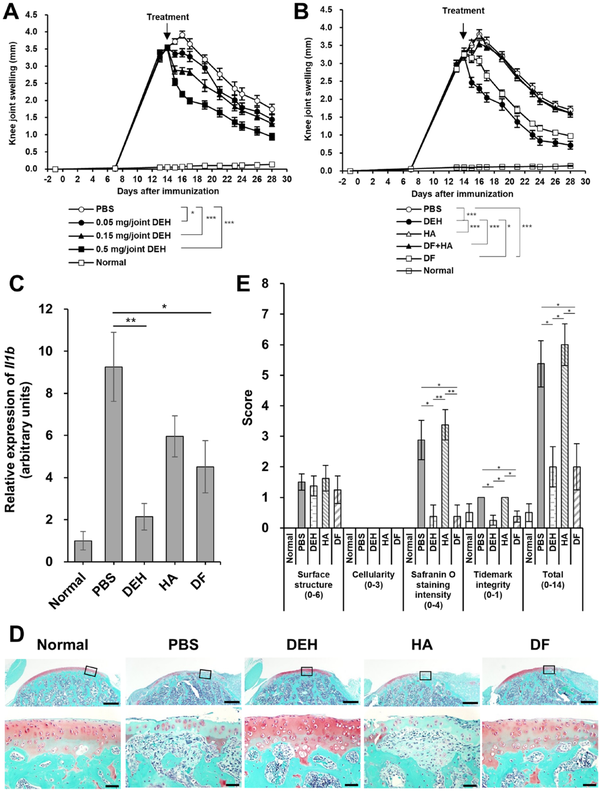
Figure 2
Anti-inflammatory and chondroprotective effects of DEH in CIA rats. (A) Dose-dependent effect of DEH on the inflammatory swelling of the knee. (B) Comparison of the effects of DEH and its chemical components (HA, a mixture of DF and HA [DF + HA], and DF) on the inflammatory swelling of the knee. (C) The expression level of Il1b mRNA in the synovial tissue. (D) Representative microscopic images showing the effects of DEH and its chemical components on cartilage degeneration. Sections of tibial cartilage were stained with safranin O and fast green. The upper images present an overview of the cartilage. The lower images represent the squared area of the corresponding upper image at high magnification. Scale bar = 500 µm (upper images) and 50 µm (lower images). (E) Tibial sections were scored for signs of cartilage degeneration based on the Mankin scoring system. *P < 0.05, **P < 0.01, ***P < 0.001. Values represent the mean ± standard error. DEH = diclofenac etalhyaluronate; CIA = collagen-induced arthritis; HA = hyaluronic acid; DF = diclofenac; PBS = phosphate-buffered saline.
Effect of DEH on Mmp3 and Mmp13 Expression in a CIA Model
To investigate the mechanisms underlying the chondroprotective effect of DEH, the Mmp3 and Mmp13 mRNA expression in the knee joints of CIA rats was measured using RT-PCR. We observed that, in this model, there was no change in Mmp3 or Mmp13 mRNA levels in the cartilage or synovial tissue 1 week after immunization. However, Mmp3 and Mmp13 mRNA levels increased 14 and 21 days after immunization compared to those in normal rats (Fig. 3). To evaluate the inhibitory effect of DEH on the expression of Mmp3 and Mmp13 mRNA, DEH was administered to the articular cavity of the knees of animals. DEH (0.5 mg/joint) significantly reduced the expression of Mmp3 mRNA in the cartilage compared with PBS (Fig. 4A). Conversely, DF administered orally for 7 days at a dose of 2 mg/kg had no effect. Although not statistically significant, the Mmp13 mRNA levels decreased in the DEH and DF groups. In synovial tissue, DEH and DF significantly reduced the expression levels of Mmp3 and Mmp13, whereas HA did not affect the expression of Mmp3 and Mmp13 mRNA in the synovial tissue or cartilage (Fig. 4B).
Figure 3
Upregulation of Mmp3 and Mmp13 mRNA in the knee tissues of rats with CIA. The expression levels of Mmp3 (left panel) and Mmp13 (right panel) in the cartilage (A) and synovial tissue (B) were determined by real-time polymerase chain reaction (RT-PCR). Results are expressed as fold changes compared with normal controls. Values represent the mean ± standard error. CIA = collagen-induced arthritis.
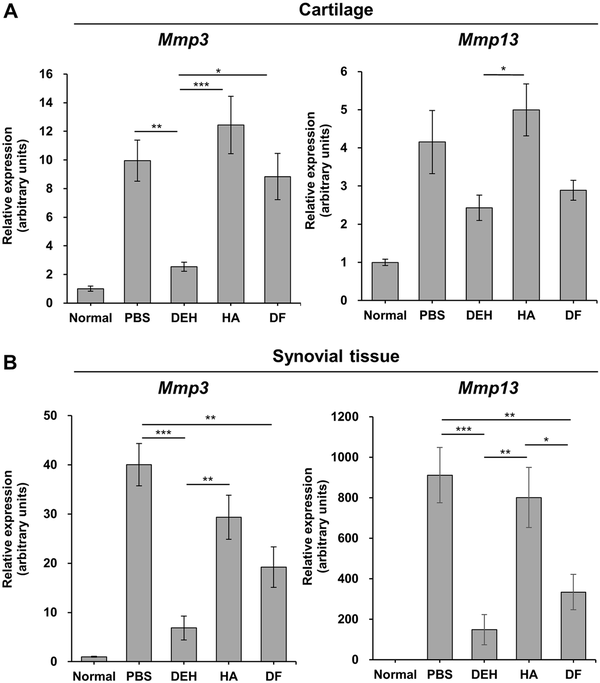
Figure 4
Reduction of Mmp3 and Mmp13 mRNA expression in CIA rats treated with DEH. The expression levels of Mmp3 (left panel) and Mmp13 (right panel) in the cartilage (A) and synovial tissue (B) were determined by RT-PCR. Results are expressed as fold changes compared with normal controls. *P < 0.05, **P < 0.01, ***P < 0.001. Values represent the mean ± standard error. CIA = collagen-induced arthritis; DEH = diclofenac etalhyaluronate; RT-PCR = real-time polymerase chain reaction.
Effect of DEH on MMP-3 and MMP-13 Production on IL-1β-Stimulated Human Chondrocytes
To further investigate the inhibitory effect of DEH on MMP production in cartilage, we analyzed the protein expression of MMP-3 and MMP-13 in IL-1β-stimulated human chondrocytes. DEH decreased the levels of MMP-3 and MMP-13 in the culture supernatant in a concentration-dependent manner (Fig. 5A). Similarly, HA reduced the expression of MMP-3 and MMP-13. The dose of DF used in the CIA rat model caused cytotoxicity (data not shown); thus, we used non-cytotoxic doses of DF (0.03-3 µg/mL) and found that MMP-3 and MMP-13 expression levels were increased in these conditions (Fig. 5B).
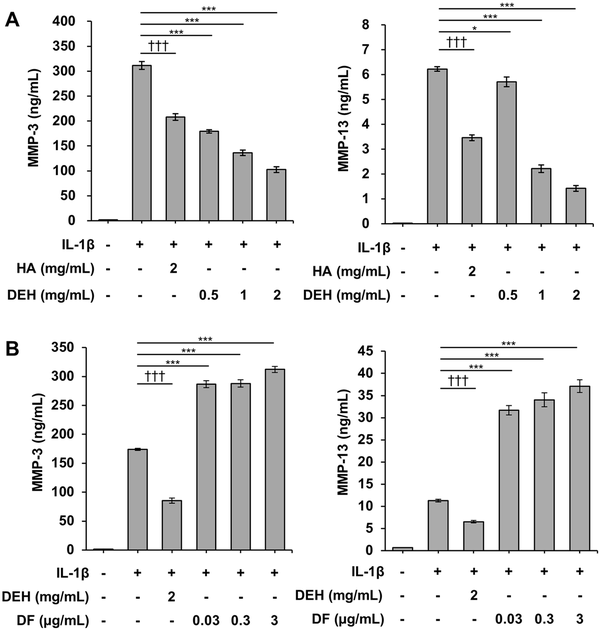
Figure 5
Inhibition of MMP-3 and MMP-13 expression by DEH in IL-1β-stimulated human chondrocytes. Concentrations of MMP-3 (left panel) and MMP-13 (right panel) in the culture supernatant were measured using an enzyme-linked immunosorbent assay (ELISA). (A) DEH reduced the production of MMP-3 and MMP-13 in a similar manner to HA. (B) By contrast, DF, a chemical component of DEH, increased the production of MMP-3 and MMP-13. *P < 0.05, **P < 0.01, ***P < 0.001, Williams’ test (vs. IL-1β-treated cells). †††P < 0.001, Student’s t test (vs. IL-1β-treated cells). The values represent the mean ± standard error. MMP = matrix metalloproteinase; DEH = diclofenac etalhyaluronate; IL-1β = interleukin-1β; HA = hyaluronic acid; DF = diclofenac; PBS = phosphate-buffered saline.
Discussion
In this study, we examined the pharmacological actions of DEH, an HA derivative chemically bound to DF, in a rat CIA model and in IL-1β-stimulated human chondrocytes. We demonstrated that DEH not only has analgesic and anti-inflammatory effects but also has a chondroprotective effect. Previously, we showed that DEH ameliorated symptoms of OA in clinical studies.- We also found that DF locally released from DEH in joints was retained in the synovial tissue for approximately 4 weeks and showed persistent anti-inflammatory effects in non-clinical studies., Whether NSAIDs such as DF have beneficial effects on structural OA lesions is controversial. Both beneficial and neutral effects of NSAIDs on cartilage have been reported in in vitro and in vivo studies-; however, negative effects of NSAIDs on the structural pathology of OA have also been observed., The present study showed, for the first time, that DEH has a protective effect against cartilage degeneration caused by inflammation.
Our findings indicated that DEH suppressed Il1b transcription and joint swelling in the rat CIA model. These anti-inflammatory effects were not observed with HA (Fig. 2B and 2C); thus, the anti-inflammatory effect of DEH was likely the result of DF release due to hydrolysis of the ester bond in DEH. Moreover, we observed anti-inflammatory effects of DEH were stronger than those of DF administered orally in multiple doses. As high doses of DF (5 mg/kg) induce gastrointestinal disorders in rats, we had to limit the DF dose that could be administered to the animals. DEH was administered locally at the sites of inflammation; therefore, DEH displayed higher efficacy than DF (2 mg/kg) without adverse gastrointestinal events (data not shown). In this study, we did not observe any anti-inflammatory effects of the DF and HA mixture (Fig. 2B). In a pharmacokinetic study using a rabbit model of arthritis, DF was found to disappear from the joint tissue earlier after the administration of a mixture of DF and HA than after the administration of DEH. Thus, we speculated that, in our study, DF administered as a mixture of DF and HA disappeared before producing an anti-inflammatory effect. Conversely, the prolonged local retention of DEH after administration and the sustained release of DF in the joint tissue may underlie the observed anti-inflammatory effects of DEH. The long-term retention of DEH in joints may be associated with the high molecular weight of DEH and the lipophilic nature of DF introduced into HA, which contributes to the interaction of DEH with the lipid bilayer membrane of cells.
Here, we found an inhibitory effect exerted by both DEH administered intra-articularly and DF administered orally on cartilage degeneration in the CIA model; no effect was observed for HA administration (Fig. 2E). Cartilage degeneration involves the disruption of biological homeostasis associated with inflammation, mechanical stress, cell death, and cellular stress. Degenerated cartilage presents with roughening/fibrosis of the cartilage surface, decreased clustering of chondrocytes, reduced proteoglycan levels, and irregular tidemark. Our findings suggest that DEH supports cartilage homeostasis by inhibiting the reduction in proteoglycan levels and the progression of cartilage degenerative diseases such as OA. HA has been reported to protect the cartilage in surgically induced OA models when administered three or five times at weekly intervals.- We speculated that HA did not show a chondroprotective effect in our study because it was administered as a single dose, similarly to DEH.
MMPs are metal-requiring proteases induced by inflammatory cytokines and mechanical stress and involved in cartilage degenerative disorders such as OA. The expression of MMP1, MMP3, MMP13, and MMP28 is increased in osteoarthritic cartilage and the production of MMP-1, MMP-3, MMP-9, and MMP-13 is increased in synovial tissue. The substrates of MMP-3 and MMP-13 include aggrecan, a core protein of proteoglycan, and collagen type II, the major component of cartilage; therefore, MMP-3 and MMP-13 are thought to be involved in cartilage degeneration. To investigate the mechanism of the chondroprotective effect of DEH, we analyzed its effect on the expression of Mmp3 and Mmp13 in the cartilage and synovial tissue. DEH inhibited the expression of Mmp3 in the cartilage and Mmp3 and Mmp13 in the synovial tissue (Fig. 4). Thus, we propose that the inhibitory effect exerted by DEH on cartilage degeneration was mediated by the decrease in MMP production in both synovial and cartilaginous tissues. Similar to DEH, DF inhibited cartilage degeneration (Fig. 2E) and the expression of Mmp3 and Mmp13 in synovial tissue (Fig. 4B). DF released from DEH in the articular cavity was speculated to exert an anti-inflammatory effect on the synovial tissue, thereby indirectly inhibiting the production of MMPs and cartilage degeneration. In contrast to DEH, DF and HA, the components of DEH, did not inhibit Mmp3 expression in the cartilage (Fig. 4A). As an effect of DEH on cartilage was not found with either DF or HA, we propose that the inhibition of Mmp3 expression in the cartilage occurred via a specific DEH-mediated mechanism. Kisukeda et al. reported that DEH uniquely regulated the transcription of genes involved in the synthesis or degradation of HA (HAS2 and HYAL2), whereas DF or a mixture of DF and HA had no observable effects on these genes. Thus, the effect of DEH on HA turnover may be involved in the inhibition of Mmp3 expression in the cartilage, observed for DEH.
To further explore the chondroprotective effect of DEH, we assessed its effects on MMP production in IL-1β-stimulated human chondrocytes and found that DEH inhibited MMP-3 and MMP-13 production (Fig. 5). HA suppresses the production of MMPs by inhibiting the phosphorylation of p38 mitogen-activated protein kinase through its receptor CD44. Similarly, DEH was speculated to act directly on chondrocytes and inhibit MMP-3 and MMP-13 production. In contrast, DF promoted the expression of MMP-3 and MMP-13 in vitro (Fig. 5B). Several studies have investigated the effects of cyclooxygenase inhibition on MMP production in chondrocytes; however, the results have been inconsistent. Cheleschi et al. and Fioravanti et al. reported inhibition of MMP-3 and MMP-13 expression by naproxen and naproxcinod, and MMP-3 expression by celecoxib, respectively. In contrast, Hashizume and Mihara reported that celecoxib and indomethacin promoted IL-1β expression in IL-1β-stimulated chondrocytes, indirectly promoting the expression of MMP-3 and MMP-13 in an autocrine manner. Our findings regarding the effects of DF were similar to those of Hashizume and Mihara, suggesting that, in chondrocytes, DF might promote MMP-3 and MMP-13 expression via the production of IL-1β.
Inflammatory mechanisms (e.g., synovitis) are known to be involved in joint disease etiology, some of which also have a non-inflammatory component (e.g., mechanical stress), such as OA. The experimental models used in this study, a rat CIA model and IL-1β-stimulated human chondrocytes, mimic the inflammatory conditions in joint diseases. Therefore, the chondroprotective effect of DEH observed in our study is limited to inflammatory conditions, and it remains unknown whether DEH affects joint diseases caused by non-inflammatory mechanisms. Age-related factors should also be considered. OA is a common disease in the elderly, and cartilage regenerative capacity is thought to be impaired in the patients. In our in vivo study, CIA was induced in young rats; therefore, the chondroprotective effect of DEH may have been exaggerated compared to what can be achieved in patients with OA patients. To comprehensively understand the effect of DEH on cartilage degeneration, its inhibitory effect on cartilage degeneration should be evaluated in a clinically relevant OA model that includes non-inflammatory and advanced-age components. Furthermore, DEH is expected to have a structure-modifying effect on the degenerated cartilage in patients with OA.
Conclusion
In this study, DEH was found to have a chondroprotective effect in addition to its known analgesic and anti-inflammatory effects. The chondroprotective effects of DEH consist of a combination of indirect outcomes associated with the anti-inflammatory effect of DF, direct effects on chondrocytes similar to those of HA, and a novel effect via a specific DEH-mediated mechanism. Our findings indicate that DEH not only reduces pain in patients with OA, as demonstrated in clinical studies, but may also inhibit cartilage degeneration in these patients.
Acknowledgments and Funding The authors thank Dr. Aisuke Nii for interpretation of the histological appearance of tissue sections and Mr. Ryoji Zuinen, Ms. Reiko Zuinen, Mr. Kazuhiro Kojima, Dr. Kei Toyama, Mr. Tomochika Kisukeda, Ms. Yaya Sugano, Ms. Yukie Saeki, and Ms. Haruka Mochizuki for their technical assistance in this study.
Author Contributions All authors have contributed to study conception and design and acquisition and interpretation of data. S.T. and K.Y. contributed to drafting and revision of the article. All the authors have reviewed and approved the final version of the manuscript. All authors have also reviewed and approved the data presented in the manuscript.
Declaration of Conflicting Interests The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors of this paper are employees of Seikagaku Corporation.
Ethical Approval Animal studies were reviewed and approved by the Animal Experiment Ethics Committee of the Seikagaku Corporation and were performed under the animal husbandry/management system in an appropriate environment for animal protection/welfare.
Shuhei Takada
https://orcid.org/0009-0006-7474-109X
Availability of Data and Materials The dataset supporting the conclusions of this study was stored at Seikagaku Corporation, Tokyo, Japan. Further inquiries regarding these data may be submitted to Shuhei Takada ([email protected]).
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
References
- 1. Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465-80.
- 2. Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461-8. doi:10.1177/1941738109350438.
- 3. Sherwood JC, Bertrand J, Eldridge SE, Dell’Accio F. Cellular and molecular mechanisms of cartilage damage and repair. Drug Discov Today. 2014;19(8):1172-7. doi:10.1016/j.drudis.2014.05.014.
- 4. Khan IM, Gilbert SJ, Singhrao SK, Duance VC, Archer CW. Cartilage integration: evaluation of the reasons for failure of integration during cartilage repair. A review. Eur Cell Mater. 2008;16:26–39. doi:10.22203/ecm.v016a04.
- 5. Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA, et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79(6):819-28. doi:10.1136/annrheumdis-2019-216515.
- 6. Aletaha D, Funovits J, Smolen JS. Physical disability in rheumatoid arthritis is associated with cartilage damage rather than bone destruction. Ann Rheum Dis. 2011;70(5):733-9. doi:10.1136/ard.2010.138693.
- 7. Kaukinen P, Podlipská J, Guermazi A, Niinimäki J, Lehenkari P, Roemer FW, et al. Magnetic resonance imaging (MRI)—defined cartilage degeneration and joint pain are associated with poor physical function in knee osteoarthritis—the Oulu Knee Osteoarthritis study. Osteoarthritis Cartilage. 2017;25(11):1829-40. doi:10.1016/j.joca.2017.07.002.
- 8. Vitaloni M, Botto-van Bemden A, Sciortino Contreras RM, Scotton D, Bibas M, Quintero M, et al. Global management of patients with knee osteoarthritis begins with quality of life assessment: a systematic review. BMC Musculoskelet Disord. 2019;20(1):493. doi:10.1186/s12891-019-2895-3.
- 9. Frizziero L, Pasquali Ronchetti I. Intra-articular treatment of osteoarthritis of the knee: an arthroscopic and clinical comparison between sodium hyaluronate (500–730 kDa) and methylprednisolone acetate. J Orthopaed Traumatol. 2002;3:89–96. doi:10.1007/s101950200034.
- 10. Goldberg VM, Buckwalter JA. Hyaluronans in the treatment of osteoarthritis of the knee: evidence for disease-modifying activity. Osteoarthritis Cartilage. 2005;13(3):216-24. doi:10.1016/j.joca.2004.11.010.
- 11. Latourte A, Kloppenburg M, Richette P. Emerging pharmaceutical therapies for osteoarthritis. Nat Rev Rheumatol. 2020;16(12):673-88. doi:10.1038/s41584-020-00518-6.
- 12. Cho Y, Jeong S, Kim H, Kang D, Lee J, Kang SB, et al. Disease-modifying therapeutic strategies in osteoarthritis: current status and future directions. Exp Mol Med. 2021;53(11):1689-96. doi:10.1038/s12276-021-00710-y.
- 13. Ishii S, Yoshioka K, Morita D. Residual property of SI-613, a novel drug for osteoarthritis, in knee joint. Yakugaku Zasshi. 2020;140(9):1141-50. doi:10.1248/yakushi.20-00008. (In Japanese)
- 14. Yoshioka K, Kisukeda T, Zuinen R, Yasuda Y, Miyamoto K. Pharmacological effects of N-[2-[[2-[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxy]ethyl]hyaluronamide (diclofenac etalhyaluronate, SI-613), a novel sodium hyaluronate derivative chemically linked with diclofenac. BMC Musculoskelet Disord. 2018;19(1):157. doi:10.1186/s12891-018-2077-8.
- 15. Nishida Y, Kano K, Nobuoka Y, Seo T. Efficacy and safety of diclofenac-hyaluronate conjugate (diclofenac etalhyaluronate) for knee osteoarthritis: a randomized phase III trial in Japan. Arthritis Rheumatol. 2021;73(9):1646-55. doi:10.1002/art.41725.
- 16. Nishida Y, Kano K, Nobuoka Y, Seo T. Sustained-release diclofenac conjugated to hyaluronate (diclofenac etalhyaluronate) for knee osteoarthritis: a randomized phase 2 study. Rheumatology. 2021;60(3):1435-44. doi:10.1093/rheumatology/keaa605.
- 17. Kubo T, Kumai T, Ikegami H, Kano K, Nishii M, Seo T. Diclofenac-hyaluronate conjugate (diclofenac etalhyaluronate) intra-articular injection for hip, ankle, shoulder, and elbow osteoarthritis: a randomized controlled trial. BMC Musculoskelet Disord. 2022;23(1):371. doi:10.1186/s12891-022-05328-3.
- 18. Mankin HJ. Biochemical and metabolic aspects of osteoarthritis. Orthop Clin North Am. 1971;2(1):19–31.
- 19. Ding F, Wang J, Zhu G, Zhao H, Wu G, Chen L. Osteopontin stimulates matrix metalloproteinase expression through the nuclear factor-κB signaling pathway in rat temporomandibular joint and condylar chondrocytes. Am J Transl Res. 2017;9(2):316-29.
- 20. Yan Z, Pan Y, Wang S, Cheng M, Kong H, Sun C, et al. Static compression induces ECM remodeling and integrin α2β1 expression and signaling in a rat tail caudal intervertebral disc degeneration model. Spine. 2017;42(8): E448-E458. doi:10.1097/BRS.0000000000001856.
- 21. Ding C. Do NSAIDs affect the progression of osteoarthritis. Inflammation. 2002;26(3):139-42. doi:10.1023/a:1015504632021.
- 22. Dingle JT. The effects of NSAID on the matrix of human articular cartilages. Z Rheumatol. 1999;58(3):125-9. doi:10.1007/s003930050161.
- 23. Smith RL, Kajiyama G, Lane NE. Nonsteroidal antiinflammatory drugs: effects on normal and interleukin 1 treated human articular chondrocyte metabolism in vitro. J Rheumatol. 1995;22(6):1130-7.
- 24. Dieppe P, Cushnaghan J, Jasani MK, McCrae F, Watt I. A two-year, placebo-controlled trial of non-steroidal anti-inflammatory therapy in osteoarthritis of the knee joint. Br J Rheumatol. 1993;32(7):595–600. doi:10.1093/rheumatology/32.7.595.
- 25. Perry TA, Wang X, Nevitt M, Abdelshaheed C, Arden N, Hunter DJ. Association between current medication use and progression of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Rheumatology (Oxford). 2021;60(10):4624-32. doi:10.1093/rheumatology/keab059.
- 26. Reijman M, Bierma-Zeinstra SM, Pols HA, Koes BW, Stricker BH, Hazes JM. Is there an association between the use of different types of nonsteroidal antiinflammatory drugs and radiologic progression of osteoarthritis? The Rotterdam Study. Arthritis Rheum. 2005;52(10):3137-42. doi:10.1002/art.21357.
- 27. Kalra BS, Chaturvedi S, Tayal V, Gupta U. Evaluation of gastric tolerability, antinociceptive and antiinflammatory activity of combination NSAIDs in rats. Indian J Dent Res. 2009;20(4):418-22. doi:10.4103/0970-9290.59439.
- 28. Weiss C. Normal and osteoarthritic articular cartilage. Orthop Clin North Am. 1979;10(1):175-89.
- 29. Kobayashi K, Amiel M, Harwood FL, Healey RM, Sonoda M, Moriya H, et al. The long-term effects of hyaluronan during development of osteoarthritis following partial meniscectomy in a rabbit model. Osteoarthritis Cartilage. 2000;8(5):359-65. doi:10.1053/joca.1999.0310.
- 30. Rezende MU, Hernandez AJ, Oliveira CR, Bolliger Neto R. Experimental osteoarthritis model by means of medial meniscectomy in rats and effects of diacerein administration and hyaluronic acid injection. Sao Paulo Med J. 2015;133(1):4–12. doi:10.1590/1516-3180.2013.6730001.
- 31. Salamanna F, Giavaresi G, Parrilli A, Martini L, Nicoli Aldini N, Abatangelo G, et al. Effects of intra-articular hyaluronic acid associated to Chitlac (arty-duo®) in a rat knee osteoarthritis model. J Orthop Res. 2019;37(4):867-76. doi:10.1002/jor.24259.
- 32. Tateiwa D, Yoshikawa H, Kaito T. Cartilage and bone destruction in arthritis: pathogenesis and treatment strategy: a literature review. Cells. 2019;8(8):818. doi:10.3390/cells8080818.
- 33. Sanchez-Lopez E, Coras R, Torres A, Lane NE, Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18(5):258-75. doi:10.1038/s41584-022-00749-9.
- 34. Mehana EE, Khafaga AF, El- Blehi SS. The role of matrix metalloproteinases in osteoarthritis pathogenesis: an updated review. Life Sci. 2019;234:116786. doi:10.1016/j.lfs.2019.116786.
- 35. Kisukeda T, Onaya J, Yoshioka K. Effect of diclofenac etalhyaluronate (SI-613) on the production of high molecular weight sodium hyaluronate in human synoviocytes. BMC Musculoskelet Disord. 2019;20(1):201. doi:10.1186/s12891-019-2586-0.
- 36. Julovi SM, Ito H, Nishitani K, Jackson CJ, Nakamura T. Hyaluronan inhibits matrix metalloproteinase-13 in human arthritic chondrocytes via CD44 and P38. J Orthop Res. 2011;29(2):258-64. doi:10.1002/jor.21216.
- 37. Cheleschi S, Pascarelli NA, Valacchi G, Di Capua A, Biava M, Belmonte G, et al. Chondroprotective effect of three different classes of anti-inflammatory agents on human osteoarthritic chondrocytes exposed to IL-1β. Int Immunopharmacol. 2015;28(1):794–801. doi:10.1016/j.intimp.2015.07.003.
- 38. Fioravanti A, Tinti L, Pascarelli NA, Di Capua A, Lamboglia A, Cappelli A, et al. In vitro effects of VA441, a new selective cyclooxygenase-2 inhibitor, on human osteoarthritic chondrocytes exposed to IL-1β. J Pharmacol Sci. 2012;120(1):6–14. doi:10.1254/jphs.12016fp.
- 39. Hashizume M, Mihara M. Desirable effect of combination therapy with high molecular weight hyaluronate and NSAIDs on MMP production. Osteoarthritis Cartilage. 2009;17(11):1513-8. doi:10.1016/j.joca.2009.04.018.
- 40. Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(10):580-92. doi:10.1038/nrrheum.2016.136.
- 41. Brady K, Dickinson SC, Hollander AP. Changes in chondrogenic progenitor populations associated with aging and osteoarthritis. Cartilage. 2015;6(suppl 2):30S-35S. doi:10.1177/1947603515574838.