Introduction
The placenta is a complex organ that contains both fetal and maternal tissues and allows the exchange of nutrients, gases, and waste materials between the mother and fetus in the intrauterine period. It is a complex organ in which the hormones required for pregnancy are made and secreted. The first special structures of the placenta that will support embryo/fetus development begin with the emergence of the cytotrophoblast and syncytiotrophoblast that appear immediately after implantation. The placenta develops from the chorion frondosum (fetal origin) and decidua basalis (maternal origin). As the main organ that supports the development of the fetus until birth, in the remaining period of pregnancy, it develops and protects its structure, allowing exchange between the mother’s and the fetal blood.
The villous trees are so named because they resemble trees, and their basic structure is established early in gestation. A primitive placenta is formed in the second to third week with the development of primary, secondary, and tertiary villi and lacunae. In the literature, in the early period, the structures with syncytiotrophoblast on the outside and cytotrophoblast on the inside are called primary villi. The structures formed by the progression of the mesoderm into the inner side of the primary villi are called secondary villi. The structures formed by the development of blood vessels inside the mesoderm of the secondary villi are called tertiary villi. Tertiary villi are also named mesenchymal villi [Jones and Fox, 1991]. When the villus stroma was examined, mesenchymal cells, reticulum cells, fibroblasts, myofibroblasts, pericytes, and Hofbauer cells were identified. Many researchers have reported an interaction between new vessel formation and trophoblast [Pavlov et al., 2014]. The cells such as fibroblasts, myofibroblasts, and pericytes located in the stroma are similar in terms of their structural and functional properties. Depending on the environment and conditions in which trophoblastic cells are present, they exhibit structural features that overlap with stromal cells, so the mentioned cell groups can easily transform into each other. It has been suggested that large cells like cytotrophoblasts have come together to form capillaries, and that large cells at the beginning have retracted to the point where they have close connections to the basal plate as they acquire endothelial cell features. It was found that the gap in between formed the capillary lumen [Pavlov et al.,2003]. Studies show that the placenta in vitro produces multilineage hematopoietic stem cells (HSCs) that arise from the labyrinthine vasculature. Placental stromal cells express pericyte markers. Since pericytes can show supportive features similar to those of mesenchymal stem cells (MSCs), they play an important role in support of HSC expansion in the placenta [Crisan et al., 2008; Çelebi-Saltik, 2018].
The cell-cell and cell-extracellular matrix interactions in vitro remain poorly understood in the placenta. Due to the challenging and ethically restricted study of human placenta in vivo, animal models have been designed. However, there are fundamental differences in placentation among mammals. This situation makes it necessary to develop a new approach. Many studies related to human placenta utilize isolated primary cells or placenta-derived cell lines, but isolated cells do not adequately recapitulate important aspects of tissue function related to cell-cell communications in vivo [Fitzgerald et al., 2018]. Three-dimensional (3D) models which maintain the cellular relationships ex vivo, can be considered as a new approach to study placenta metabolic activity in normal and complicated pregnancies. According to Haider et al. [2018], EGF signaling, inhibition of TGF-beta, and reinforcement of the Wnt pathway are required for long-term expansion of first trimester cytotrophoblast organoid cultures. Removal of Wnt stimulators decreases villous cytotrophoblast self-renewal and induces development of proliferative invasive extravillous trophoblast progenitors lacking nuclear beta-catenin and T-cell factors, key transcription factors of canonical Wnt signaling, as could be observed in the different trophoblast subtypes. This reveals the importance of stimulators and/or inhibitors in the culture condition for expansion, differentiation, and regeneration of the placental cells [Haider et al., 2018].
Wong et al. [2018] reported that thicker Matrigel surfaces induced the self-assembly of trophoblast cells into 3D spheroids that exhibited thickness-dependent changes in viability, proliferation, syncytial fusion, and gene expression profiles compared to 2D cultures. Their data suggest that a critical surface thickness is required for spheroid formation, and variations in thickness can regulate spheroid phenotype. It has also been shown that 3D chitosan-gelatine scaffolds can preserve the placenta-derived stem cells in their native immunological state [Azizian et al., 2018]. In this 3D environment, it is important to investigate other parameters that affect cell behavior. Microcirculation of oxygen, pressure, wall shear stress distribution, and blood flow regulation highly depend on the vascular network anatomy, and therefore, detailed structural information is required. Microfluidics and printing approaches have been previously used to model human placental functions, such as transport and invasion [Abbas et al., 2017; Kuo et al., 2019]. These 3D structures are used not only in invasion or transport studies but also in toxicity studies [Fry et al., 2019; Nishiguchi et al., 2019]. Yin et al. [2019] developed an in vitro 3D placental barrier-on-a-chip microdevice to acquire a deep understanding of nanoparticle (NP) exposure and subsequent responses at the placental barrier. This review summarizes the nature of the placenta with its 3D composition which has been called niche. We also discuss the systems and approaches used in the creation of current 3D placental models.
3D Structure of the Placenta
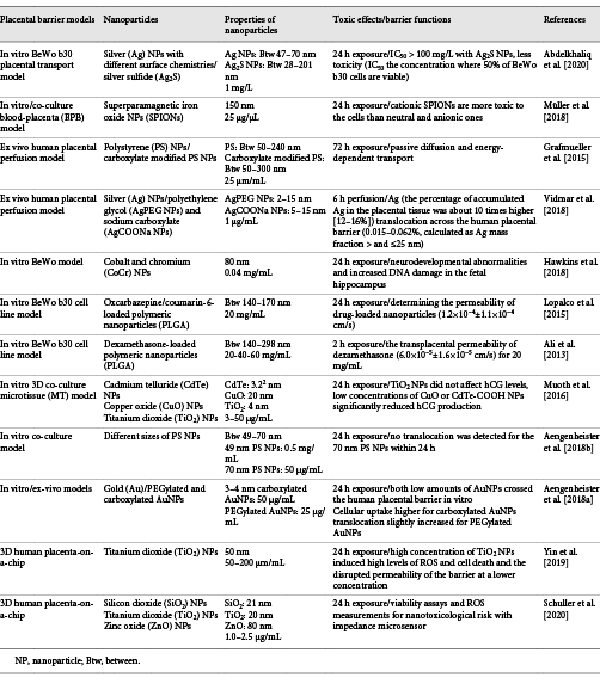
Macroscopically, the placenta is a disc-shaped organ and has a maternal and fetal face. The maternal face is called the basal plate, has a lobule structure, and each lobule is called cotyledon. There are approximately 10–40% cotyledons in the maternal portion. Cotyledons are nonfunctional structures. The fetal face is called chorionic plate and has a smooth surface. The umbilical cord emerges from the fetal face and connects the placenta to the fetus [Koblinsky et al., 2012]. The placenta can be divided into 3 parts. The first part is composed of the fetal amniotic membranes that encapsulate the fetus and the amniotic cavity. The second part consists of the tree-like placental villi which are lined by a syncytial trophoblast and cytotrophoblast layers. These layer cells are in direct contact with maternal blood. The last part, on the maternal side of the placenta, is the basal plate, which is responsible for the maternal-fetal crosstalk, immune tolerance, and defense. This part consists of several cell types such as maternal immune cells, endothelial cells, and fetal trophoblasts, which extravasate from the placental villi to invade the basal plate and promote spiral artery remodeling to facilitate the flow of blood to the fetal side of the placenta [Parnell et al., 2017]. The maternal placenta is separated from the uterine endometrium by spiral arteries, and the fetal placenta is formed by the cells originating from the trophectoderm and allantoic mesoderm as shown in Figure 1 [Khodadi et al., 2016].
Fig. 1
a Interaction between the fetal and maternal blood circulation and placental components of the human placenta during pregnancy. The decidual compartment of the placenta includes maternal immune cells (T cells, NK cells, macrophages) and stromal cells. b The cell components of the villus. Hofbauer cells are placental villous macrophages of fetal origin that are present throughout pregnancy. Syncytiotrophoblast cells are placental epithelial cells in direct communication with the maternal endometrial vasculature at the maternal-fetal interface. The cytotrophoblast (or layer of Langhans) is the inner layer of the trophoblast. It is interior to the syncytiotrophoblast and external to the wall of the blastocyst in a developing embryo. Extravillous cytotrophoblast cells that erupt from the villi and contact the decidua are the first fetally derived cells to encounter maternal immune cells.
Placental Development and Niche
Formation of the placenta begins very early in pregnancy, while the embryo is in the blastocyst stage. The placenta is delivered with the fetus at birth. Human placental development starts at the time of implantation around day 6 to 7 after conception, as soon as the blastocyst starts to attach to the uterine surface epithelium and invasion of the endometrium. At this stage, the blastocyst consists of an outer vesicular cellular cover, called the trophoblast, whereas the placenta and the membranes are mainly derived, and of an inner cell mass, called the embryoblast that develops into the umbilical cord and the fetus and adds the mesenchyme to the placenta. The trophoblast is the precursor of the epithelial parts of the feto-maternal barrier of the placenta, whereas the embryoblast contributes placental mesenchyme and the fetal vascular system [Koffler, 1981]. The trophoblast has 2 cell populations: undifferentiated cytotrophoblasts and fully differentiated syncytiotrophoblasts. The syncytiotrophoblast (specialized layer of epithelial cells), the outermost layer of the human placenta, is in direct contact with maternal blood and is the main site of exchange for drugs and metabolites, nutrients, waste products, and gases. Cytotrophoblast cells continually differentiate into syncytiotrophoblast cells, and they are stem cells for syncytiotrophoblasts. Similar to cancer invasion, embryo implantation requires invasion, degradation of extracellular matrix, promotion of angiogenesis, migration, and escape from the maternal immune system by placental cells [Murray and Lessey, 1999; D'Souza and Wagner, 2014]. After cytotrophoblast invasion into the uterine spiral arteries, endothelial lining and musculoelastic tissue in these vessels are lost. This phenomenon is important for placental vascular remodeling in the early stages of the implantation process [Wang and Zhao, 2010]. The trophoblast is metabolically active and accounts for ∼40% of oxygen consumption by the feto-placental unit. A large surface area and a thin membrane between the maternal and fetal circulation are needed to enhance diffusional exchange. The villous trees branch repeatedly to generate a surface area of 12–14 m2. Besides trophoblast cells, the mesodermal part of the placenta contains a range of cells present within the stromal core of the villi that support the structure; pericytes, smooth muscle cells, endothelial cells, and erythrocytes that are responsible of the fetal placental vasculature, then macrophages that are responsible for immune surveillance. Most of the leukocytes in the placental bed are innate immune cells such as NK cells and macrophages, which may comprise about 90% of all leukocytes, and 10% of the immune cells are T cells in early human pregnancy.
The placental niche supports blood stem cells by producing hematopoietic progenitor cells (HPCs) and expansion of HSCs without differentiation. HPCs are formed in the chorioallantoic mesenchyme of large vessels and expand in the placental vascular labyrinth. Trophoblast and endothelial cells have PDGF and EPO receptors that can cause HSC expansion. Pericytes, mesenchymal stem cells (MSCs), and endothelial cells reside in a vascular niche in the placenta and support the development of various progenitors in the placenta [Khodadi et al., 2016]. Stromal cells and MSCs have a crucial function in the development of the HSCs in the placenta via SCF/Kit signaling in the placental vascular niche. Placental stromal cells show characteristics similar to those of bone marrow stromal cells and express pericyte markers supporting HPC expansion. Placental MSCs are positive for CD105, CD146, 3G5, CD106, STRO-1, CD49a, and alpha-SMA, but negative for HSC markers (CD34 and CD117) and endothelial markers (CD34, vWF) [Khodadi et al., 2016]. Pericytes are the subpopulation of the MSCs in the chorionic villi and play an important role in placental vasculature maturation and stability.
3D Human Placenta and Placental Barrier Models
It is not yet fully understood how the communication between the mother and the fetus is formed in the placenta and which parameters trigger this communication. Different human placenta models have been created for the simulation of this information exchange between mother and fetus [Nishiguchi et al., 2019]. Ex vivo models have come to the forefront to investigate human placental structure and placental transport. These models are based on the perfusion and isolation of the human placenta. With these models, it is possible to understand both the placenta integrity and the complex structure [Saunders, 2009]. But the biggest challenge in ex vivo studies is the placental transport. In addition, the use of large amounts of material for the study is another challenge that restricts ex vivo models. The circulatory system of the fetus is separated from the maternal blood in the placenta by the fetal capillary endothelium and placental trophoblast known as the placental barrier. This barrier is a very important tool for fetal development between the mother and the fetus [Syme et al., 2004]. Toxic effects and bacterial/viral infection contaminants are the most frequently studied areas for fetal development. The toxic effects of nanostructured particles, especially prepared by engineering methods, on the placental barrier is one of the most important research areas recently [Wick et al., 2010].
Investigation of the material transport for the placental barrier has been reported by using different in vitro conventional methods [Liu et al., 1997]. BeWo human trophoblastic cells have been seeded on monolayer cultures which consisted of polymer porous membrane [Hutson et al., 2011]. It has been proven that culturing targeted tissue cells in in vitro platforms differs in 2D and 3D environments through studies in tissue engineering applications. In a study published by Gupta et al. [2016], cells were shown to grow in single layer and exhibit a flat morphology especially in 2D culture condition. But it has been shown that the same cells exhibit a more cubic morphology in the 3D condition and can mimic their physiological environment [Gupta et al., 2016]. In particular, poor physiological outputs of studies on existing animal models highlighted the use of in vitro models [Moffett and Loke, 2006]. The use of cell lines in a transwell model (barrier model with membrane) has emerged as an alternative. BeWo, Jar, and JEG-3 cells were used in this model system [Li et al., 2013]. The response of the placental barrier, especially against toxic agents, has been carefully investigated. For this purpose, diffusion of the different toxic substances through the placental barrier has been examined, and the results have been published. Especially short- and long-term toxic results of nanostructured materials on human placenta were investigated [Buerki-Thurnherr et al., 2012]. The toxic effects of silver NPs (AgNPs) on placenta were investigated using embryonic stem cells and BeWo in the transwell model [Abdelkhaliq et al., 2020]. In another published study, the transfer mechanism and related parameters of magnetic NPs on the in vitro human placenta model were reported. This model was designed as a blood-placental barrier, and the interaction of NPs within the cells with this model was shown [Müller et al., 2018]. Grafmueller et al. [2015] also developed a human placenta perfusion model to investigate the transfer and transfer mechanism of polystyrene NPs from the placental barrier. Another ex vivo human placental perfusion model has been presented by Vidmar et al. [2018] to show the accumulation of silver in the placental system with AgNPs. Cobalt and chromium NPs that caused a toxicity during pregnancy were studied, and the toxicity mechanism was also explained [Hawkins et al., 2018]. To understand the transfer mechanism between the blood and the placental barrier, studies in which the drugs are carried in biodegradable structures were performed. A study was conducted using antiepileptic drug-loaded polymeric NPs in which their effects on the in vitro human placental trophoblast model were examined, and blood-placental barrier permeability was monitored [Lopalco et al., 2015]. In another study, polymeric NPs loaded with dexamethasone were prepared and carried on the in vitro human placenta model [Ali et al., 2013]. Another study investigated the transfer mechanism of Zika virus which causes serious neurological damage both in adults and fetuses on the placental barrier [Chiu et al., 2020]. Again, chemicals such as bisphenol A (BPA) and phthalates that cause disruption of the placental structure during pregnancy and their toxic effects on the endocrine system have been tried to elucidate by in vivo and in vitro toxicology studies [Gingrich et al., 2020]. Mørck et al. [2010] used different models such as the human-derived BeWo trophoblast cell line, placental explant cultures, and placental perfusions to examine the toxic and in vitro translocation effects of BPA exposure during pregnancy. Muoth et al. [2016] developed a placental microtissue platform to evaluate the NP toxicological risk on fetus development. The scaffold-free hanging drop technology was applied in this study to optimize reproducibility of the 3D co-culture system. The nanotoxicity assessment was determined by using cadmium telluride (CdTe), copper oxide (CuO), and titanium dioxide (TiO2) NPs for 2D placental monoculture and 3D co-culture models. Acute toxicity of NPs in 3D culture medium was found to be less compared to 2D monoculture medium. CdTe and CuO NPs have been shown to reduce tissue viability. This model can be used to determine acute toxicity effects rapidly after any NP exposure [Muoth et al., 2016]. Current in vitro models proposed in placental transfer studies of NPs ignore the effects of placental endothelial cells on translocation [Aengenheister et al., 2018b]. These authors developed an in vitro human placental barrier culture platform to investigate NP and macromolecule transfer mechanisms with placental endothelial cells which were seeded on translocation pathway for barrier. Here, Na-F, FITC-dextran (40 kDa), antipyrine, indomethacin, and different sizes of polystyrene NPs were used as model compounds and NPs to show their efficiency on translocation by prepared novel human placental barrier co-cultures with different conditions called static and shaken. Translocation studies of macromolecules and NPs have been successfully carried out with the prepared multi-cell model [Aengenheister et al., 2018b]. The same group also presented in vitro and ex vivo human placental barrier platforms to explain translocation of gold NPs (AuNPs) which were prepared with some modifications in different models. Surface modifications were applied to obtain PEGylated and carboxylated AuNPs. Polycarbonate transwell was used for monoculture of HPEC-A2 and BeWo b30 cells to evaluate in vitro placental barrier formation. The ex vivo placental models were chosen from uncomplicated term pregnancies. These models and different cell types were applied to determine the distribution of AuNPs. It was provided that these models can be used to understand translocatıon of the AuNPs [Aengenheister et al., 2018a]. An overview of studies on translocation and toxic effects of NPs within the placental barrier is presented in Table 1.
Different models have also been proposed to investigate the diffusion of viruses from the placental barrier [Tuntland et al., 1999]. It has been known that 3D morphology of the placenta plays a key role in the overall transfer. Perazzolo et al. [2015] evaluated the effects of maternal blood flow in the intervillous space on the placental transfer of solutes, modeling both maternal flow as well as solute transport, using 3D image-based models of the placental microstructure. According to these authors, mathematical modeling of the flux through the villous barrier alone was 2.4 ± 0.4 times higher compared to the simulations with maternal flow under physiological conditions. They highlighted that maternal flow and villous structure affect the efficiency of placental transfer [Perazzolo et al., 2015]. In the light of this information, new fluid systems should be designed.
Placenta-on-a-Chip
Organ-on-a-chip systems, which are widely used as 3D microfabrication methods and where micro-models of tissues and organs are made, offer testable in vitro environments for many tissue engineering applications [Huh et al., 2013]. Soft lithography techniques are used to design microfluidic chip systems. Polydimethylsiloxane (PDMS) is widely used to create these platforms because of its biocompatibility and transparency [Bhatia and Ingber, 2014]. Reproductive medicine and placental function studies on chip systems provide very important data for both mother and baby health [Blundell et al., 2018]. In the early applications, especially the human trophoblast culture system was intended to be cultured in vitro. These cells were observed to be unable to perform many of the human placenta functions in vitro, and studies focused on designing different in vitro platforms as shown in Figure 2. Based on this aim, the first placental model in a bioreactor system was published by Ma et al. [1999] in which the mother and baby circulatory systems were simulated. In this study, cells that were cultured on chemically modified poly(ethylene terephthalate) fibers showed natural 3D organization within this system. A novel placenta-on-a-chip platform was developed by Lee et al. [2016] to understand the biological function of the human placenta. This platform was composed of a thin extracellular matrix membrane and microfluidic channels. Human JEG-3 and human umbilical vein endothelial cells were used to mimic the placental barrier under dynamic flow. Biological permeability of the placental barrier was determined by measuring glucose transport, glucose metabolism variables were calculated, and comparable consistent in vivo results were obtained [Lee et al., 2016]. In another study, Blundell et al. [2016] developed a new 3D microphysiological platform that mimics the human placental barrier. On this placenta-on-a-chip platform, human trophoblast and human fetal endothelial cells were cultured together. The chip platform was divided into 2 microlayers, called upper and lower channels, that were separated from each other by a membrane. With the chip system prepared, the aim is to grow human placental cells under dynamic flow conditions and to transform the natural structure of the mother-fetus barrier into a multi-layer tissue [Blundell et al., 2016]. The same group also fabricated another human placenta-on-a-chip model to show the placental drug transfer pathway. Here, simulation of the drug glyburide was investigated by using the in vitro placental barrier chip platform. The designed placenta-on-a-chip device provides an important model for investigating the effects of drugs on the fetus during pregnancy [Blundell et al., 2018]. In another application, Pemathilaka et al. [2019] improved a new placenta-on-a-chip model to understand transportation of caffeine from the placental barrier. This platform was divided into 2 layers which were prepared by using PDMS to separate maternal site and fetal site with porous membranes for blood circulation. The caffeine transport rate for the placenta was measured by analyzing the rate and duration of the intake of caffeine given to the mother at a certain concentration from the placental barrier [Pemathilaka et al., 2019]. The protective feature of the human placental barrier against environmental harmful effects for the fetus is also being investigated. Studies in mice investigating the long-term effects of NPs found a negative effect on the placenta. However, the differences between mouse and human significantly limit the evaluation of the results [Yin et al., 2019]. The authors suggested a placental barrier-on-a-chip model to evaluate the toxicity effect of NPs on the human placental barrier. The prepared 3D microsystem was used to investigate placental responses such as oxidative stress and barrier transmissivity after exposure to the different NPs. TiO2 NPs, a common nanomaterial, have been used to investigate the placental response. The results showed that placental barrier integrity and maternal immune cell behavior were particularly affected by exposure to NPs. Schuller et al. [2020] fabricated a new placental lab-on-a-chip platform which included integrated biosensor array to determine toxicity of the nanomaterials for the placental barrier. Here, the nanotoxicological risk of the NPs was determined by using electrochemical biosensing and organ-on-chip platform together. The chip system was prepared by using porous PET membrane and electrode array. Silicon dioxide (SiO2), TiO2, and zinc oxide (ZnO) NPs were selected to investigate their toxicity effect on the placental cytotrophoblast (BeWo) [Schuller et al., 2020].
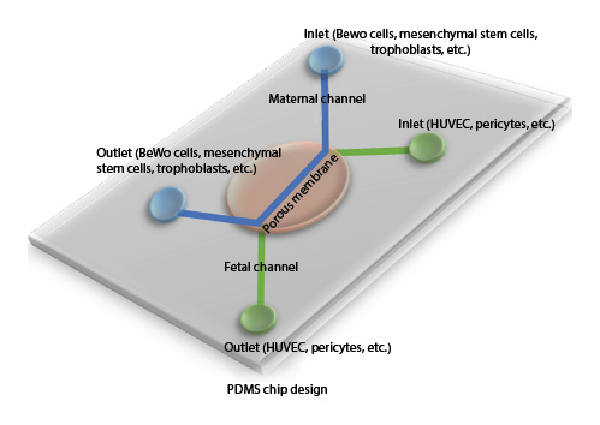
Fig. 2
Human placental barrier lab-on-a-chip model. PDMS-based chip design of placenta with co-culture of different cell types.
Placental inflammation is an important problem causing placental damage. Understanding the different arrangements of placental inflammation requires careful consideration of possible infection pathways and maternal-fetal interfaces through which they pass. Placenta and its implantation area contain 2 most prominent inflammatory cell populations: large granular lymphocytes in the decidualized endometrium and Hofbauer cells of the villous stroma that regulate placental development and function [Weindling, 1994]. Besides, rapid mobilization of myeloid cells, derived from both the mother and the fetus, and expression of antibacterial proteins in endometrial glandular secretions and amniotic fluid play critical roles in maternal-fetal interfaces of the placenta [Redline, 2004]. Microorganisms that enter fetal tissues may catch out immune responses specific to the fetal antigen, but these responses are often immature and ineffective. Responses specific to maternal antigen in the placenta are usually pathological and not directed to microbial antigens. Local maternal immune responses to fetoplacental antigen can lead to recurrent abortion and adverse pregnancy outcome. Infectious organisms in the placenta elicit primary innate immune responses. Spreading of the infection into the placenta can be from vagina to cervix and cavity, via placenta, intra-abdominal route or iatrogenic (during amniocentesis) [Boyle et al., 2017; Singh et al., 2018]. Acute chorioamnionitis, chronic histiocytic intervillositis, chronic placentitis, idiopathic (villitis of unknown etiology), chronic deciduitis, and fetal vasculitis can be classified under the microorganism-driven processes and non-replicating antigen-driven processes [Redline, 2004; Kim et al., 2015]. Additionally, it has been shown that microorganisms have the potential to influence the epigenetic machinery and cause differential DNA methylation in the human placenta that might also affect neurological development [Tomlinson et al., 2019]. Traditional cell and animal models are insufficient for an in-depth understanding of the inflammatory mechanism. The placenta structure and function cause great differences in the application of traditional methods. In a study, the placental barrier-on-a-chip system was prepared, and inflammatory responses caused by bacterial infection were investigated. Zhu et al. [2018] fabricated this microdevice which was composed of multi-layers of the human placental barrier structures. Escherichia coli were selected as model bacteria for the inflammatory responses. This simple and dynamic placental barrier model has been shown to understand the mechanism of inflammatory responses for bacterial infection [Zhu et al., 2018]. Studies in this area need to be further expanded.
Conclusion
The early maternal-fetal interface provides an essential resource for understanding normal and pathological pregnancies. Trophoblasts, stromal cells, and endothelial cells represent groups of cells that retain their potential to be transduced to each other. Despite their importance, the molecular mechanisms governing human placental formation have been poorly unraveled, mostly due to the lack of appropriate cellular model systems. However, development of organoid culture systems, placental barrier models, and placenta-on-a-chip models might overcome this limitation. The development of these 3D systems can then be a suitable platform for mimicking disease models and for the solution that can be developed against them. As a perspective, some new techniques such as printing, microfluidic system, organoid cultures, epigenetic modification, or gene therapy should be combined and studied to mimic human placental development and used to overcome placental diseases. Studies in this area are currently progressing and will likely contribute to an increased confidence in the use of gene, protein, and metabolic data using in vitro omics technologies in conjunction with in silico approaches to better understand the relationships between prenatal exposure to chemicals, placental toxicity, and development outcomes.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors had no funding support for this manuscript.
Author Contributions
Both authors contributed equally to this study.
References
- 1. Abbas Y, Oefner CM, Polacheck WJ, Gardner L, Farrell L, Sharkey A, et al. A microfluidics assay to study invasion of human placental trophoblast cells. J R Soc Interface. 2017;14(130):20170131.http://dx.doi.org/10.1098/rsif.2017.0131
- 2. Abdelkhaliq A, van der Zande M, Peters RJB, Bouwmeester H. Combination of the BeWo b30 placental transport model and the embryonic stem cell test to assess the potential developmental toxicity of silver nanoparticles. Part Fibre Toxicol. 2020;17(1):11.http://dx.doi.org/10.1186/s12989-020-00342-6
- 3. Aengenheister L, Dietrich D, Sadeghpour A, Manser P, Diener L, Wichser A, et al. Gold nanoparticle distribution in advanced in vitro and ex vivo human placental barrier models. J Nanobiotechnology. 2018a;16(1):79.http://dx.doi.org/10.1186/s12951-018-0406-6
- 4. Aengenheister L, Keevend K, Muoth C, Schönenberger R, Diener L, Wick P, et al. An advanced human in vitro co-culture model for translocation studies across the placental barrier. Sci Rep. 2018b;8(1):5388.http://dx.doi.org/10.1038/s41598-018-23410-6
- 5. Ali H, Kalashnikova I, White MA, Sherman M, Rytting E. Preparation, characterization, and transport of dexamethasone-loaded polymeric nanoparticles across a human placental in vitro model. Int J Pharm. 2013;454(1):149–57.http://dx.doi.org/10.1016/j.ijpharm.2013.07.010
- 6. Azizian S, Khatami F, Modaresifar K, Mosaffa N, Peirovi H, Tayebi L, et al. Immunological compatibility status of placenta-derived stem cells is mediated by scaffold 3D structure. Artif Cells Nanomed Biotechnol. 2018;46(Suppl 1):876–84.http://dx.doi.org/10.1080/21691401.2018.1438452
- 7. Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32(8):760–72.http://dx.doi.org/10.1038/nbt.2989
- 8. Blundell C, Tess ER, Schanzer AS, Coutifaris C, Su EJ, Parry S, et al. A microphysiological model of the human placental barrier. Lab Chip. 2016;16(16):3065–73.http://dx.doi.org/10.1039/c6lc00259e
- 9. Blundell C, Yi YS, Ma L, Tess ER, Farrell MJ, Georgescu A, et al. Placental Drug Transport-on-a-Chip: A Microengineered In Vitro Model of Transporter-Mediated Drug Efflux in the Human Placental Barrier. Adv Healthc Mater. 2018;7(2).http://dx.doi.org/10.1002/adhm.201700786
- 10. Boyle AK, Rinaldi SF, Norman JE, Stock SJ. Preterm birth: Inflammation, fetal injury and treatment strategies. J Reprod Immunol. 2017;119:62–6.http://dx.doi.org/10.1016/j.jri.2016.11.008
- 11. Buerki-Thurnherr T, von Mandach U, Wick P. Knocking at the door of the unborn child: engineered nanoparticles at the human placental barrier. Swiss Med Wkly. 2012;142:w13559.http://dx.doi.org/10.4414/smw.2012.13559
- 12. Çelebi-Saltik B. Pericytes in Tissue Engineering. Adv Exp Med Biol. 2018;1109:125–37.
- 13. Chiu CF, Chu LW, Liao IC, Simanjuntak Y, Lin YL, Juan CC, et al. The Mechanism of the Zika Virus Crossing the Placental Barrier and the Blood-Brain Barrier. Front Microbiol. 2020;11:214.http://dx.doi.org/10.3389/fmicb.2020.00214
- 14. Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–13.http://dx.doi.org/10.1016/j.stem.2008.07.003
- 15. D'Souza AW, Wagner GP. Malignant cancer and invasive placentation: A case for positive pleiotropy between endometrial and malignancy phenotypes. Evol Med Public Health. 2014;2014(1):136–45.http://dx.doi.org/10.1093/emph/eou022
- 16. Fitzgerald W, Gomez-Lopez N, Erez O, Romero R, Margolis L. Extracellular vesicles generated by placental tissues ex vivo: A transport system for immune mediators and growth factors. Am J Reprod Immunol. 2018;80(1):e12860.http://dx.doi.org/10.1111/aji.12860
- 17. Fry RC, Bangma J, Szilagyi J, Rager JE. Developing novel in vitro methods for the risk assessment of developmental and placental toxicants in the environment. Toxicol Appl Pharmacol. 2019;378:114635.http://dx.doi.org/10.1016/j.taap.2019.114635
- 18. Gingrich J, Ticiani E, Veiga-Lopez A. Placenta Disrupted: Endocrine Disrupting Chemicals and Pregnancy. Trends Endocrinol Metab. 2020;31(7):508–24.http://dx.doi.org/10.1016/j.tem.2020.03.003
- 19. Grafmueller S, Manser P, Diener L, Diener PA, Maeder-Althaus X, Maurizi L, et al. Bidirectional Transfer Study of Polystyrene Nanoparticles across the Placental Barrier in an ex Vivo Human Placental Perfusion Model. Environ Health Perspect. 2015;123(12):1280–6.http://dx.doi.org/10.1289/ehp.1409271
- 20. Gupta N, Liu JR, Patel B, Solomon DE, Vaidya B, Gupta V. Microfluidics-based 3D cell culture models: Utility in novel drug discovery and delivery research. Bioeng Transl Med. 2016;1(1):63–81.http://dx.doi.org/10.1002/btm2.10013
- 21. Haider S, Meinhardt G, Saleh L, Kunihs V, Gamperl M, Kaindl U, et al. Self-Renewing Trophoblast Organoids Recapitulate the Developmental Program of the Early Human Placenta. Stem Cell Reports. 2018;11(2):537–51.http://dx.doi.org/10.1016/j.stemcr.2018.07.004
- 22. Hawkins SJ, Crompton LA, Sood A, Saunders M, Boyle NT, Buckley A, et al. Nanoparticle-induced neuronal toxicity across placental barriers is mediated by autophagy and dependent on astrocytes. Nat Nanotechnol. 2018;13(5):427–33.http://dx.doi.org/10.1038/s41565-018-0085-3
- 23. Huh D, Kim HJ, Fraser JP, Shea DE, Khan M, Bahinski A, et al. Microfabrication of human organs-on-chips. Nat Protoc. 2013;8(11):2135–57.http://dx.doi.org/10.1038/nprot.2013.137
- 24. Hutson JR, Garcia-Bournissen F, Davis A, Koren G. The human placental perfusion model: a systematic review and development of a model to predict in vivo transfer of therapeutic drugs. Clin Pharmacol Ther. 2011;90(1):67–76.http://dx.doi.org/10.1038/clpt.2011.66
- 25. Jones CJ, Fox H. Ultrastructure of the normal human placenta. Electron Microsc Rev. 1991;4(1):129–78.http://dx.doi.org/10.1016/0892-0354(91)90019-9
- 26. Khodadi E, Shahrabi S, Shahjahani M, Azandeh S, Saki N. Role of stem cell factor in the placental niche. Cell Tissue Res. 2016;366(3):523–31.http://dx.doi.org/10.1007/s00441-016-2429-3
- 27. Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl l):S53–69.http://dx.doi.org/10.1016/j.ajog.2015.08.041
- 28. Koblinsky M, Chowdhury ME, Moran A, Ronsmans C. Maternal morbidity and disability and their consequences: neglected agenda in maternal health. J Health Popul Nutr. 2012;30(2):124–30.http://dx.doi.org/10.3329/jhpn.v30i2.11294
- 29. Koffler H. Fetal and neonatal physiology. Clin Obstet Gynecol. 1981;24(2):545–53.http://dx.doi.org/10.1097/00003081-198106000-00017
- 30. Kuo CY, Shevchuk M, Opfermann J, Guo T, Santoro M, Fisher JP, et al. Trophoblast-endothelium signaling involves angiogenesis and apoptosis in a dynamic bioprinted placenta model. Biotechnol Bioeng. 2019;116(1):181–92.http://dx.doi.org/10.1002/bit.26850
- 31. Lee JS, Romero R, Han YM, Kim HC, Kim CJ, Hong JS, et al. Placenta-on-a-chip: a novel platform to study the biology of the human placenta. J Matern Fetal Neonatal Med. 2016;29(7):1046–54.http://dx.doi.org/10.3109/14767058.2015.1038518
- 32. Li H, van Ravenzwaay B, Rietjens IM, Louisse J. Assessment of an in vitro transport model using BeWo b30 cells to predict placental transfer of compounds. Arch Toxicol. 2013;87(9):1661–9.http://dx.doi.org/10.1007/s00204-013-1074-9
- 33. Liu F, Soares MJ, Audus KL. Permeability properties of monolayers of the human trophoblast cell line BeWo. Am J Physiol. 1997;273(5 Pt 1):C1596–604.http://dx.doi.org/10.1152/ajpcell.1997.273.5.C1596
- 34. Lopalco A, Ali H, Denora N, Rytting E. Oxcarbazepine-loaded polymeric nanoparticles: development and permeability studies across in vitro models of the blood-brain barrier and human placental trophoblast. Int J Nanomedicine. 2015;10:1985–96.http://dx.doi.org/10.2147/IJN.S77498
- 35. Ma T, Yang ST, Kniss DA. Development of an in vitro human placenta model by the cultivation of human trophoblasts in a fiber-based bioreactor system. Tissue Eng. 1999;5(2):91–102.http://dx.doi.org/10.1089/ten.1999.5.91
- 36. Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6(8):584–94.http://dx.doi.org/10.1038/nri1897
- 37. Mørck TJ, Sorda G, Bechi N, Rasmussen BS, Nielsen JB, Ietta F, et al. Placental transport and in vitro effects of Bisphenol A. Reprod Toxicol. 2010;30:131–7.http://dx.doi.org/10.1016/j.reprotox.2010.02.007
- 38. Müller EK, Grafe C, Wiekhorst F, Bergemann C, Weidner A, Dutz S, et al. Magnetic Nanoparticles Interact and Pass an In Vitro Co-Culture Blood-Placenta Barrier Model. Nanomaterials (Basel). 2018;8(2):108.
- 39. Muoth C, Wichser A, Monopoli M, Correia M, Ehrlich N, Loeschner K, et al. A 3D co-culture microtissue model of the human placenta for nanotoxicity assessment. Nanoscale. 2016;8(39):17322–32.http://dx.doi.org/10.1039/c6nr06749b
- 40. Murray MJ, Lessey BA. Embryo implantation and tumor metastasis: common pathways of invasion and angiogenesis. Semin Reprod Endocrinol. 1999;17(3):275–90.http://dx.doi.org/10.1055/s-2007-1016235
- 41. Nishiguchi A, Gilmore C, Sood A, Matsusaki M, Collett G, Tannetta D, et al. In vitro placenta barrier model using primary human trophoblasts, underlying connective tissue and vascular endothelium. Biomaterials. 2019;192:140–8.http://dx.doi.org/10.1016/j.biomaterials.2018.08.025
- 42. Parnell LA, Briggs CM, Mysorekar IU. Maternal microbiomes in preterm birth: Recent progress and analytical pipelines. Semin Perinatol. 2017;41(7):392–400.http://dx.doi.org/10.1053/j.semperi.2017.07.010
- 43. Pavlov N, Hatzi E, Bassaglia Y, Frendo JL, Evain Brion D, Badet J. Angiogenin distribution in human term placenta, and expression by cultured trophoblastic cells. Angiogenesis. 2003;6(4):317–30.http://dx.doi.org/10.1023/B:AGEN.0000029412.95244.81
- 44. Pavlov N, Frendo JL, Guibourdenche J, Degrelle SA, Evain-Brion D, Badet J. Angiogenin expression during early human placental development; association with blood vessel formation. Biomed Res Int. 2014;2014:781632.http://dx.doi.org/10.1155/2014/781632
- 45. Pemathilaka RL, Caplin JD, Aykar SS, Montazami R, Hashemi NN. Placenta-on-a-Chip: In Vitro Study of Caffeine Transport across Placental Barrier Using Liquid Chromatography Mass Spectrometry. Glob Chall. 2019;3(3):1800112.http://dx.doi.org/10.1002/gch2.201800112
- 46. Perazzolo S, Hirschmugl B, Wadsack C, Desoye G, Lewis RM, Sengers BG. Computational modelling of fatty acid transport in the human placenta. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:8054–7.http://dx.doi.org/10.1109/EMBC.2015.7320262
- 47. Redline RW. Placental inflammation. Semin Neonatol. 2004;9(4):265–74.http://dx.doi.org/10.1016/j.siny.2003.09.005
- 48. Saunders M. Transplacental transport of nanomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(6):671–84.http://dx.doi.org/10.1002/wnan.53
- 49. Schuller P, Rothbauer M, Kratz SRA, Holl G, Taus P, Schinnerl M, et al. A lab-on-a-chip system with an embedded porous membrane-based impedance biosensor array for nanoparticle risk assessment on placental Bewo trophoblast cells. Sensor Actuat B-Chem. 2020;312:127946.
- 50. Singh AM, Sherenian MG, Kim KY, Erickson KA, Yang A, Mestan K, et al. Fetal cord blood and tissue immune responses to chronic placental inflammation and chorioamnionitis. Allergy Asthma Clin Immunol. 2018;14:66.http://dx.doi.org/10.1186/s13223-018-0297-y
- 51. Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43(8):487–514.http://dx.doi.org/10.2165/00003088-200443080-00001
- 52. Tomlinson MS, Lu K, Stewart JR, Marsit CJ, O'Shea TM, Fry RC. Microorganisms in the Placenta: Links to Early-Life Inflammation and Neurodevelopment in Children. Clin Microbiol Rev. 2019;32(3):e00103-18.http://dx.doi.org/10.1128/CMR.00103-18
- 53. Tuntland T, Odinecs A, Pereira CM, Nosbisch C, Unadkat JD. In vitro models to predict the in vivo mechanism, rate, and extent of placental transfer of dideoxynucleoside drugs against human immunodeficiency virus. Am J Obstet Gynecol. 1999;180(1 Pt 1):198–206.http://dx.doi.org/10.1016/s0002-9378(99)70175-4
- 54. Vidmar J, Loeschner K, Correia M, Larsen EH, Manser P, Wichser A, et al. Translocation of silver nanoparticles in the ex vivo human placenta perfusion model characterized by single particle ICP-MS. Nanoscale. 2018;10(25):11980–91.http://dx.doi.org/10.1039/c8nr02096e
- 55. Wang Y, Zhao S. Vascular Biology of the Placenta. San Rafael (CA): Morgan & Claypool Life Sciences; 2010.
- 56. Weindling AM. Fetal and neonatal pathology. Arch Dis Child. 1994;71(4):390.http://dx.doi.org/10.1136/adc.71.4.390
- 57. Wick P, Malek A, Manser P, Meili D, Maeder-Althaus X, Diener L, et al. Barrier capacity of human placenta for nanosized materials. Environ Health Perspect. 2010;118(3):432–6.http://dx.doi.org/10.1289/ehp.0901200
- 58. Wong MK, Shawky SA, Aryasomayajula A, Green MA, Ewart T, Selvaganapathy PR, et al. Extracellular matrix surface regulates self-assembly of three-dimensional placental trophoblast spheroids. PLoS One. 2018;13(6):e0199632.http://dx.doi.org/10.1371/journal.pone.0199632
- 59. Yin F, Zhu Y, Zhang M, Yu H, Chen W, Qin J. A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier. Toxicol In Vitro. 2019;54:105–13.http://dx.doi.org/10.1016/j.tiv.2018.08.014
- 60. Zhu Y, Yin F, Wang H, Wang L, Yuan J, Qin J. Placental Barrier-on-a-Chip: Modeling Placental Inflammatory Responses to Bacterial Infection. ACS Biomater Sci Eng. 2018;4(9):3356–63.http://dx.doi.org/10.1021/acsbiomaterials.8b00653
Both authors contributed equally to this manuscript.