Introduction
A wound is defined as an injury to the cellular and anatomic continuity of living tissue by a chemical or physical external agent such as surgery [Bennett, 1988]. Wounds may be classified as closed and open wounds. With closed wounds, the protective body surface is not hurt, and the damaged tissue is thus not exposed to the external environment. With open wounds, the protective body surface has been broken which permits the entry of foreign materials (such as contamination) into the tissue and causes subsequent problems in the process of healing. Under normal conditions, the wound-healing process can be divided into 4 overlapping phases [Gosain and DiPietro, 2004]:
Hemostasis
Inflammation
Proliferation
Maturation or remodeling.
The hemostatic phase occurs following vascular constriction, platelet aggregation, degranulation, and fibrin formation. In the inflammatory phase, blood clotting and the inflammatory process are predominant events. This phase is characterized by the activation of clotting systems, platelet aggregation, vasoconstriction, and vasodilation [Balbino et al., 2005; Rodrigues et al., 2011]. The proliferation phase (or fibroblastic phase) and the deposition of extracellular matrix is characterized by granulation, contraction, angiogenesis, fibroplasia, collagen deposition, and epithelialization of the wound. The maturation (or remodeling) phase remains for months or years. In this phase, acute and chronic inflammatory responses as well as angiogenesis and fibroplasia processes gradually decrease. The cellular activity reduces, and unnecessary vessels formed in the granulation tissue are removed by apoptosis [Greenhalgh, 1998]. In addition, the amount of collagen synthesis equals that of collagen degradation [Greenhalgh, 1998; Balbino et al., 2005; Rodrigues et al., 2011].
Administration of drugs either locally (topical) or systemically (oral or parenteral) is carried out to reduce the healing time and/or the adverse effects of the drug [Myers et al., 1980; Savant et al., 1998]. Important advantages of topical drug delivery are the bypassing of first-pass metabolism, the enhancement of therapeutic efficiency, and the reduction of systemic adverse effects [Marwah et al., 2016]. The topical agents used include antiseptics (e.g., boric acid), antibiotics (e.g., gentamicin), desloughing agents, and wound-healing promoters (e.g., herbal oils) [Raina et al., 2008]. Several natural products such as triterpenes, alkaloids, and flavonoids have been found to promote the wound-healing process [Sumitra et al., 2005]. Wound-healing herbals as topical agents can encourage blood clotting, fight infection, and accelerate angiogenesis and the healing of wounds [Maciel et al., 2002; Raina et al., 2008; Estevão et al., 2013]. For example, the topical administration of microspheres containing asiaticoside (isolated from Centella asiatica) significantly accelerates reepithelization, collagen synthesis, and proangiogenesis in rat wounds [Zhang et al., 2016].
Lemon, sesame, and olive oils are widely used in medicine due to their allelopathic compounds [Komiya et al., 2006; Guasch-Ferré et al., 2014; Vaghardoost et al., 2018]. Lemon [Grassmann et al., 2001; Komiya et al., 2006], sesame [Kang et al., 1998] and olive [Visioli et al., 2002; Waterman and Lockwood, 2007] oils have an antioxidant effect. Studies have shown that antioxidant agents can help the wound-healing process [Fitzmaurice et al., 2011]. Previous studies have documented the potential of these oils in wound healing. For example, Ahmad et al. [2013] demonstrated that lemon extract has healing potential for the treatment of chronic wounds in diabetic and nondiabetic rats. Vitamin C (in lemon extract) can accelerate cutaneous wound healing [Lee et al., 2012]. Sesame oil is also effective in the healing of excision and burn wounds when used orally or topically [Kiran and Asad, 2008]. Topical application improves the wound-healing process in the treatment of second-degree burns [Tehrani et al., 2016]. Furthermore, olive oil has been reported to improve and accelerate wound healing in cutaneous excisional lesions by the synthesis of reactive oxygen species (ROS) and nitric oxide (NO) [Rosa et al., 2014; Donato-Trancoso et al., 2016]. In one clinical study, when olive oil was administered orally to patients with burns, there was accelerated wound healing [Najmi et al., 2015].
The aim of this study was to prepare topical ointments containing lemon, sesame, and olive oils, and then evaluate their influence in the various phases of healing of infected full-thickness wounds in rats. This is the first report of its type that compares the efficacy of these oils and wound control in vivo.
Materials and Methods
Microorganisms and Chemicals
Lemon oil (pharmaceutical grade, from Citrus aurantifolia) was purchased from Barij Essence (Kashan, Iran), sesame (pharmaceutical grade, from Sesamum indicumL.), and olive oil (pharmaceutical grade) were obtained from Farabekr (Tehran, Iran). Eucerin was gifted by Sepidaj Pharmaceutical Co. (Tehran, Iran). Staphylococcus aureus (ATCC 25923) and Pesudomonas aeruginosa (ATCC 27853) were obtained from the Pasteur Institute of Iran (Tehran, Iran). PBS, glycerol, nutrient broth and agar, MTT, penicillin, streptomycin, DMEM, DMSO, and formalin were purchased from Merck Chemicals Co. (Darmstadt, Germany). FBS was obtained from Gibco (Germany).
Microorganisms and Growth Condition
According to previous studies, the most common isolates from infected wounds are Pseudomonas spp. (29.9%) and S. aureus (27.5%) [Thanni et al., 2003; Ogba et al., 2014]. We thus selected P. aeruginosa (Gram-negative) and S. aureus (Gram-positive) for our study.
P. aeruginosa and S. aureus had been stored at –20°C in 10% glycerol and were cultured in nutrient broth and nutrient agar at 37°C for 16 h. From the stock cultures, a single bacterial colony was selected and cultured onto nutrient agar medium overnight prior to testing. Subsequently, at a turbidity of approximately 0.5 McFarland standard, the bacterial suspension was prepared by inoculation of a few bacterial colonies into 10 mL of physiological serum (0.85% NaCl).
Antimicrobial Assays
The antimicrobial activity of lemon, sesame, and olive oils was measured against P. aeruginosa and S. aureus by the broth dilution method using microtubes. Briefly, all microtubes were filled with 100 μL of sterile nutrient broth (2X). Afterwards, 100 μL of oil (lemon, sesame, or olive) was added to the first microtube. Two-fold serial dilutions were done from microtubes 1–5 to obtain final oil concentrations of 2.98, 5.95, 11.90, 23.81, and 47.61% (v/v) in the microtubes. Then, 10 μL of 0.5 McFarland of bacterial suspension was added to each microtube and incubated for 24 h at 37°C. The lowest concentration showing no turbidity was considered as the minimum inhibitory concentration (MIC) [Perumal et al., 2012]. For the determination of minimum bactericidal concentration (MBC), 10 μL of each dilution was placed onto an 8-cm Petri dish containing nutrient agar, and then incubated at 37°C for 24 h. The lowest concentration that showed no growth of bacteria was taken as the MBC. The experiments were repeated to verify the findings.
Cell Viability Studies
L929 mouse fibroblast cells were seeded at a density of 5 × 103 cells/well into 96-well plates in DMEM culture medium supplemented with 10% FBS. Cells were incubated at 37°C and a humidified 5% CO2 atmosphere overnight in an incubator. After incubation, DMEM was replaced by DMEM + 10% FBS containing different amounts of oil (i.e., 0.5, 1, 2.5, 5, 7.5, and 10% [v/v]). In controls, the culture medium was replaced by fresh DMEM + 10% FBS (i.e., no oil). Cells were incubated for 24 h, treated with a 0.5-mg/mL solution of MTT (100 µL/well), and then incubated for another 4 h. Subsequently, 100 µL of DMSO was added and incubated for another 5 min. Optical density was then read using a microplate spectrophotometer (Bio Tek Instruments, USA) at a wavelength of 570 nm, and the percentage of the cell viability was calculated as the mean ± standard deviation (SD).
Animal Studies
Male albino Wistar rats, weighing 200 ± 30 g were obtained from the Animal House, Tehran University of Medical Sciences (Tehran, Iran) and randomly divided into control and treatment groups. The animals were kept under standard laboratory conditions, normal 12-h day/night cycles, at 25°C and a humidity of 55–65%.
Wound Creation
Wounds were created on day 0 for all groups. The dorsal surface of rats was shaved after anesthetizing with an intraperitoneal injection of ketamine hydrochloride (25 mg/kg) and xylazine hydrochloride (10 mg/kg). After creating wound sites by means of a stamp on the dorsum of rats (Fig. 2a), 2 incisions were made on the rats in the control groups and 3 on those in the treatment groups. These were full-thickness incisions, extending up to the adipose tissue, by cutting out a circular piece (1 cm in diameter) from the shaved areas A, B, and C.
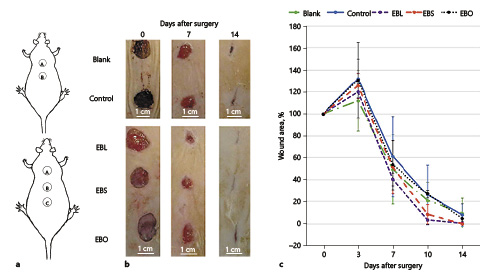
Fig. 2
In vivo wound healing in Wistar rats. a Images of wound sites in control (upper) and treatment (lower) group animals. b Wound contraction images on days 0, 7, and 14. c Percentage of wound area and time-course of wound closure on days post-surgery. Rats treated with EBL and EBS had a faster rate of wound closure than EBO and control groups.
Wound Infection
The wounds of the animals were inoculated with a bacterial suspension of S. aureus as a common pathogenic agent in wounds [Ogba et al., 2014], and 24 h after inoculation, these wounds were checked for signs of infection.
Preparation and Administration of Eucerin-Based Ointment
Eucerin-based ointments of lemon (EBL), sesame (EBS), and olive (EBO) oils were prepared by dissolving 33% (w/w) of the oil in Eucerin (as a suitable base for ointment preparation [Hemmati et al., 2015; Jabbari et al., 2015a, 2015b]) and mixing this until a homogenized sample was obtained. These samples were examined for proper viscosity so that they would remain in place following application.
Rate of Wound Contraction and Period of Epithelialization
Randomly, the rats were divided into 2 groups (n = 6). Group 1 was taken as the control, and Group 2 received Eucerin-based oils. In Group 1, A was blank and B was treated with Eucerin (as control). In Group 2, the sites A, B, and C were treated with EBL, EBS, and EBO, respectively. Both groups were treated twice a day for 14 days and their wounds were covered with sterile 4 × 4 cm gauze and bandages after each treatment.
A wound-healing study was carried out for 14 days. A photograph was taken using a mounted digital camera (GT-N5100, Samsung, China) on days 0, 3, 7, 10, and 14. Wound-closure kinetics were identified using image-processing software (Digimizer v4.1.1.0, MedCalc, Belgium). Results were expressed as a mean (SD) percentage of the remaining area of the original wound. Epithelialization period was expressed as mean (SD) number of days taken for complete epithelialization.
Histological Studies
Five animals from each group were euthanized 14 days posttreatment. Their skin tissues were harvested, and fixed immediately in 10% neutral-buffered formalin (pH 7.26) for 48 h. The fixed tissue samples were processed, embedded in paraffin, and sectioned into 5-µm-thick segments. These sections were stained with Masson’s trichrome (MT) and hematoxylin and eosin (H&E). Histological slides were evaluated by an independent reviewer using light microscopy (BX51, Olympus, Japan). Epithelialization, inflammatory cell infiltration, fibroplasia, and the formation of granulation tissue were assessed and compared in the different groups. A magnification of ×400 was employed for counting the cells. The percentage of collagen density was quantified using ImageJ software using a method published previously [Rangan and Tesch, 2007]. This was calculated by detecting the blue-green area under the magnification fields of each group (6 fields/section were evaluated in each group).
Histomorphometric Analysis
Epithelialization on day 14 was assessed semiquantitatively: 0 (no new epithelialization), 1 (25% epithelialization), 2 (50% epithelialization), 3 (75% epithelialization), and 4 (100% epithelialization). Moreover, the number of inflammatory cells and newly formed blood vessels were recorded in each group. Results were validated by means of comparative analysis by one independent observer blinded to the treatment groups.
Statistical Analysis
All results were compared using Kruskal-Wallis analysis, and expressed as mean ± SD. p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software v20.0 (SPSS Inc., USA).
Results
Antimicrobial Assays
The effect of lemon, sesame, and olive oils was tested on S. aureus and P. aeruginosa using the agar dilution method. It was observed that both bacterial strains were susceptible to lemon oil, showing no growth at concentrations of 47.61 and 23.8% (v/v) against P. aeruginosa and S. aureus, respectively. However, both bacterial strains were resistant to sesame and olive oils using the concentrations studied. Lemon oil showed MBC at 47.61 concentration against S. aureus only, whilesesame and olive oils did not show MBC at all concentrations.
MTT Assay
The cytotoxicity of lemon, sesame, and olive oils was evaluated on L929 mouse fibroblast cells by measuring the percentage of viable cells after 24 h of exposure. Cytotoxicity of oil samples was demonstrated by a decrease in cell viability in a dose-dependent manner (Fig. 1). A significant decrease in cell viability was observed at all concentrations of lemon oil (p < 0.001). Sesame oil also showed a significant decrease at concentrations of 0.5–5% (v/v), but no significant decrease was observed at concentrations of 7.5% and 10% (v/v) (p > 0.05). Olive oil had a significant negative effect on cell viability at concentrations of 0.5, 1, and 5% (v/v), but no significant change at concentrations of 2.5, 7.5, and 10% (v/v) (p > 0.05).
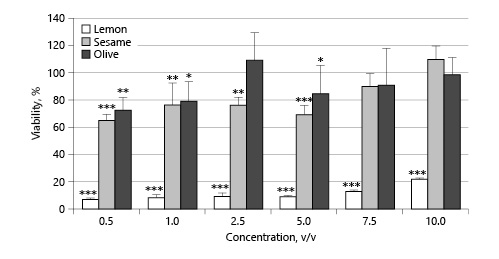
Fig. 1
Cell viability (%) of lemon, sesame, and olive oils. * p <0.05, ** p <0.01, *** p < 0.001, treatment groups versus blank group. Data normalized to the control.
Macroscopic Assessment of Wound Healing
Changes from baseline (wound area at day 0) are shown in Figure 2. Wound contraction was observed to start faster in the EBL and EBS groups than in the EBO and control groups. Normally, incisional wounds tend to stretch and expand during the first day after wound creation [Mack et al., 2012]. From the data, on day 3, the mean wound size was largest in the control (132%) and EBO (131%) groups, and smallest in the blank group (112%). On day 7, mean wound size was largest (61%) in the control group and smallest (40%) in the EBL group. On day 10, the EBL and EBS groups showed significantly better wound contraction than the other groups (96.47 and 91.51%, respectively). On day 14 after surgery, wound contraction was 98.91 and 98.77% in the EBL and EBS groups, respectively, and 92.59, 90.40, and 92.07% in the EBO, blank, and control groups, respectively.
Histological Analysis
Histological analysis of the skin wounds was performed by H&E staining (Fig. 3). In the blank and control groups, histopathological evaluation on day 14 posttreatment showed infiltration of mononuclear inflammatory cells and the formation of granulation tissue. However, the wounds were covered by a crusty scab and the epidermal layer was not observed (Fig. 3).
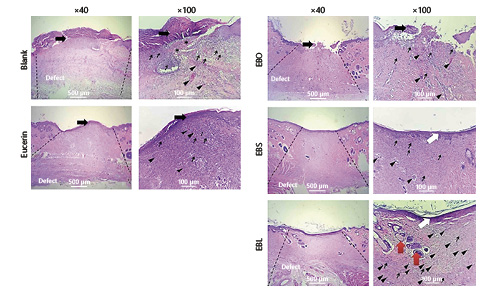
Fig. 3
Microscopic sections of healed incisions 14 days post-surgery. Thin arrows show infiltration of inflammatory cells, white arrows indicate re-epithelialization and arrowheads present rejuvenation of skin appendages. H&E. ×40, ×100.
Micrographs of the EBO group on day 14 posttreatment revealed a close similarity to the control group. The re-epithelialization process was incomplete and there was no important difference between this group and the blank/control groups in terms of inflammation and angiogenesis (Fig. 3e, f). Histological findings were similar in the EBS and EBL groups. In the EBS group, the proliferation of epithelial cells initiated the re-epithelialization process which was nearly completed by day 14. The number of inflammatory cells was also substantially reduced in comparison with the blank/control groups (Fig. 3g, h).
Of particular interest, histopathological evaluation of the wounds treated by EBL showed a considerable reduction of inflammatory cells on day 14 in comparison with the other groups (Fig. 3i, j). A complete epithelial layer with rete pegs (extensions of the epithelium which develop into the underlying connective tissues) was formed. In total, EBL treatment showed the best results compared to the controls and the other groups.
Histomorphometric Analysis
Histomorphometric analysis was performed 14 days after initiation of the skin injury and the results are presented in Table 1 and Figure 4. Re-epithelialization in the blank and control groups was minimal and the wounds were mostly filled with immature granulation tissue. The best re-epithelialization process was observed in the EBL group, followed by the EBS and EBO groups. Moreover, the total number of inflammatory cells in the EBL and EBS groups was significantly reduced in comparison with the other groups on day 14 posttreatment (p < 0.001). Overall, the healing condition of EBL-treated wounds was satisfactory, with normal thickness of epidermal layer and rejuvenation of the hair follicles as well as other skin appendages.
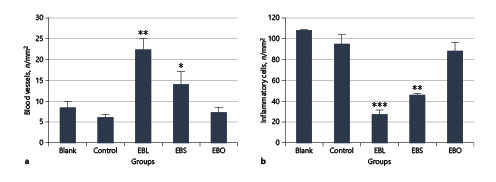
Fig. 4
Histomorphometric analysis of wounds on day 14 post-treatment (n = 5). a Number of blood vessels in each treatment group. b Number of inflammatory cells in each treatment group. * p < 0.05, ** p < 0.01, *** p < 0.001, in treatment groups versus blank group.
Figure 5 shows the results of MT staining in the different groups under study. Collagen fibers stain blue-green; the intensity of this color corresponds to the relative amount of deposited total collagen and reflects the ability to synthesize or remodel collagen. The results indicated that the EBS group had the greatest collagen synthesis (90.72 ± 1.64%), followed by the EBL group (76.43 ± 2.20%). The EBO group showed lower collagen density (63.15 ± 1.16%) than the EBS and EBL groups. On the other hand, the rate of collagen synthesis and deposition in wounds was minimum in the blank and control groups (35.42 ± 1.13 and 51.69 ± 1.67%, respectively).
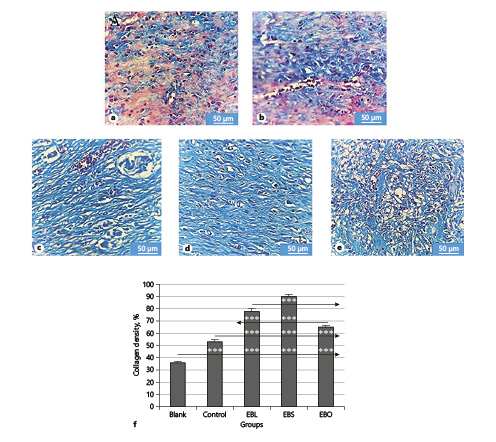
Fig. 5
Representative MT stained microscopic sections of healed incisions in rats on day 14. a Blank. b Control. c EBL. d EBS. e EBO. MT. ×400. Scale bar, 20 µm. f Quantitative analysis of the percentage of collagen density in each group by ImageJ. *** p < 0.001.
Discussion
This study was designed to evaluate the effects of ointments containing lemon, sesame, or olive oil in the healing of infected wounds in rats. Contamination makes the inflammatory phase longer [Guo and DiPietro, 2010]. In the first step, the antimicrobial activity of the oils against S. aureus and P. aeruginosa was studied. Lemon oil showed better antimicrobial activity than the other oils. Previous studies showed that lemon oil has antibacterial activity against Escherichia coli and S. aureus [Baratta et al., 1998; Teixeira et al., 2013]. It has also been reported that α-pinene and β-pinene (2 major components of lemon oil) have antibacterial effects [Leite et al., 2007; da Silva et al., 2012].
The lemon oil in this study showed the highest cytotoxicity. This could be related to some of its components, e.g., α-pinene and (+)-β-pinene. da Silva et al. [2012] showed that 250 µg/mL of (+)-α-pinene and (+)-β-pinene added to Swiss mouse peritoneal macrophages reduced cell viability to 66.8 and 57.7%, respectively.
After creating the wound, a complex process will occur that helps to restore damaged tissues [Chithra et al., 1998; Estevão et al., 2013]. In the process of wound healing, the production and organization of collagen (as the main structural protein) play an integral role [Chithra et al., 1998; Dwivedi et al., 2015]. Our results showed that ointments containing lemon and sesame oils improved synthesis of collagen compared with control groups. Other studies show that, in several types of injury (e.g., skin ulcers), lipid peroxidation is an important process that will be damaging to the cell membrane [Ostrea et al., 1985; Kiran and Asad, 2008]. Agents that inhibit the lipid peroxidation process can decrease the degradation of collagen fibrils and fibers, thereby leading to the prevention of cell damage [Senel et al., 1997]. Antioxidants may inhibit the lipid peroxidation process [Huang et al., 2002].
Previous studies reported that the antioxidant activity of lemon and sesame oils may help the wound-healing process [Misharina and Samusenko, 2008; Hobson, 2016; Khalesi et al., 2016]. The antioxidant activity of lemon oil is related to the presence of high levels of unsaturated and oxygen-functionalized terpenes [Grassmann, 2005; Nguyen et al., 2009]. Studies show that terpenes have antioxidant activity [Grassmann, 2005] and wound-healing effects [Barreto et al., 2014]. Moreover, an important component of lemon oil is limonene. Keskin et al. [2017] showed that limonene enhances wound healing. Their results demonstrated a significant increase in re-epithelialization, angiogenesis, and the thickness of granulation tissue as well as the deposition of collagen fibers. They suggested that anti-inflammatory properties of limonene accelerate the formation of granulation tissue. Our results showed that lemon oil has anti-inflammatory and healing effects, which help repair the skin faster. Moreover, lemon oil reduced the number of inflammatory cells significantly in comparison to the control group on day 14 postsurgery (p < 0.001). Studies also show that lemon oil has a strong immunostimulating activity due to the presence of α-pinene [Kedzia et al., 1998]. A possible mechanism by which lemon oil imparts its activation on macrophages is via α-pinene, which may promote the speed of healing [Rodero and Khosrotehrani, 2010]. In addition, lemon oil can accelerate the production of white blood cells which can shorten the healing time by eliminating the bacterial infection [Ali et al., 2015].
The vitamin E found in sesame oil has an antioxidant effect. It also mediates cell signaling and the regulation of gene expression, which affect cell proliferation, platelet aggregation, and NADPH-oxidase activation [Azzi et al., 2004]. These activities of vitamin E may influence the wound-healing process [Hobson, 2016]. Musalmah et al. [2002] showed that α-tocopherol (a type of vitamin E) increased glutathione peroxidase activity and accelerated the rate of wound closure in treated rats. It has been suggested that other components of sesame oil such as sesamolin, sesamolinol, and sesaminol reduce lipid oxidation by antioxidant activity [Kiran and Asad, 2008]. Our results showed that sesame oil reduced the number of inflammatory cells significantly in comparison to the control group (Fig. 4b). Previous studies showed that oleic acid and linoleic acid in sesame oil [Moalla Rekik et al., 2016] have proinflammatory effects [Pereira et al., 2008; Cardoso et al., 2011; Rodrigues et al., 2012], which may speed up the wound-healing process by increasing protein and DNA amounts and wound mass [Pereira et al., 2008]. Oleic and linoleic acids also stimulate neutrophils to migrate into wounded tissue and release VEGF-α, IL-1β, and CINC-2α/β that help to reduce the time wounds take to heal [Pereira et al., 2008].
Olive oil also has wound-healing effects [Najmi et al., 2015; Donato-Trancoso et al., 2016]. It is the richest source of oleic and linoleic acids (about 79 %) among oils [Zarrouk et al., 2009]. These acids help the wound-healing process by reducing the inflammatory response and the oxidative damage at the wound site [Pereira et al., 2008; Rodrigues et al., 2012]. However, our results showed that in the EBO group, the rate of wound healing was similar to the blank and control groups, and slower than the EBL and EBS groups. In another study [Sharif et al., 2013], sesame and olive oils were used for healing dermal wounds in rats. Their results showed that sesame oil had a better wound-healing effect than olive oil.
Conclusion
This study shows that lemon oil was effective in inhibiting the growth of the pathogenic bacteria tested. Additionally, our findings suggest that lemon and sesame oils might be useful for accelerating the healing of acute wounds by inhibiting the infiltration of inflammatory cells while increasing collagen synthesis and connective tissue formation in the repaired tissue. In addition, topical application of lemon and sesame oils enhances the re-epithelialization and angiogenesis that contribute to the loss of necrotic tissue and the closure of lesions. This study showed that lemon and sesame oil ointments could be considered as interesting alternatives for wound dressings to enhance tissue repair processes and shorten the healing time.
Acknowledgments
The authors would like to thank Dr. Kamali for pathological interpretation.
Statement of Ethics
Animal experiments were done according to the guidelines for the care and use laboratory animals. The experimental protocol was approved by the Ethics Committee of Tehran University of Medical Sciences (approval No. IR.TUMS.VCR.REC.1396.3553, dated 24.09.2017).
Disclosure Statement
The authors declare that there are no conflicts of interest regarding the publication of this manuscript.
Funding Source
This study was supported by the Tehran University of Medical Science and Health Services grant No. 96–01–87–34142.
Author Contributions
A.V., M.S., and M.R.P., performed the antibacterial investigations and analyzed data; A.V and A.A. performed the MTT assay and analyzed data; A.V. and H.S. performed the statistical analysis of the obtained data; A.A. designed and coordinated the research; A.V. and A.A. wrote the paper; all authors approved the final version of the article before submission.
References
- 1. Ahmad M, Ansari MN, Alam A, Khan TH. Oral dose of citrus peel extracts promotes wound repair in diabetic rats. Pak J Biol Sci. 2013;16(20):1086–94.
- 2. Ali B, Al-Wabel NA, Shams S, Ahamad A, Khan SA, Anwar F. Essential oils used in aromatherapy: A systemic review. Asian Pac J Trop Biomed. 2015;5(8):601–11.
- 3. Azzi A, Gysin R, Kempná P, Munteanu A, Negis Y, Villacorta L, et al Vitamin E mediates cell signaling and regulation of gene expression. Ann N Y Acad Sci. 2004;1031(1):86–95.
- 4. Balbino CA, Pereira LM, Curi R. Mecanismos envolvidos na cicatrização: uma revisão. Revista Brasileira de Ciências Farmacêuticas. 2005;41(1):27–51.
- 5. Baratta MT, Dorman HJ, Deans SG, Figueiredo AC, Barroso JG, Ruberto G. Antimicrobial and antioxidant properties of some commercial essential oils. Flavour Fragrance J. 1998;13(4):235–44.
- 6. Barreto RS, Albuquerque-Júnior RL, Araújo AA, Almeida JR, Santos MR, Barreto AS, et al A systematic review of the wound-healing effects of monoterpenes and iridoid derivatives. Molecules. 2014;19(1):846–62.
- 7. Bennett RG. Fundamentals of cutaneous surgery. CV Mosby Company; 1988.
- 8. Cardoso CR, Favoreto S Jr, Oliveira LL, Vancim JO, Barban GB, Ferraz DB, et al Oleic acid modulation of the immune response in wound healing: a new approach for skin repair. Immunobiology. 2011;216(3):409–15.
- 9. Chithra P, Sajithlal GB, Chandrakasan G. Influence of aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol. 1998;59(3):195–201.
- 10. da Silva AC, Lopes PM, de Azevedo MM, Costa DC, Alviano CS, Alviano DS. Biological activities of α-pinene and β-pinene enantiomers. Molecules. 2012;17(6):6305–16.
- 11. Donato-Trancoso A, Monte-Alto-Costa A, Romana-Souza B. Olive oil-induced reduction of oxidative damage and inflammation promotes wound healing of pressure ulcers in mice. J Dermatol Sci. 2016;83(1):60–9.
- 12. Dwivedi S, Singh D, Deshmukh PT, Soni R, Trivedi R. Healing Potential of Ferulic Acid on Dermal Wound in Diabetic Animals. Asian Journal of Molecular Modeling. 2015;1:1.
- 13. Estevão LR, Mendonça FS, Baratella-Evêncio L, Simões RS, Barros ME, Arantes RM, et al Effects of aroeira (Schinus terebinthifoliu Raddi) oil on cutaneous wound healing in rats. Acta Cir Bras. 2013;28(3):202–9.
- 14. Fitzmaurice SD, Sivamani RK, Isseroff RR. Antioxidant therapies for wound healing: a clinical guide to currently commercially available products. Skin Pharmacol Physiol. 2011;24(3):113–26.
- 15. Gosain A, DiPietro LA. Aging and wound healing. World J Surg. 2004;28(3):321–6.
- 16. Grassmann J, Schneider D, Weiser D, Elstner EF. Antioxidative effects of lemon oil and its components on copper induced oxidation of low density lipoprotein. Arzneimittelforschung. 2001;51(10):799–805.
- 17. Grassmann J. Terpenoids as plant antioxidants. Vitam Horm. 2005;72:505–35.
- 18. Greenhalgh DG. The role of apoptosis in wound healing. Int J Biochem Cell Biol. 1998;30(9):1019–30.
- 19. Guasch-Ferré M, Hu FB, Martínez-González MA, Fitó M, Bulló M, Estruch R, et al Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014;12(1):78.
- 20. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29.
- 21. Hemmati AA, Houshmand G, Nemati M, Bahadoram M, Dorestan N, Rashidi-Nooshabadi MR, et al Wound healing effects of persian Oak (Quercus brantii) ointment in rats. Jundishapur J Nat Pharm Prod. 2015;10(4).
- 22. Hobson R. Vitamin E and wound healing: an evidence-based review. Int Wound J. 2016;13(3):331–5.
- 23. Huang HY, Appel LJ, Croft KD, Miller ER 3rd, Mori TA, Puddey IB. Effects of vitamin C and vitamin E on in vivo lipid peroxidation: results of a randomized controlled trial. Am J Clin Nutr. 2002;76(3):549–55.
- 24. Jabbari N, Asghari MH, Ahmadian H, Mikaili P. Developing a commercial air ultrasonic ceramic transducer to transdermal insulin delivery. J Med Signals Sens. 2015a ;5(2):117–22.
- 25. Jabbari N, Asghari MH, Mikaili P. The efficiency of transdermal insulin deliveryby using different concentrations of insulin ointment in hyperglycemic rats. International Journal of Research in Applied and Basic Medical Sciences. 2015b;1(1):20–6.
- 26. Kang MH, Katsuzaki H, Osawa T. Inhibition of 2,2′-azobis(2,4-dimethylvaleronitrile)-induced lipid peroxidation by sesaminols. Lipids. 1998;33(10):1031–6.
- 27. Kedzia B, Jankowiak J, Holonska J, Krzyzaniak M. Investigation of essential oils and components with immunostimulating activity. Herba Pol. 1998;44:126–35.
- 28. Keskin I, Gunal Y, Ayla S, Kolbasi B, Sakul A, Kilic U, et al Effects of Foeniculum vulgare essential oil compounds, fenchone and limonene, on experimental wound healing. Biotech Histochem. 2017;92(4):274–82.
- 29. Khalesi S, Paukste E, Nikbakht E, Khosravi-Boroujeni H. Sesame fractions and lipid profiles: a systematic review and meta-analysis of controlled trials. Br J Nutr. 2016;115(5):764–73.
- 30. Kiran K., M. Asad (2008) Wound healing activity of Sesamum indicum L seed and oil in rats.
- 31. Komiya M, Takeuchi T, Harada E. Lemon oil vapor causes an anti-stress effect via modulating the 5-HT and DA activities in mice. Behav Brain Res. 2006;172(2):240–9.
- 32. Lee YH, Chang JJ, Chien CT, Yang MC, Chien HF. Antioxidant sol-gel improves cutaneous wound healing in streptozotocin-induced diabetic rats. Exp Diabetes Res. 2012;2012:504693.
- 33. Leite AM, Lima EO, Souza EL, Diniz MF, Trajano VN, Medeiros IA. Inhibitory effect of beta-pinene, alpha-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Revista Brasileira de Ciências Farmacêuticas. 2007;43(1):121–6.
- 34. Maciel MA, Pinto AC, Veiga V Jr, Grynberg NF, Echevarria A. Plantas medicinais: a necessidade de estudos multidisciplinares. Quim Nova. 2002;25(3):429–38.
- 35. Mack JA, Feldman RJ, Itano N, Kimata K, Lauer M, Hascall VC, et al Enhanced inflammation and accelerated wound closure following tetraphorbol ester application or full-thickness wounding in mice lacking hyaluronan synthases Has1 and Has3. J Invest Dermatol. 2012;132(1):198–207.
- 36. Marwah H, Garg T, Goyal AK, Rath G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016;23(2):564–78.
- 37. Misharina TA, Samusenko AL. [Antioxidant properties of essential oils from lemon, grapefruit, coriander, clove, and their mixtures]. Prikl Biokhim Mikrobiol. 2008;44(4):482–6.
- 38. Moalla Rekik D, Ben Khedir S, Ksouda Moalla K, Kammoun NG, Rebai T, Sahnoun Z. Evaluation of Wound Healing Properties of Grape Seed, Sesame, and Fenugreek Oils. Evid Based Complement Alternat Med. 2016;2016:7965689.
- 39. Musalmah M, Fairuz AH, Gapor MT, Ngah WZ, Zurinah W. Effect of vitamin E on plasma malondialdehyde, antioxidant enzyme levels and the rates of wound closures during wound healing in normal and diabetic rats. Asia Pac J Clin Nutr. 2002;11(s7Suppl 7):S448–51.
- 40. Myers K, Freidin J, Marshall RD. Principles of pathology in surgery. Blackwell; 1980.
- 41. Najmi M, Vahdat Shariatpanahi Z, Tolouei M, Amiri Z. Effect of oral olive oil on healing of 10-20% total body surface area burn wounds in hospitalized patients. Burns. 2015;41(3):493–6.
- 42. Nguyen H, Campi EM, Jackson WR, Patti AF. Effect of oxidative deterioration on flavour and aroma components of lemon oil. Food Chem. 2009;112(2):388–93.
- 43. Ogba O, Olorode O, Adie G. Bacterial pathogens associated with wound infections in Calabar, Nigeria. J Med. 2014;13(1):26–33.
- 44. Ostrea EM Jr, Cepeda EE, Fleury CA, Balun JE. Red cell membrane lipid peroxidation and hemolysis secondary to phototherapy. Acta Paediatr Scand. 1985;74(3):378–81.
- 45. Pereira L.M., E. Hatanaka, E.F. Martins, F. Oliveira, E.A. Liberti, S.H. Farsky, R. Curi, T.C. Pithon‐Curi (2008) Effect of oleic and linoleic acids on the inflammatory phase of wound healing in rats. Cell Biochemistry and Function: Cellular biochemistry and its modulation by active agents or disease 26(2): 197-204.
- 46. Perumal S, Pillai S, Cai LW, Mahmud R, Ramanathan S. Determination of minimum inhibitory concentration of Euphorbia hirta (L.) extracts by tetrazolium microplate assay. Journal of Natural Products. 2012;5(2).
- 47. Raina R, Parwez S, Verma P, Pankaj N. Medicinal plants and their role in wound healing. Online Veterinary J. 2008;3(1):21.
- 48. Rangan GK, Tesch GH. Quantification of renal pathology by image analysis. Nephrology (Carlton). 2007;12(6):553–8.
- 49. Rodero MP, Khosrotehrani K. Skin wound healing modulation by macrophages. Int J Clin Exp Pathol. 2010;3(7):643–53.
- 50. Rodrigues FV, Hochman B, Wood VT, Simões MJ, Juliano Y, Ferreira LM. Effects of lidocaine with epinephrine or with buffer on wound healing in rat skin. Wound Repair Regen. 2011;19(2):223–8.
- 51. Rodrigues HG, Vinolo MA, Magdalon J, Vitzel K, Nachbar RT, Pessoa AF, et al Oral administration of oleic or linoleic acid accelerates the inflammatory phase of wound healing. J Invest Dermatol. 2012;132(1):208–15.
- 52. Rosa AS, Bandeira LG, Monte-Alto-Costa A, Romana-Souza B. Supplementation with olive oil, but not fish oil, improves cutaneous wound healing in stressed mice. Wound Repair Regen. 2014;22(4):537–47.
- 53. Savant S, Atal-Shan R, Gore D. Text Book and Atlas of Dermatosurgery & Cosmetology. Indian J Dermatol. 1998;43(03):142.
- 54. Senel O, Cetinkale O, Ozbay G, Ahçioğlu F, Bulan R. Oxygen free radicals impair wound healing in ischemic rat skin. Ann Plast Surg. 1997;39(5):516–23.
- 55. Sharif MR, Alizargar J, Sharif A. Evaluation of the wound healing activity of sesame oil extract in rats. World Journal of Medical Sciences. 2013;9(2):74–8.
- 56. Sumitra M, Manikandan P, Suguna L. Efficacy of Butea monosperma on dermal wound healing in rats. Int J Biochem Cell Biol. 2005;37(3):566–73.
- 57. Tehrani S, Lotfi P, Tehrani S, Jangholi E, Aryan H, Aidun A. Healing effect of sesame ointment on second-degree burn wound in rats. Galen Med J. 2016;5(2):56–62.
- 58. Teixeira B, Marques A, Ramos C, Neng NR, Nogueira JM, Saraiva JA, et al Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind Crops Prod. 2013;43:587–95.
- 59. Thanni LO, Osinupebi OA, Deji-Agboola M. Prevalence of bacterial pathogens in infected wounds in a tertiary hospital, 1995-2001: any change in trend?J Natl Med Assoc. 2003;95(12):1189–95.
- 60. Vaghardoost R, Mousavi Majd SG, Tebyanian H, Babavalian H, Malaei L, Niazi M, et al The Healing Effect of Sesame Oil, Camphor and Honey on Second Degree Burn Wounds in Rat. World J Plast Surg. 2018;7(1):67–71.
- 61. Visioli F, Poli A, Gall C. Antioxidant and other biological activities of phenols from olives and olive oil. Med Res Rev. 2002;22(1):65–75.
- 62. Waterman E, Lockwood B. Active components and clinical applications of olive oil. Altern Med Rev. 2007;12(4):331–42.
- 63. Zarrouk W, Baccouri B, Taamalli W, Trigui A, Daoud D, Zarrouk M. Oil fatty acid composition of eighteen Mediterranean olive varieties cultivated under the arid conditions of Boughrara (southern Tunisia). Grasas Aceites. 2009;60(5):500–8.
- 64. Zhang CZ, Niu J, Chong YS, Huang YF, Chu Y, Xie SY, et al Porous microspheres as promising vehicles for the topical delivery of poorly soluble asiaticoside accelerate wound healing and inhibit scar formation in vitro &in vivo. Eur J Pharm Biopharm. 2016;109:1–13.