Introduction
Statins are the widely used medications to lower the serum low-density lipoprotein cholesterol (LDL-C) level. They inhibit the HMG-CoA reductase enzyme, the rate-limiting step in cholesterol biosynthesis in the liver and other tissues [Istvan and Deisenhofer, 2001; Sirtori, 2014]. Their cholesterol-lowering effect makes them highly effective in reducing the morbidity and mortality from coronary heart and other cardiovascular diseases [Baigent et al., 2005]. Beside lowering LDL-C, statins have shown promising therapeutic effects such as anti-inflammatory, anticancer, and immunomodulatory effects [Weitz-Schmidt et al., 2001; Chow, 2009; Pradelli et al., 2013; Kany et al., 2018]. Additionally, statins promote angiogenesis and bone formation [Kureishi et al., 2000; Ishihara et al., 2017]. Moreover, Zissimopoulos et al. [2017] have associated statins with reduced risk for Alzheimer’s disease. In the United States, the annual statin prescription increased by 65% over the period 2002–2013 [Salami et al., 2017]. According to these data, it should be expected that the number of statin prescriptions is continually rising due to the increased risk for cardiovascular diseases, the ongoing research to identify therapeutic uses of statins in noncardiovascular diseases, and changes in the 2013 American College of Cardiology/American Heart Association (ACC/AHA) blood cholesterol management guidelines, an important driver for increasing statin use [Stone et al., 2014].
Myopathy is a well-documented and the most serious adverse effect of statins. The number of patients with statin-induced myopathy varies in different types of studies. In this regard, in an observational study conducted in a population of hyperlipidemic patients receiving high doses of statins for 3 months, muscle-related symptoms were reported by 10.5% of patients, which is two times higher than that observed in randomized controlled trials (RCTs) involving statins (1–5%) [Thompson et al., 2003; Bruckert et al., 2005]. This discrepancy between the two types of studies could be attributed to patient selection in many RCTs. For example, patients with a history of statin-induced myopathy were excluded in many RCTs. Additionally, patients at high risk for statin-induced myopathy such as elderly, women, and patients with comorbidity are underrepresented in RCTs [Fernandez et al., 2011; Maningat and Breslow, 2011]. Moreover, most studies used ten-time elevation in serum creatine kinase (CK) level as a marker for statin-induced myopathy while excluding myalgia [Sinzinger et al., 2002]. Therefore, the exclusion of these patients from many RCTs leads to underestimation of the incidence of statin myopathy in these trials.
Statin-induced myotoxic events range from myalgia to muscle necrosis with or without CK increase. In severe cases, life-threatening rhabdomyolysis develops approximately in 3.4/100,000 patients treated with statins and is manifested by muscle destruction and release of myoglobin [Graham et al., 2004; Armitage, 2007]. Myalgia is the most common adverse event associated with statin treatment [Ucar et al., 2000]. Unfortunately, statin-associated myopathy has a great impact on patients’ physical activities and quality of life. Recently, the ACC/AHA blood cholesterol management guidelines recommended discontinuation of statin therapy if severe myotoxicity develops [Stone et al., 2014]. Discontinuation of statin therapy increases the risk of cardiovascular disease morbidity and mortality in highly susceptible patients. Therefore, scientific research should find a way to help these patients adhere to their statin therapy in order to decrease their risk for cardiovascular events.
The exact mechanism of statin-induced myopathy is still unknown. However, several mechanisms have been hypothesized. In this regard, statins have been associated with inhibition of skeletal muscle mitochondria complex III and lead to mitochondrial dysfunction [Kaufmann et al., 2006; Schirris et al., 2015]. Additionally, simvastatin (SIM) administration increases the muscle and serum levels of oxidative stress markers [Kwak et al., 2012; Ghalwash et al., 2018]. Moreover, muscle biopsies taken from patients receiving SIM showed reduced muscle coenzyme Q10 (CoQ10) levels [Päivä et al., 2005].
CoQ10, also known as ubiquinone, is a potent antioxidant and a cofactor in the electron transport chain [Lenaz and Genova, 2010; Tian et al., 2014]. Statins inhibit the mevalonate pathway, a common pathway for cholesterol and CoQ10 biosynthesis [Mas and Mori, 2010]. The reduction in CoQ10 level during statin treatment disrupts cellular respiration and possibly causes myopathy [Tomaszewski et al., 2011]. Interestingly, Vaughan et al. [2013] demonstrated that addition of CoQ10 to statin-treated myotubes rescued mitochondrial functions and myotube viability. Additionally, treating myopathic rats with CoQ10 improves mitochondrial functions and alleviates myopathy [El-Ganainy et al., 2016]. Moreover, several clinical studies show that statins decrease serum CoQ10 levels [Bargossi et al., 1994; Davidson et al., 1997; Mortensen et al., 1997], an effect which is probably due to a reduction in circulating LDL [Marcoff and Thompson, 2007]. Furthermore, muscle CoQ10 concentrations are reduced in patients treated with high doses of SIM [Päivä et al., 2005]. On the other hand, Fukami et al. [1993] reported no change in muscle CoQ10 levels in SIM-treated animals despite severe muscle lesions. Additionally, the muscle CoQ10 level is reduced in rabbits treated with SIM without reduction in mitochondrial enzyme activities [Nakahara et al., 1998]. Moreover, normal muscle CoQ10 and ATP contents were reported in patients treated with SIM for 6 months [Laaksonen et al., 1996]. Furthermore, CoQ10 supplementation to patients with myopathy did not affect severity of muscle pain or strength [Taylor et al., 2015]. These data point to the inconsistent response of muscle CoQ10 levels to statin treatment and/or variable effects of CoQ10 supplementation on statin-induced myopathy.
Nitric oxide (NO) is a well-known signaling molecule in various cellular pathways. Its potential impacts on different organ systems have been extensively studied [Gow, 2006]. NO regulates the mitochondrial complex IV activities and ATP synthesis [Cooper and Giulivi, 2007; Kim et al., 2012]. During oxidative stress, NO reacts with superoxide anion and generates peroxynitrite. This highly reactive nitrogenous species reacts with cellular components and induces membrane lipid peroxidation and protein modification [Thomas et al., 2008; Maiti et al., 2017]. Additionally, Gupta et al. [2002] demonstrated that NO overproduction in skeletal muscle of rats treated with acetylcholinesterase inhibitor leads to mitochondrial dysfunction, depletion of energy stores, and muscle necrosis. On the other hand, inhibition of NO synthase (NOS) reduces acetylcholinesterase-induced skeletal muscle myopathy [Jeyarasasingam et al., 2000]. Moreover, increased NO production has been associated with skeletal muscle myopathy induced by organophosphorous pesticides [Venkatesh et al., 2009]. Interestingly, the expression of skeletal muscle NOSs was elevated in rats treated with SIM [Goodman et al., 2015]. These data suggest that NO could be implicated in the development of statin-induced myopathy.
The standardized leaf extracts of ginkgo biloba (EGb761) are a widely used traditional herbal medicine for various disease conditions. Two major fractions are found in these extracts: flavonoids and terpenes [Smith and Luo, 2004]. EGb761 has been tried in several conditions, including Parkinson’s disease, cognitive decline, headache, Alzheimer’s disease, and ischemic stroke [Vellas et al., 2012; Oskouei et al., 2013; Dalla Libera et al., 2014; Li et al., 2017; Banjari et al., 2018]. Additionally, it has been shown that EGb761 has antioxidant effects through its free radical scavenging properties protecting the mitochondria from oxidative damage [Eckert et al., 2003]. Moreover, ginkgo biloba extracts have a vasoregulatory activity, increase microcirculatory perfusion, and antagonize platelet aggregation [Suter et al., 2011; El-Boghdady, 2013; Hirsch et al., 2017]. Furthermore, Kaur et al. [2018] demonstrated that ginkgo biloba treatment protected the hippocampal neurons from trimethyltin-induced inflammation and oxidative stress. These data highlight the antioxidant and mitochondrial stabilizing effects of EGb761.
Based on the previously mentioned data, we designed the present study to unravel the relationship between oxidative stress, NO generation, CoQ10 level, and SIM-induce myopathy. We also aimed to investigate the ameliorative effects of EGb761 on SIM-induced changes in these parameters in rat gastrocnemius muscle.
Materials and Methods
Drugs and Chemicals
Drugs were obtained from the following sources: SIM: Sigma-Aldrich Co., USA; EGb761 powder: Amriya Pharmaceutical Industries, Egypt; carboxymethyl cellulose (CMC): Sigma Chemical Company, USA.
Animals
Male adult Wistar rats weighing 180–220 g were purchased from the animal housing facility of the Faculty of Medicine, Assiut University, Assiut, Egypt. Rats were accommodated to the atmosphere of the animal house for 1 week before the beginning of experiments. Animals were kept at 25°C and a 12/12 h light/dark cycle. They were kept free on a standard diet and tap water ad libitum.
Experimental Design
Sixty rats were used in this study and were divided randomly into six groups, 10 rats each. The sample size was calculated using the G*Power 3 software [Faul et al., 2007]. A calculated minimum sample of 54 rats (divided into six equal groups) were needed to detect an effect size of 0.3 in the variance of CK level and/or oxidative stress markers, with an error probability of 0.05 and 80% power. On expecting 10% attrition, the final sample size will be 10 animals per group (n = 60). Group I served as the control group and animals in this group received saline orally for 30 days and were then sacrificed. Rats of group II (vehicle group) were treated daily with 0.5% CMC by oral gavage for 30 days and then sacrificed. Group III (SIM group) animals received SIM orally at a dose of 80 mg/kg/day (0.1% suspension in 0.5% CMC in saline) for 30 days and were then sacrificed. Rats of group IV (SIM withdrawal group) received 80 mg/kg/day SIM orally in 0.5% CMC for 16 days and were sacrificed 14 days after discontinuing SIM. Group V (EGb761-100 group) included rats receiving 80 mg/kg/day SIM orally for 30 days with oral administration of 100 mg/kg/day EGb761 (4% suspension in 0.5% CMC in saline) starting on the 17th day until the end of the experiments. Rats of group VI (EGb761-200) received SIM at a dose of 80 mg/kg/day orally for 30 days, with oral administration of 200 mg/kg/day EGb761 starting on the 17th day and continued until the end of the experiments. Dose and duration of treatment with SIM and EGb761 were selected depending on our preliminary evaluation and following previously published reports [Westwood et al., 2005; Abdel-Zaher et al., 2018].
Assessments of Muscle Performance (Rotarod Test)
Muscle performance was assessed using an accelerating rat rotarod apparatus (Ugo Basile, Italy) as previously published [Dunham and Miya, 1957]. Before the start of the experiments, animals were trained on the rotarod apparatus daily for 3 days. The latency to drop from a rotating rod at a speed of 16 rpm was recorded in all experimental groups. Rats remaining on the rotarod bar for 180 s (cutoff time) were included in the study.
Preparation of Blood and Tissue Samples
At the end of the experiments, rats were anesthetized with an intraperitoneal injection of thiopental sodium (50 mg/kg) and killed by decapitation. Blood samples and gastrocnemius muscles were obtained from each animal. Blood samples were centrifuged at 3,000 rpm for 10 min, and the supernatant was collected for estimation of levels of CK, NO, and CoQ10. Samples were stored at –20°C until use. The right gastrocnemius muscle of each animal was used for histopathological examination while the left muscle was divided into two portions. The first portion was washed well in ice-cold saline, fat and other tissues were removed, then it was cut into small pieces, blotted, weighed, and homogenized in phosphate buffer (10% w/v) at pH 7.4 by using a motor-driven Teflon pestle (Glas-Col, USA). After homogenization and centrifugation at 10,000 rpm for 15 min, the supernatant was used for estimation of malondialdehyde (MDA) level as well as superoxide dismutase (SOD) and catalase (CAT) activities. The second portion of the muscle was processed for electron microscopy.
Biochemical Measurements
Estimation of Serum CK Activity. Serum CK activity was determined using a commercial kit purchased from Egyptian Company for Biotechnology, Cairo, Egypt (Ref.: 238 002) and we followed the manufacturer’s instructions.
Estimation of Serum and Muscle CoQ10 Levels. Serum and muscle CoQ10 levels were assessed using a commercially available sandwich ELISA kit (MyBioSource Inc., San Diego, CA, USA, Cat. No. MBS9304873) following the manufacturer’s instructions.
Estimation of Serum and Muscle NO Level. Serum and muscle concentrations of the nitrite end product were measured as an indicator of NO production according to the previously published method [Montogomery and Dymock, 1961]. We used a kit purchased from Biodiagnostic, Giza, Egypt (Cat. No. NO 25 33) and followed the manufacturer’s instructions.
Estimation of Muscle MDA. Muscle MDA level, an indicator of lipid peroxidation, was determined according to Mihara and Uchiyama [1978].
Assessment of Muscle SOD Activity. Muscle SOD activity was estimated according to Marklund [1985]. The principle of the reaction is that SOD inhibits pyrogallol autoxidation. The inhibition is directly proportional to the activity of SOD in the tested sample.
Assessment of Muscle CAT Activity. Muscle CAT activity was estimated using a commercially available kit from Biodiagnostic (Cat. No. CA 25 17) following the manufacturer’s instructions.
Histological Analysis
Light Microscopic Examination. Muscle samples for light microscopic examination were fixed in 10% neutral buffered formaldehyde solution. Tissues were then washed, dehydrated with ascending grades of ethyl alcohol, cleared with xylene, and embedded in paraffin. Five-micrometer-thick sections were obtained by a rotatory microtome. Sections were stained with Harris’ hematoxylin and eosin (H&E) according to previously published methods [Drury and Wallington, 1980]. We assessed the mean cross-sectional areas in transversely sectioned muscle fibers stained with H&E the using ImageJ software. Briefly, we measured the cross-sectional areas in ten nonoverlapping images of randomly chosen fields of each slide prepared. Images were captured at 400× magnification. In each image, 15 randomly selected muscle fibers were used to assess the cross-sectional areas. Data were analyzed using one-way analysis of variance. The Bonferroni corrections test was used for pairwise comparisons.
Electron Microscopic Examination. Muscle samples for electron microscopy were fixed in 4% glutaraldehyde in cacodylate buffer for 24 h. Samples were postfixed in osmium tetroxide in phosphate buffer for 2 h, dehydrated in graded alcohols, and embedded in epoxy resin blocks. Semithin sections (1 μm) were cut, stained with toluidine blue, and examined using a light microscope. Ultrathin sections (500–800 A) were prepared from selected areas in semithin sections, mounted on copper grids, and contrasted with uranyl acetate and lead citrate according to previously published methods [Bozzola and Russell, 1999]. Sections were examined and photographed using a JEOL100 CX Japan transmission electron microscope at 80 kV at the Assiut University Electron Microscopic Unit.
Statistical Analysis
Statistical analysis was done using one-way analysis of variance followed by Newman-Keuls post hoc test for multiple comparisons. A p value <0.05 was deemed to be statistically significant. All statistics were carried out using the GraphPad Prism 7 software (GraphPad, San Diego, CA, USA) and SPSS 25.0 for Windows (SPSS Inc., USA). Data were expressed as the mean ± standard error of the mean.
Results
Lipid soluble statins including SIM are well known for the development of muscle toxicity, also called myopathy, in susceptible patients [Hoffman et al., 2012]. This adverse effect is characterized by pain, muscle weakness, and elevated CK levels and leads to discontinuation of statin therapy [McKenney et al., 2006]. To help these patients stick to their therapy, we sought to test whether EGb761 could reverse statin-induced muscle toxicity in rats.
EGb761 Reversed SIM-Induced Changes in Muscle Performance
To test the effect of EGb761 on SIM-induced changes in muscle performance, rats were posttreated with 100 or 200 mg/kg/day starting on the 17th day until the end of the experiments. After acclimatization, rats were allowed to run on a rat rotarod device on days 23 and 29 of the experiments. On day 23, we noticed that SIM significantly decreased the time rat stayed on the rotarod compared to the control and vehicle-treated groups (p < 0.05). In contrast, EGb761-treated rats stayed for significantly longer times on the rotarod compared to SIM-treated animals, with higher values obtained by animals receiving 200 mg/kg/day (p < 0.05). Interestingly, rats treated with SIM for 16 days and then discontinued until the end of the experiments (SIM withdrawal group) did not show a significant change on the time they stayed on the rotarod compared to SIM-treated animals. On day 29, SIM further decreased the time rats stayed on the rotarod device, while posttreating animals with EGb761 at the dose of 100 or 200 mg/kg/day significantly increased the time rats stayed on the rotarod (p < 0.05). SIM withdrawal did not cause significant changes in this time compared to the SIM group. These observations suggested that posttreatment with EGb761 improved muscle performance deteriorated by SIM in a dose-dependent manner (Fig. 1a).
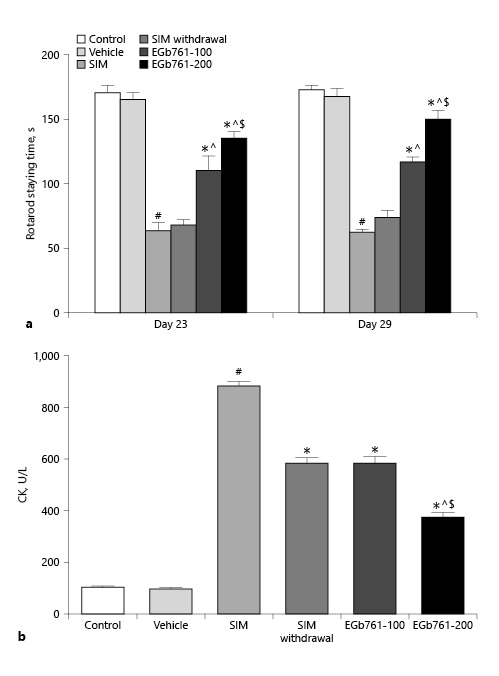
Fig. 1
Effect of posttreatment with EGb761 (100 or 200 mg/kg/day) on stain-induced changes in the time rats stayed on rotarod (a) and serum CK levels (b). Data are expressed as mean ± standard error of the mean (n = 10). #Significant difference compared to the vehicle-treated group (p < 0.05). *Significant difference compared to the SIM-treated group (p < 0.05). ^Significant difference compared to the SIM withdrawal group (p < 0.05). $Significant difference compared to the EGb761-100-treated group (p < 0.05). CK, creatine kinase; EGb761, standardized leaf extracts of ginkgo biloba; SIM, simvastatin.
EGb761 Reversed SIM-Induced Changes in Serum CK Activity
CK is a marker of muscle damage with plasma membrane injury. Here, we measured the serum level of CK activity. We observed that treating animals with SIM orally at a dose of 80 mg/kg/day for 30 days significantly increased the serum CK activity (p < 0.05) compared to control and vehicle-treated animals. In contrast, administration of SIM (80 mg/kg/day orally) for 30 days with EGb761 starting on day 17 reduced the SIM-induce changes in serum CK activity in a dose-dependent manner (Fig. 1b). On the other hand, animals treated with SIM for 16 days and sacrificed 14 days later (SIM withdrawal group) showed a significant reduction in serum CK activity compared to the SIM-treated group. This observation was correlated with the effect of EGb761 at a dose of 100 mg/kg/day on serum CK activity (Fig. 1b). These results suggested that EGb761 protected rat gastrocnemius muscle from the damaging effect of SIM.
EGb761 Reversed SIM-Induced Changes in Serum and Muscle CoQ10 Level
By analyzing RCTs, Qu et al. [2018b] showed that statin treatment reduced the circulating CoQ10 levels. Therefore, we tested the effect of two different doses of EGb761 on SIM-induced changes in serum CoQ10 levels. We found that repeated treatment of rats with SIM at a dose of 80 mg/kg/day orally for 30 days significantly reduced the serum CoQ10 level compared to the control and vehicle-treated groups (p < 0.05). This reduction was reversed by treating myopathic rats with EGb761 orally at a dose of 100 mg/kg/day starting on the 17th day until the end of the experiments (p < 0.05). Additionally, posttreatment of rats with EGb761 orally at a dose of 200 mg/kg/day further elevated the serum level of CoQ10 reduced by SIM treatment. We also observed that the reduction effect of SIM on serum CoQ10 level was inhibited by its withdrawal for 14 days. However, the serum level of CoQ10 in the SIM withdrawal group was significantly lower than that of the EGb761-200 group (p < 0.05) (Fig. 2a).
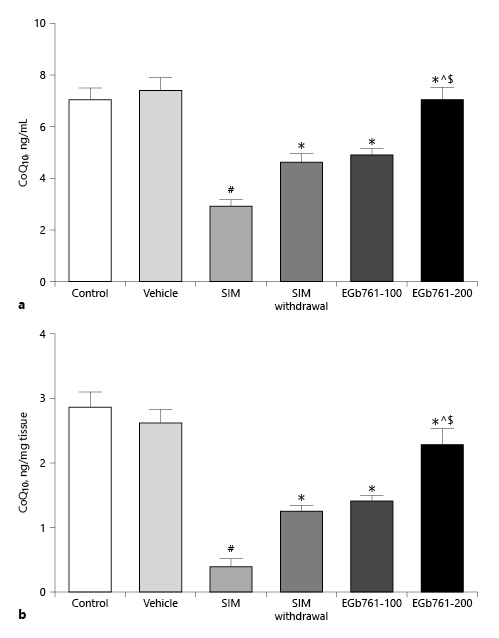
Fig. 2
Mean ± standard error of the mean of serum (a) and muscle (b) CoQ10 levels (n = 10). Animals were treated orally with saline (control), carboxymethyl cellulose (vehicle), SIM (80 mg/kg/day orally for 16 days), SIM withdrawal for 14 days, or EGb761 (at doses of 100 or 200 mg/kg/day orally from day 17 until the end of the experiments) in combination with SIM (80 mg/kg/day). #Significant difference compared to the vehicle-treated group (p < 0.05). *Significant difference compared to the SIM-treated group (p < 0.05). ^Significant difference compared to the SIM withdrawal group (p < 0.05). $Significant difference compared to the EGb761-100-treated group (p < 0.05). CoQ10, coenzyme Q10; EGb761, standardized leaf extracts of ginkgo biloba; SIM, simvastatin.
We also assessed muscle CoQ10 concentrations. As shown in Figure 2b, administration of SIM for 30 days significantly decreased muscle CoQ10 concentration compared to the control and vehicle-treated groups (p < 0.05). This effect was reversed by treating myopathic rats with EGb761 in a dose-dependent manner. Interestingly, the muscle CoQ10 concentration in the SIM withdrawal group was significantly lower than that of rats treated with EGb761 at a dose of 200 mg/kg/day (p < 0.05) (Fig. 2b).
EGb761 Reversed SIM-Induced Changes in Serum and Tissue Nitrite Levels
Increased NO production has been associated with various forms of muscle injuries [Ozkan et al., 2015; Li et al., 2016]. Here, we investigated the serum level of nitrite, an NO end product, as indicative of NO production. As illustrated in Figure 3a, the SIM-treated group displayed a significant elevation in serum nitrite level relative to the control and vehicle-treated groups (p < 0.05). In contrast, oral administration of either 100 or 200 mg/kg/day of EGb761, starting on the 17th day and continued with 80 mg/kg/day SIM until the end of the experiments, significantly decreased serum nitrite levels compared to SIM-treated animals. Additionally, the elevation of serum nitrite level induced by oral administration of SIM (80 mg/kg/day) was significantly reduced after SIM withdrawal for 14 days. The higher dose of EGb761 (200 mg/kg/day orally) appeared to be more effective in decreasing the serum nitrite level compared to the EGb761-100 and SIM withdrawal groups (p < 0.05) (Fig. 3a).
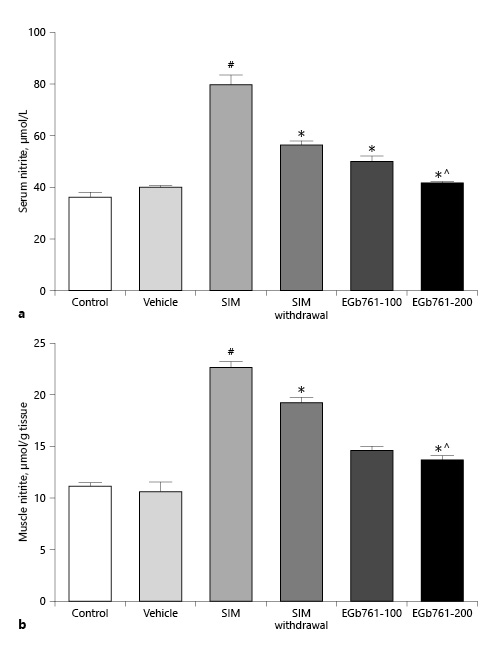
Fig. 3
Effect of posttreatment with EGb761 on statin-induced changes in serum (a) and muscle (b) nitrite levels. SIM increased both serum and muscle nitrite levels, while EGb761 (100 or 200 mg/kg/day) posttreatment significantly lowered serum and muscle nitrite levels compared to the SIM group. EGb761 at a dose of 200 mg/kg/day significantly lowered serum and muscle nitrite levels compared to the SIM withdrawal group. Data are expressed as mean ± standard error of the mean (n = 10). #Significant difference compared to the vehicle-treated group (p < 0.05). *Significant difference compared to the SIM-treated group (p < 0.05). ^Significant difference compared to the SIM withdrawal group (p < 0.05). EGb761, standardized leaf extracts of ginkgo biloba; SIM, simvastatin.
We also assessed the gastrocnemius muscle nitrite levels. As expected, administration of SIM for 30 days increased the muscle nitrite level by twofold compared to the control and vehicle-treated groups. However, SIM withdrawal for 14 days significantly reduced muscle nitrite levels compared to the SIM-treated group (p < 0.05). Interestingly, the administration of EGb761 at 100 or 200 mg/kg/day started on day 17 significantly reduced muscle nitrite levels compared to the SIM and SIM withdrawal groups (p < 0.05). We did not detect a significant difference between the effects of the two doses of EGb761 (Fig. 3b).
EGb761 Reversed SIM-Induced Changes in Gastrocnemius Muscle Oxidative Stress Markers and Antioxidant Enzymes
Previously, it was shown that EGb761 alleviated renal damage in a rat model of diabetic nephropathy [Zayed et al., 2018]. In this report, we investigated whether EGb761 exerted its protective effect on statin-induced myopathy via antagonizing the oxidative stress. Homogenates of gastrocnemius muscle collected from rats treated with SIM at a dose of 80 mg/kg/day for 30 days showed a significant increase in MDA level compared to the control and vehicle-treated groups (Fig. 4a). In contrast, concomitant oral administration of 80 mg/kg/day SIM with EGb761 at a dose of 100 or 200 mg/kg/day, from day 17 until the end of the experiments, significantly decreased the elevated MDA level caused by administration of SIM alone. We next investigated the effect of EGb761 on SIM-induced changes in muscle antioxidant enzymes (SOD and CAT activities). We showed that treatment of rats with SIM for 30 days significantly decreased SOD and CAT activities (p < 0.05) (Fig. 4b, c). On the other hand, oral administration of 80 mg/kg/day SIM for 30 days with administration of either 100 or 200 mg/kg/day of EGb761 orally from day 17 until the end of the experiments significantly increased SOD and CAT activities compared to the SIM-treated group (p < 0.05). Importantly, we did not detect a significant difference in MDA levels or SOD and CAT activities of muscles taken from animals treated with SIM for 16 days and sacrificed 14 days later (SIM withdrawal group) when compared to the SIM-treated group (Fig. 4a–c). These data suggest that EGb761 could ameliorate statin-induced lipid peroxidation and the decrease in antioxidant enzyme status.
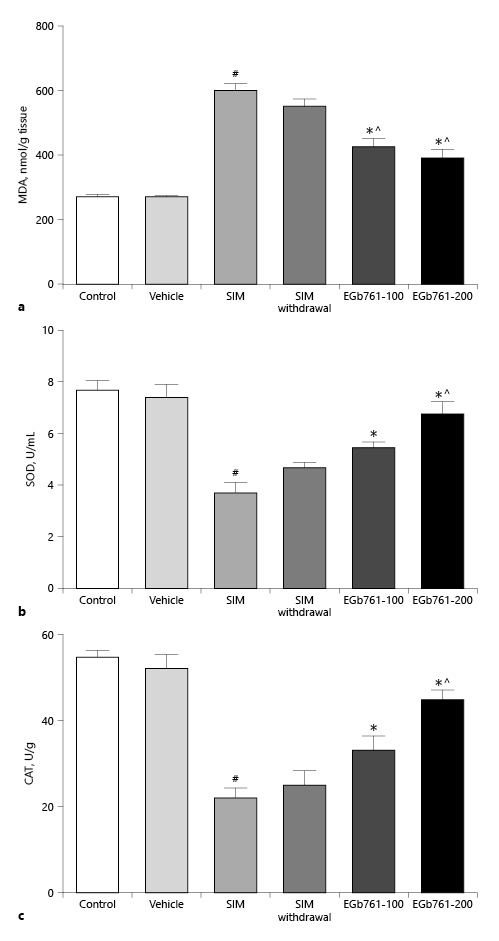
Fig. 4
a Effects of EGb761 (100 and 200 mg/kg/day) on statin-induced changes in gastrocnemius muscle MDA levels. Muscle MDA levels significantly increased after SIM administration while posttreatment with EGb761 significantly decreased muscle MDA levels. b, c Effects of posttreatment with two doses of EGb761 on SIM-induced changes in muscle SOD (b) and CAT (c) activities. Posttreating rats with EGb761 (100 or 200 mg/kg/day) significantly increased SOD and CAT activities compared to the SIM-treated group. Withdrawal of SIM for 14 days did not improve muscle SOD or CAT activities. Data are expressed as mean ± standard error of the mean (n = 6–8). #Significant difference compared to the vehicle-treated group (p < 0.05). *Significant difference compared to the SIM-treated group (p < 0.05). ^Significant difference compared to the SIM withdrawal group (p < 0.05). CAT, catalase; EGb761, standardized leaf extracts of ginkgo biloba; MDA, malondialdehyde; SIM, simvastatin; SOD, superoxide dismutase.
Histological Analysis
To further verify the effect of EGb761 on SIM-induced muscle changes, gastrocnemius muscle sections were stained with H&E. Longitudinal sections from gastrocnemius muscles of control and vehicle-treated animals showed bundles of muscle fibers with multiple elongated peripheral nuclei and acidophilic striated sarcoplasm (Fig. 5a, b, arrows). Fibers were separated by thin endomysium (Fig. 5a, b, arrowheads). In contrast, muscle sections from SIM-treated animals showed fibers with vacuolated (Fig. 5c, double-headed arrows) and fragmented (Fig. 5c, stars) sarcoplasm while others showed splitting (Fig. 5c, thick arrows). Fibers were separated by wide endomysium (Fig. 5c, arrowheads). Additionally, we also observed some fibers with central rather than peripheral nuclei (Fig. 5c, arrows). Muscle sections from animals of the SIM withdrawal group showed split fibers with centrally located nuclei (Fig. 5d, arrows) and fragmented sarcoplasm (Fig. 5d, star). On the other hand, muscle sections from the EGb761-100 group showed fibers with intact acidophilic sarcoplasm and peripherally located elongated nuclei (Fig. 5e, arrows). However, there were vacuolated fibers (Fig. 5e, double-headed arrow) and others with fragmented sarcoplasm (Fig. 5e, stars). Additionally, few fibers were separated by wide endomysium (Fig. 5e, arrowheads). We also observed a few fibers with central nuclei (Fig. 5e, thick arrows). In muscle sections taken from the EGb761-200 group, the fibers mostly restored their normal morphological structure. Muscle fibers stacked together into bundles with minimal endomysial spaces between them (Fig. 5f, arrowheads). These fibers appeared intact, and their sarcoplasm was nonfragmented and had peripheral elongated nuclei (Fig. 5f, arrows). Nevertheless, we observed a few fibers with central nuclei (Fig. 5f, thick arrow).
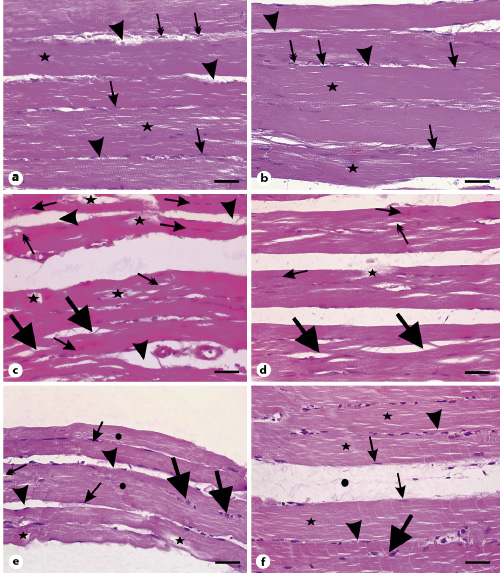
Fig. 5
Photomicrographs of longitudinal sections of gastrocnemius muscles. a Muscle fibers from the control group with multiple elongated nuclei (arrows) and acidophilic sarcoplasm (stars). Arrowheads point to the endomysium. Note the flat nuclei of fibroblasts in the endomysium (double-headed arrows). b Muscle section from the vehicle group showing also cylindrical muscle fibers with peripheral elongated multiple nuclei (arrows) and nuclei of fibroblasts in the endomysium (double-headed arrow). Arrowheads point to the endomysium. c Muscle section from the SIM-treated group showing vacuolations (double-headed arrows), fragmentation of sarcoplasm (stars) of muscle fibers, and splitting of many muscle fibers (thick arrows). Arrows show fibers with central nuclei while arrowheads point to the wide endomysium. d Muscle sections from the SIM withdrawal group with splitting (thick arrows), fragmented fibers (star), and fibers with central nuclei (arrows). Note the presence of vacuolated fibers (double-headed arrow). e Muscle sections from the EGb761-100 group showing muscle fibers with multiple peripheral elongated nuclei (arrows) and intact acidophilic sarcoplasm (circles). Arrowheads reveal wide endomysium and the thick arrows points to fiber with central nuclei. Note the presence of vacuolated fibers (double-headed arrow). f Muscle sections from the EGb761-200 group showing bundles of nonsplitting or fragmented muscle fibers with acidophilic sarcoplasm (stars) and multiple elongated peripheral nuclei (arrows). Arrowheads point to endomysium. Note the wide perimysium (circle) between muscle fiber bundles. Scale bars, 30 µm. EGb761, standardized leaf extracts of ginkgo biloba; SIM, simvastatin.
In transverse sections, muscle fibers of the control and vehicle-treated rats were polygonal with peripheral nuclei (Fig. 6a, b, arrows) and acidophilic sarcoplasm (Fig. 6a, b, stars). They were surrounded by endomysium (Fig. 6a, b, arrowheads). Bundles of fibers were enclosed in perimysium (Fig. 6a, b, circles). In contrast, muscle sections from SIM-treated rats varied greatly in size, with the appearance of rounded rather than polygonal fibers (Fig. 6c, double-headed arrows). Their sarcoplasm appeared fragmented (Fig. 6c, stars), with many of them having central rather than peripheral nuclei (Fig. 6c, arrows). Additionally, we observed wide endomysium separating muscle fibers (Fig. 6c, arrowheads). By withdrawing SIM for 14 days, muscle fibers appeared more or less homogenous in size (Fig. 6d). The fibers were rounded and separated by wide endomysium (Fig. 6d, arrowheads). Few fibers possess eosinophilic sarcoplasm and peripheral nuclei (Fig. 6d, arrows), while others had fragmented sarcoplasm (Fig. 6d, stars). We also observed muscle fibers with central nuclei (Fig. 6d, double-headed arrows). On the other hand, muscle fibers of the EGb761-100 group were polygonal and separated by relatively narrow endomysium (Fig. 6e, arrowheads). Most of the fibers had eosinophilic sarcoplasm with peripheral nuclei (Fig. 6e, arrows). Few fibers were rounded (Fig. 6e, thick arrows) and others had their sarcoplasm fragmented (Fig. 6e, star). We also observed a few fibers with central nuclei (Fig. 6e, double-headed arrows). In muscle sections taken from animals of the EGb761-200 group, muscle fibers were polygonal (Fig. 6f). Their sarcoplasm was eosinophilic and their nuclei were peripherally located (Fig. 6f, arrows). Nevertheless, few fibers with fragmented sarcoplasm (Fig. 6f, stars) and others with central nuclei were seen (Fig. 6f, double-headed arrow). Muscle fibers of this group were separated by narrow endomysium (Fig. 6f, arrowheads) compared to the SIM-treated group. These observations suggested that EGb761 could reverse SIM-induced muscle toxicity.
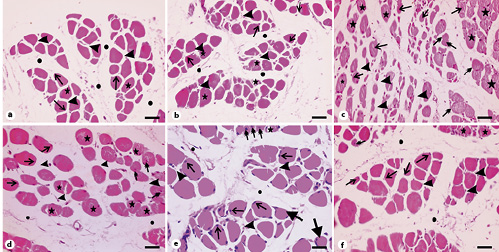
Fig. 6
Photomicrographs of transverse sections of gastrocnemius muscles stained with hematoxylin and eosin. a Section from the control group showing polygonal muscle fibers with peripheral nuclei (arrows) and acidophilic sarcoplasm (stars). Note the endomysium between the fibers (arrowheads) and the perimysium between the muscle bundles (circles). b Muscle section from the vehicle-treated group showing bundles of polygonal muscle fibers with acidophilic sarcoplasm (stars) and peripheral nuclei (arrows). Arrowheads point to the endomysium while circles mark the perimysium. c Muscle section from the SIM-treated group showing fibers varying in shape and size. Stars indicate fragmented sarcoplasm, arrows mark fibers with central nuclei, and double-headed arrows represent rounded muscle fibers. Note the wide endomysium between muscle fibers (arrowheads). d Muscle sections from the SIM withdrawal group with less variability in fibers size. Arrows mark fibers with peripheral nuclei, stars refer to fragmented fibers, and double-headed arrows point to fibers with central nuclei. Note the narrow endomysium between fibers (arrowheads). e Muscle section of the EGb761-100 group with peripheral nuclei (arrows) but few fibers having central nuclei (double-headed arrows). The star indicates muscle fibers with fragmented sarcoplasm while arrowheads depict endomysium. Note the presence of rounded fibers (thick arrows). f Muscle section from the EGb761-200 group showing polygonal fibers with peripheral nuclei (arrows). Arrowheads indicate endomysium. Note the presence of fibers with central nuclei (double-headed arrow) and fibers with fragmented cytoplasm (stars). Circles depict perimysium. Scale bars, 50 µm. EGb761, standardized leaf extracts of ginkgo biloba; SIM, simvastatin.
To confirm our observations, we assessed the mean cross-sectional area using the ImageJ software. As shown in Figure 7, administration of SIM for 30 days significantly reduced the mean cross-sectional areas of muscle fibers compared to the vehicle-treated groups (p < 0.05). Withdrawal of SIM for 14 days significantly increased the mean cross-sectional area compared to SIM-treated animals. As expected, treating myopathic animals with EGb761 at a dose of 100 mg/kg/day significantly increased the mean cross-sectional areas compared to the SIM-treated group (p < 0.05). Additionally, treating myopathic rats with EGb761 at a dose of 200 mg/kg/day further increased the cross-sectional areas of muscle fibers compared to SIM-treated animals as well as the SIM withdrawal group (p < 0.05).
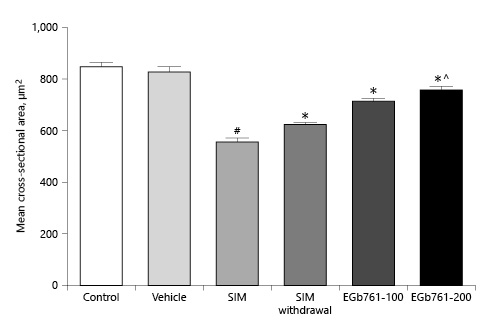
Fig. 7
Mean cross-sectional areas of transversely sectioned skeletal muscle fibers. Animals were treated orally with either saline (control), carboxymethyl cellulose (vehicle), SIM (80 mg/kg/day for 30 days), SIM for 16 days then withdrawn for 14 days or SIM for 30 days (SIM withdrawal), or EGb761 (100 or 200 mg/kg/day) from day 17 until the end of the experiments. Each column represents the mean ± standard error of the mean (n = 6–7). #Significant difference compared to the vehicle-treated group (p < 0.05). *Significant difference compared to the SIM-treated group (p < 0.05). ^Significant difference compared to the SIM withdrawal group (p < 0.05). EGb761, standardized leaf extracts of ginkgo biloba; SIM, simvastatin.
Myofiber changes were also assessed using the transmission electron microscope. Muscle sections taken from the control group showed longitudinal parallel myofibrils with mitochondria and glycogen granules in the intermyofibrillar spaces (Fig. 8a). Each myofibril was formed of sarcomeres that extended between two successive Z lines. In the sarcomere, three banding patterns are observed: I band, A band, and H band. Similar observations were seen in muscle samples taken from vehicle-treated animals (Fig. 8b). In the SIM-treated group, myofibrils appeared disorganized and split. Additionally, we observed disruption of the Z lines, loss of sarcomeres, disintegration of myofilaments and their separation from the Z line, and loss of myofibrillar banding pattern (Fig. 8c). We also observed widening of the intermyofibrillar region (Fig. 8c, asterisks) together with vacuolations and loss of mitochondria (Fig. 8c). In the SIM withdrawal group, we observed preservation of sarcomeres and banding patterns in few myofibrils, while most of the myofibrils showed destruction (Fig. 8d, asterisks). We also found mitochondria of variable sizes and shapes in the intermyofibrillar region (Fig. 8d, arrows). On the other hand, muscle sections from the EGb761-100 group showed parallel myofibrils with intact sarcomeres and banding patterns. However, some myofibrils had areas of myofilament destruction. Additionally, widening of the intermyofibrillar region and few mitochondria were noticed in some areas (Fig. 8e). In muscle sections taken from the EGb761-200 group, myofibrils had restored their normal structure with intact sarcomeres and banding patterns. We also noticed glycogen granules and mitochondria in the intermyofibrillar region (Fig. 8f).
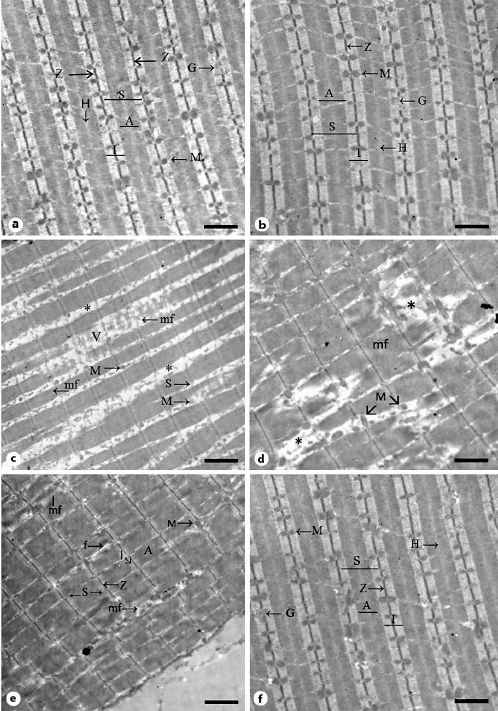
Fig. 8
Representative electron micrographs of longitudinally sectioned gastrocnemius muscles. a Muscle section from the control group showing several longitudinal parallel myofibrils (mf) with light (I), dark (A), and H bands. The sarcomeres (S) extend between two Z lines (Z). Note the intermyofibrillar mitochondria (M) and glycogen (G). b Muscle section from the vehicle-treated group with l, A, and H bands in the myofibrils. Sarcomeres extend between two Z lines. Note the intermyofibrillar mitochondria (M) and glycogen (G). c Muscle section from the SIM-treated group showing disintegration of myofibrils (mf) with disruption of Z lines and areas of loss of myofilaments. Asterisks depict widely separated myofibrils. Note the destruction of the mitochondria (M) and the presence of vacuolations (V) in the intermyofibrillar region. d Section from the SIM withdrawal group showing partial loss of myofilaments and Z lines (asterisks). Mitochondria (M) of many sizes and shapes are noticed. e Section from the EGb761-100 group. Note the parallel arrangement of myofibrils with sarcomeres (S) extending between two successive Z lines (Z) and the presence of dark (A) and light (I) bands. Note the regions of myofibrillar disruption, wide separation between myofibrils (f), and few mitochondria (M) in the intermyofibrillar region. f Section from the EGb761-200 group with a parallel arrangement of myofibrils and their banding patterns (I, A, and H bands) and intact sarcomeres (S). Glycogen (G) and mitochondria (M) are noticed in the intermyofibrillar regions. Scale bars, 2 µm. EGb761, standardized leaf extracts of ginkgo biloba; SIM, simvastatin.
Discussion
Statin-induced muscle toxicity, known as myopathy, is a well-established adverse effect during statin treatment [Hoffman et al., 2012]. This adverse effect is the major cause of nonadherence to statin therapy [Brown and Watson, 2018]. The aim of this study was to explore the effect of two different doses of EGb761 on SIM-induced myopathy in rats. We showed that treatment of myopathic rats with EGb761 improved muscle performance deteriorated by SIM. Additionally, we showed that EGb761 reversed SIM-induced changes in serum CK as well as serum and muscle CoQ10. We also demonstrated that administration of EGb761 after the establishment of myopathy in rats alleviated SIM-induced changes in serum and tissue nitrites. Moreover, we showed that EGb761 improved the antioxidant status deteriorated by SIM. Finally, we showed that EGb761 ameliorated the histopathological changes induced by SIM administration.
The data of the present study showed that treating rats with SIM (80 mg/kg/day orally for 30 days) resulted in a significant elevation in the serum level of CK (p < 0.05) compared to the vehicle-treated group. Our observations are in line with several animal and clinical studies [Carvalho et al., 2004; Guis et al., 2006; Pierno et al., 2006]. This effect of SIM could be due to increased sarcolemmal permeability that allows diffusion of CK into the circulation. This impaired sarcolemmal permeability could be due to statin-induced imbalance between membrane cholesterol and phospholipids, as discussed by Jamal et al. [2004], and/or membrane damage due to SIM-induced oxidative stress. In this work, we have shown that treating myopathic rats with EGb761 resulted in a dose-dependent decline in serum CK activity when compared to the SIM-treated group. This effect could be due to a membrane-stabilizing effect of EGb761 and/or its free radical scavenging properties suggested by previously published reports [Punkt et al., 1999; Saini et al., 2014].
Our results suggest that SIM-induced skeletal muscle toxicity was associated with oxidative stress. We have shown that administration of SIM at a dose of 80 mg/kg/day for 30 days significantly elevated muscle MDA levels. Additionally, SIM significantly decreased gastrocnemius muscle SOD and CAT activities. In agreement with our observations, Ghalwash et al. [2018] showed that SIM administration induced oxidative stress and decreased muscle contractile capacity, leading to muscle dysfunction. Moreover, several in vitro studies have associated oxidative stress with SIM treatment [Kwak et al., 2012; Essid et al., 2019]. On the other hand, we showed that EGb761 administration to myopathic rats decreased muscle MDA levels. This result is in line with the observations of Chen et al. [2019] who recently demonstrated that EGb761 protected rat myocardium from ischemia/reperfusion-induced oxidative stress. The lowering effect of EGb761 on MDA levels could be explained by its protective role against membrane-lipid peroxidation, an effect attributed to its free radical scavenging properties [Boveris et al., 2007]. Moreover, we have shown that EGb761 increased muscle SOD and CAT activities. This effect can be also attributed to its potent free radical scavenging properties as excessive production of reactive oxygen species would overwhelm the activities of these antioxidant enzymes [Hasegawa et al., 1992]. Together, these data suggest potent antioxidant activities for EGb761.
It has been well established that statin treatment is associated with impaired CoQ10 synthesis [Sirvent et al., 2008]. In this report, we have shown that SIM treatment markedly decreased serum and muscle CoQ10 levels. In agreement with our observations, Qu et al. [2018b] demonstrated a reduction in circulating CoQ10 in statin-treated patients through a meta-analysis of RCTs. Additionally, our results are in agreement with the findings of Larsen et al. [2013] who demonstrated that patients treated with SIM had lower muscle CoQ10 levels, which were associated with impaired muscle oxidative phosphorylation capacity. The impaired oxidative phosphorylation capacity could explain the muscle pain and weakness of those patients. This also can explain the reduced performance of SIM-treated rats on the rotarod reported here. CoQ10 deficiency may also explain the mitochondrial changes we observed in our histological analysis. Previous data have attributed reduced serum CoQ10 to the reduction in the blood level of its carrier, LDL-C [Tomasetti et al., 1999]. However, a randomized trial conducted on patients with SIM-induced myalgia revealed that CoQ10 supplementation raised the serum CoQ10 level without affecting the lipid-lowering effect of SIM [Taylor et al., 2015]. Additionally, administration of ezetimibe did not affect blood CoQ10 levels despite its LDL-lowering effect [Berthold et al., 2006]. Therefore, it is likely that SIM-induced reduction in serum CoQ10 level is due to SIM-induced impairment of CoQ10 biosynthesis rather than a consequence of its LDL-lowering effect.
Our results also showed that treating myopathic rats with EGb761 reversed SIM-induced changes in serum and muscle CoQ10 levels. These observations could lead to speculation that EGb761 enhanced the mevalonate pathway and increased cholesterol synthesis. However, a recent meta-analysis revealed that ginkgo biloba leaf extract augmented the LDL-C-lowering effect of statins [Fan et al., 2018]. Additionally, experiments by Aberg et al. [1994] suggested that various drugs can augment the production of one mevalonate end product while inhibiting others. Moreover, our histological analysis showed that treating myopathic rats with EGb761 reversed mitochondrial damage. Furthermore, Abdel-Kader et al. [2007] reported that EGb761 restored mitochondrial functions in an in vitro model of aging. Recently, a meta-analysis of RCTs showed that CoQ10 supplementation alleviated statin-induced muscle symptoms [Qu et al., 2018a]. Altogether, it is likely that EGb761 elevated CoQ10 levels, possibly through augmenting CoQ10 biosynthesis. This effect probably contributes to restoring the mitochondrial structure, function, and performance on rotarod in response to EGb761 posttreatment.
Additionally, our results show that administration of SIM (80 mg/kg/day orally) for 30 days elevated serum and muscle nitrite levels, indicative of excessive NO production. These findings are consistent with those of Goodman et al. [2015], who demonstrated that SIM-induced muscle damage was associated with increased NOS expression and increased NO production. High NO levels lead to the production of a potent oxidant, peroxynitrite [Pryor and Squadrito, 1995; Goodman et al., 2015]. On the other hand, we showed that treating myopathic rats with EGb761 resulted in a significant decrease in serum and muscle nitrite levels in a dose-dependent manner. In the same context, Marcocci et al. [1994] reported that EGb761 can scavenge NO in a dose-responsive manner and limited nitrite accumulation. Moreover, Punkt et al. [2001] demonstrated that EGb761 alleviated muscle dysfunction in diabetic rats through upregulation of muscle neuronal and endothelial NOS expressions. Furthermore, Abdel-Zaher et al. [2018] showed that EGb761 protected rat kidneys against injury by hypertension and hypercholesterolemia by lowering nitrite and inducible NOS expression. Altogether, EGb761 maintains NO within its physiological levels through upregulation of neuronal and endothelial NOS, downregulation of inducible NOS expressions, and/or its NO scavenging activities as an antioxidant.
Our histological data reveal that muscle sections from SIM-treated animals showed muscle damage in the form of vacuolation, fragmentation of sarcoplasm, and variation of muscle size in transversely sectioned fibers. Additionally, electron microscopy examination of muscle sections from SIM-treated rats showed disruption of sarcomeres, fragmentation of myofilaments and their separation from the Z lines, disruption of banding patterns, and loss of mitochondria. These findings suggest myofiber destruction. Previously, Goodman et al. [2015] reported that SIM treatment activated the ubiquitin ligases: muscle atrophy F-box or atrogin-1 and muscle ring finger-1 (MuRF-1). Activated MuRF-1 disrupts Z lines and destroys titin and other protein-linking myofilaments to the Z lines, which leads to dissociation of actin and myosin myofilaments from the Z lines [Wray et al., 2003]. Additionally, both MuRF-1 and atrogin-1 are involved in the process of intracellular proteolysis [Bhat et al., 2013]. These data could explain the dissociation of myofilaments from the Z lines we observed in our electron micrographs. Therefore, SIM-induced myofibrillar degradation could be explained, at least in part, by SIM-induced activations of the ubiquitin ligases MuRF-1 and atrogin-1.
There is accumulating evidence that the ubiquitin ligases atrogin-1 and MuRF-1 are regulated by protein kinase B, also known as Akt. In this regard, phosphorylation of Akt inhibits protein degradation via phosphorylation of FOXO3 transcription factor. Phosphorylated FOXO3 cannot translocate into the nucleus to activate atrogin-1 and MuRF-1 [Brunet et al., 1999; Sandri et al., 2004; Stitt et al., 2004]. Interestingly, Mallinson et al. [2009] showed that SIM inhibited Akt phosphorylation and activated FOXO transcription factors. Additionally, Zheng et al. [2019] demonstrated that activation of PI3K/Akt/mTOR signaling inactivates the FOXO3 transcription factor and its downstream effectors. On the other hand, Bonifacio et al. [2017] reported that coadministration of IGF-1 and SIM activated IGF-1/Akt signaling and inhibited FOXO3 translocation to the nucleus in vitro. Altogether, activation of the IGF-1/PI3K/Akt/mTOR pathway inhibits activation of the ubiquitin ligases.
Our histological data also showed that treating myopathic rats with EGb761 decreased splitting of muscle fibers, vacuolation, fragmentation of sarcoplasm, and sarcomere changes induced by SIM administration. Also, we showed that EGb761 at a dose of 200 mg/kg/day rescued the mitochondria and decreased endomysial spaces. These data suggest that treating myopathic animals with EGb761 decreased myofiber damaged induced by SIM treatment. Interestingly, a recent animal study showed that EGb761 increased Akt phosphorylation, protecting myocardium from ischemia/reperfusion injury [Chen et al., 2019]. Additionally, Koh [2010] showed that EGb761 exhibited a neuroprotective effect through activation of Akt/mTOR signaling in a rat model of brain ischemia. Therefore, EGb761 could reverse SIM-induced muscle changes by activation of the Akt signaling pathway, which ultimately blocks the activation of atrophy genes. This role of EGb761 needs further exploration.
It is well documented that statin-induced myopathy is reversible upon drug cessation [Needham et al., 2007; Armour and Zhou, 2013]. In the current study, we have shown that SIM withdrawal for 14 days after induction of myopathy resulted in a significant reduction in serum CK activity as well as serum and muscle nitrite levels with significant elevation of serum and muscle CoQ10 levels compared to the SIM-treated group; however, these parameters did not reach normal values. Therefore, the persistence of these mild myopathic changes after SIM cessation suggests partial recovery. On the other hand, muscle performance on the rotarod, MDA levels, as well as SOD and CAT activities did not significantly change after cessation of SIM for 14 days compared with the SIM-treated group. These parameters may need a longer time to be recovered.
A limitation of our study should be noted. The dose of the SIM used in the present study was high relative to the human dose. We used this high dose of SIM to induce myopathy in rats. The same dose was used by Westwood et al. [2005] to induce myopathy in their animals. Additionally, Mallinson et al. [2009] treated their animals with SIM at a dose of 88 mg/kg/day for 12 days to induce muscle toxicity. Not only SIM, but also other statins were administered at the maximally tolerated dose to induce myopathy in experimental animals [Waclawik et al., 1993; El-Ganainy et al., 2016, 2017]. Therefore, we and others used statins at the maximally tolerated doses to induce myopathy rather than for therapeutic purposes.
In conclusion, this study revealed that oxidative stress as well as changes in NO and CoQ10 levels were the major contributors to the pathophysiology of myopathy during SIM treatment. Our study also denotes that posttreating myopathic rats with EGb761 ameliorated SIM-induced muscle toxicity. This suggests, under experimental conditions, that cotreatment with EGb761 could be a complementary approach in statin-induced myopathy. Further animal research is needed to support this suggestion.
Statements of Ethics
All experiments in the present study were managed according to the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) Guide for the Care and Use of Laboratory Animals and according to Animal Care and Use Committee at the Faculty of Medicine, Assiut University, Assiut, Egypt. The study protocol was approved by the Institutional Research Committee at the Faculty of Medicine, Assiut University, Assiut, Egypt.
Disclosure Statement
The authors declare no conflicts of interest.
Funding Sources
This work was supported by the Assiut University Faculty of Medicine Grants Unit (reference code: 2015-12-005).
Author Contributions
E.O. Kamel, M.A. Ahmed, E.A. Ahmed, and T.H. Abd-Elhamid formulated the study design. A.R. Mahmoud, E.O. Kamel, M.A. Ahmed, E.A. Ahmed, and T.H. Abd-Elhamid participated in data collection, data analysis, manuscript preparation, and review processes. All authors approved the final manuscript.
References
- 1. Abdel-Kader R, Hauptmann S, Keil U, Scherping I, Leuner K, Eckert A, et al Stabilization of mitochondrial function by Ginkgo biloba extract (EGb 761). Pharmacol Res. 2007;56(6):493–502.
- 2. Abdel-Zaher A.O., H.S.M. Farghaly, A.E.M. El-Refaiy, A.M. Abd-Eldayem (2018) Protective effect of the standardized leaf extract of Ginkgo biloba (EGb761) against hypertension-induced renal injury in rats. Clinical and experimental hypertension (New York, NY : 1993) 40(8): 703-714.
- 3. Aberg F, Zhang Y, Appelkvist EL, Dallner G. Effects of clofibrate, phthalates and probucol on ubiquinone levels. Chem Biol Interact. 1994;91(1):1–14.
- 4. Armitage J. The safety of statins in clinical practice. Lancet. 2007;370(9601):1781–90.
- 5. Armour R, Zhou L. Outcomes of statin myopathy after statin withdrawal. J Clin Neuromuscul Dis. 2013;14(3):103–9.
- 6. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et alCholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–78.
- 7. Banjari I, Marček T, Tomić S, Waisundara VY. Forestalling the Epidemics of Parkinson’s Disease Through Plant-Based Remedies. Front Nutr. 2018;5:95.
- 8. Bargossi AM, Grossi G, Fiorella PL, Gaddi A, Di Giulio R, Battino M. Exogenous CoQ10 supplementation prevents plasma ubiquinone reduction induced by HMG-CoA reductase inhibitors. Mol Aspects Med. 1994;15Suppl:s187–93.
- 9. Berthold HK, Naini A, Di Mauro S, Hallikainen M, Gylling H, Krone W, et al Effect of ezetimibe and/or simvastatin on coenzyme Q10 levels in plasma: a randomised trial. Drug Saf. 2006;29(8):703–12.
- 10. Bhat M, Kalam R, Qadri SS, Madabushi S, Ismail A. Vitamin D deficiency-induced muscle wasting occurs through the ubiquitin proteasome pathway and is partially corrected by calcium in male rats. Endocrinology. 2013;154(11):4018–29.
- 11. Bonifacio A, Sanvee GM, Brecht K, Kratschmar DV, Odermatt A, Bouitbir J, et al IGF-1 prevents simvastatin-induced myotoxicity in C2C12 myotubes. Arch Toxicol. 2017;91(5):2223–34.
- 12. Boveris AD, Galleano M, Puntarulo S. In vivo supplementation with Ginkgo biloba protects membranes against lipid peroxidation. Phytother Res. 2007;21(8):735–40.
- 13. Bozzola JJ. Electron Microscopy: Principles and Techniques for Biologists. USA: Jones and Bartlett Publishers, Inc.; 1998.
- 14. Brown AS, Watson KE. Statin Intolerance. Rev Cardiovasc Med. 2018;19S1:S9–19.
- 15. Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19(6):403–14.
- 16. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–68.
- 17. Carvalho AA, Lima UW, Valiente RA. Statin and fibrate associated myopathy: study of eight patients. Arq Neuropsiquiatr. 2004;62(2a2A):257–61.
- 18. Chen XJ, Ren SM, Dong JZ, Qiu CG, Chen YW, Tao HL. Ginkgo biloba extract-761 protects myocardium by regulating Akt/Nrf2 signal pathway. Drug Des Devel Ther. 2019;13:647–55.
- 19. Chow SC. Immunomodulation by statins: mechanisms and potential impact on autoimmune diseases. Arch Immunol Ther Exp (Warsz). 2009;57(4):243–51.
- 20. Cooper CE, Giulivi C. Nitric oxide regulation of mitochondrial oxygen consumption II: molecular mechanism and tissue physiology. Am J Physiol Cell Physiol. 2007;292(6):C1993–2003.
- 21. Dalla Libera D., B. Colombo, G. Pavan, G. Comi (2014) Complementary and alternative medicine (CAM) use in an Italian cohort of pediatric headache patients: the tip of the iceberg. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 35 Suppl 1: 145-148.
- 22. Davidson M, McKenney J, Stein E, Schrott H, Bakker-Arkema R, Fayyad R, et alAtorvastatin Study Group I. Comparison of one-year efficacy and safety of atorvastatin versus lovastatin in primary hypercholesterolemia. Am J Cardiol. 1997;79(11):1475–81.
- 23. Drury RA, Wallington EA. Carleton’s Histological Technique. Oxford, New York, Toronto: Oxford University Press; 1980.
- 24. Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc. 1957;46(3):208–9.
- 25. Eckert A, Keil U, Kressmann S, Schindowski K, Leutner S, Leutz S, et al Effects of EGb 761 Ginkgo biloba extract on mitochondrial function and oxidative stress. Pharmacopsychiatry. 2003;36Suppl 1:S15–23.
- 26. El-Boghdady NA. Increased cardiac endothelin-1 and nitric oxide in adriamycin-induced acute cardiotoxicity: protective effect of Ginkgo biloba extract. Indian J Biochem Biophys. 2013;50(3):202–9.
- 27. El-Ganainy SO, El-Mallah A, Abdallah D, Khattab MM, Mohy El-Din MM, El-Khatib AS. Elucidation of the mechanism of atorvastatin-induced myopathy in a rat model. Toxicology. 2016;359-360:29–38.
- 28. El-Ganainy SO, El-Mallah A, Abdallah D, Khattab MM, Mohy El-Din MM, El-Khatib AS. Rosuvastatin safety: an experimental study of myotoxic effects and mitochondrial alterations in rats. Toxicol Lett. 2017;265:23–9.
- 29. Essid SM, Bevington A, Brunskill NJ. Proinsulin C-Peptide Enhances Cell Survival and Protects against Simvastatin-Induced Myotoxicity in L6 Rat Myoblasts. Int J Mol Sci. 2019;20(7):E1654.
- 30. Fan Y, Jin X, Man C, Gong D. Does Adjuvant Treatment With Ginkgo Biloba to Statins Have Additional Benefits in Patients With Dyslipidemia?Front Pharmacol. 2018;9:659.
- 31. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91.
- 32. Fernandez G, Spatz ES, Jablecki C, Phillips PS. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78(6):393–403.
- 33. Fukami M, Maeda N, Fukushige J, Kogure Y, Shimada Y, Ogawa T, et al Effects of HMG-CoA reductase inhibitors on skeletal muscles of rabbits. Res Exp Med (Berl). 1993;193(5):263–73.
- 34. Ghalwash M, Elmasry A, El-Adeeb N. Effect of L-carnitine on the skeletal muscle contractility in simvastatin-induced myopathy in rats. J Basic Clin Physiol Pharmacol. 2018;29(5):483–91.
- 35. Goodman CA, Pol D, Zacharewicz E, Lee-Young RS, Snow RJ, Russell AP, et al Statin-Induced Increases in Atrophy Gene Expression Occur Independently of Changes in PGC1α Protein and Mitochondrial Content. PLoS One. 2015;10(5):e0128398.
- 36. Gow AJ. The biological chemistry of nitric oxide as it pertains to the extrapulmonary effects of inhaled nitric oxide. Proc Am Thorac Soc. 2006;3(2):150–2.
- 37. Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, et al Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292(21):2585–90.
- 38. Guis S, Figarella-Branger D, Mattei JP, Nicoli F, Le Fur Y, Kozak-Ribbens G, et al In vivo and in vitro characterization of skeletal muscle metabolism in patients with statin-induced adverse effects. Arthritis Rheum. 2006;55(4):551–7.
- 39. Gupta RC, Milatovic D, Dettbarn WD. Involvement of nitric oxide in myotoxicity produced by diisopropylphosphorofluoridate (DFP)-induced muscle hyperactivity. Arch Toxicol. 2002;76(12):715–26.
- 40. Hasegawa T, Kaneko F, Niwa Y. Changes in lipid peroxide levels and activity of reactive oxygen scavenging enzymes in skin, serum and liver following UVB irradiation in mice. Life Sci. 1992;50(24):1893–903.
- 41. Hirsch GE, Viecili PR, de Almeida AS, Nascimento S, Porto FG, Otero J, et al Natural Products with Antiplatelet Action. Curr Pharm Des. 2017;23(8):1228–46.
- 42. Hoffman KB, Kraus C, Dimbil M, Golomb BA. A survey of the FDA’s AERS database regarding muscle and tendon adverse events linked to the statin drug class. PLoS One. 2012;7(8):e42866.
- 43. Ishihara T., M. Miyazaki, N. Notani, S. Kanezaki, M. Kawano, H. Tsumura (2017) Locally Applied Simvastatin Promotes Bone Formation in a Rat Model of Spinal Fusion. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 35(9): 1942-1948.
- 44. Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292(5519):1160–4.
- 45. Jamal SM, Eisenberg MJ, Christopoulos S. Rhabdomyolysis associated with hydroxymethylglutaryl-coenzyme A reductase inhibitors. Am Heart J. 2004;147(6):956–65.
- 46. Jeyarasasingam G, Yeluashvili M, Quik M. Nitric oxide is involved in acetylcholinesterase inhibitor-induced myopathy in rats. J Pharmacol Exp Ther. 2000;295(1):314–20.
- 47. Kany S, Woschek M, Kneip N, Sturm R, Kalbitz M, Hanschen M, et al Simvastatin exerts anticancer effects in osteosarcoma cell lines via geranylgeranylation and c-Jun activation. Int J Oncol. 2018;52(4):1285–94.
- 48. Kaufmann P, Török M, Zahno A, Waldhauser KM, Brecht K, Krähenbühl S. Toxicity of statins on rat skeletal muscle mitochondria. Cell Mol Life Sci. 2006;63(19-20):2415–25.
- 49. Kaur S, Sharma N, Nehru B. Anti-inflammatory effects of Ginkgo biloba extract against trimethyltin-induced hippocampal neuronal injury. Inflammopharmacology. 2018;26(1):87–104.
- 50. Kim KH, Song K, Yoon SH, Shehzad O, Kim YS, Son JH. Rescue of PINK1 protein null-specific mitochondrial complex IV deficits by ginsenoside Re activation of nitric oxide signaling. J Biol Chem. 2012;287(53):44109–20.
- 51. Koh PO. Gingko biloba extract (EGb 761) prevents cerebral ischemia-induced p70S6 kinase and S6 phosphorylation. Am J Chin Med. 2010;38(4):727–34.
- 52. Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, et al The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6(9):1004–10.
- 53. Kwak HB, Thalacker-Mercer A, Anderson EJ, Lin CT, Kane DA, Lee NS, et al Simvastatin impairs ADP-stimulated respiration and increases mitochondrial oxidative stress in primary human skeletal myotubes. Free Radic Biol Med. 2012;52(1):198–207.
- 54. Laaksonen R, Jokelainen K, Laakso J, Sahi T, Harkonen M, Tikkanen MJ, et al The effect of simvastatin treatment on natural antioxidants in low-density lipoproteins and high-energy phosphates and ubiquinone in skeletal muscle. Am J Cardiol. 1996;77(10):851–4.
- 55. Larsen S, Stride N, Hey-Mogensen M, Hansen CN, Bang LE, Bundgaard H, et al Simvastatin effects on skeletal muscle: relation to decreased mitochondrial function and glucose intolerance. J Am Coll Cardiol. 2013;61(1):44–53.
- 56. Lenaz G., M.L. Genova (2010) Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Antioxidants & redox signaling 12(8): 961-1008.
- 57. Li TH, Lee PC, Lee KC, Hsieh YC, Tsai CY, Yang YY, et al Down-regulation of common NFκB-iNOS pathway by chronic Thalidomide treatment improves Hepatopulmonary Syndrome and Muscle Wasting in rats with Biliary Cirrhosis. Sci Rep. 2016;6(1):39405.
- 58. Li S, Zhang X, Fang Q, Zhou J, Zhang M, Wang H, et al Ginkgo biloba extract improved cognitive and neurological functions of acute ischaemic stroke: a randomised controlled trial. Stroke Vasc Neurol. 2017;2(4):189–97.
- 59. Maiti AK, Saha NC, More SS, Panigrahi AK, Paul G. Neuroprotective Efficacy of Mitochondrial Antioxidant MitoQ in Suppressing Peroxynitrite-Mediated Mitochondrial Dysfunction Inflicted by Lead Toxicity in the Rat Brain. Neurotox Res. 2017;31(3):358–72.
- 60. Mallinson JE, Constantin-Teodosiu D, Sidaway J, Westwood FR, Greenhaff PL. Blunted Akt/FOXO signalling and activation of genes controlling atrophy and fuel use in statin myopathy. J Physiol. 2009;587(1):219–30.
- 61. Maningat P, Breslow JL. Needed: pragmatic clinical trials for statin-intolerant patients. N Engl J Med. 2011;365(24):2250–1.
- 62. Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun. 1994;201(2):748–55.
- 63. Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49(23):2231–7.
- 64. Marklund SL. Superoxide dismutase isoenzymes in tissues and plasma from New Zealand black mice, nude mice and normal BALB/c mice. Mutat Res. 1985;148(1-2):129–34.
- 65. Mas E, Mori TA. Coenzyme Q(10) and statin myalgia: what is the evidence?Curr Atheroscler Rep. 2010;12(6):407–13.
- 66. McKenney JM, Davidson MH, Jacobson TA, Guyton JRNational Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97(88A):89C–94C.
- 67. Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271–8.
- 68. Montogomery HA, Dymock JF. The determination of nitrite in water: colorimetric method of nitric oxide assay. Analyst (Lond). 1961;86:414.
- 69. Mortensen SA, Leth A, Agner E, Rohde M. Dose-related decrease of serum coenzyme Q10 during treatment with HMG-CoA reductase inhibitors. Mol Aspects Med. 1997;18Suppl:S137–44.
- 70. Nakahara K, Kuriyama M, Sonoda Y, Yoshidome H, Nakagawa H, Fujiyama J, et al Myopathy induced by HMG-CoA reductase inhibitors in rabbits: a pathological, electrophysiological, and biochemical study. Toxicol Appl Pharmacol. 1998;152(1):99–106.
- 71. Needham M, Fabian V, Knezevic W, Panegyres P, Zilko P, Mastaglia FL. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscul Disord. 2007;17(2):194–200.
- 72. Oskouei D.S., R. Rikhtegar, M. Hashemilar, H. Sadeghi-Bazargani, M. Sharifi-Bonab, E. Sadeghi-Hokmabadi, S. Zarrintan, E. Sharifipour (2013) The effect of Ginkgo biloba on functional outcome of patients with acute ischemic stroke: a double-blind, placebo-controlled, randomized clinical trial. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association 22(8): e557-563.
- 73. Ozkan H, Ekinci S, Uysal B, Akyildiz F, Turkkan S, Ersen O, et al Evaluation and comparison of the effect of hypothermia and ozone on ischemia-reperfusion injury of skeletal muscle in rats. J Surg Res. 2015;196(2):313–9.
- 74. Päivä H, Thelen KM, Van Coster R, Smet J, De Paepe B, Mattila KM, et al High-dose statins and skeletal muscle metabolism in humans: a randomized, controlled trial. Clin Pharmacol Ther. 2005;78(1):60–8.
- 75. Pierno S, Didonna MP, Cippone V, De Luca A, Pisoni M, Frigeri A, et al Effects of chronic treatment with statins and fenofibrate on rat skeletal muscle: a biochemical, histological and electrophysiological study. Br J Pharmacol. 2006;149(7):909–19.
- 76. Pradelli D, Soranna D, Scotti L, Zambon A, Catapano A, Mancia G, et al Statins and primary liver cancer: a meta-analysis of observational studies. Eur J Cancer Prev. 2013;22(3):229–34.
- 77. Pryor WA, Squadrito GL. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol. 1995;268(5 Pt 1):L699–722.
- 78. Punkt K, Psinia I, Welt K, Barth W, Asmussen G. Effects on skeletal muscle fibres of diabetes and Ginkgo biloba extract treatment. Acta Histochem. 1999;101(1):53–69.
- 79. Punkt K, Zaitsev S, Park JK, Wellner M, Buchwalow IB. Nitric oxide synthase isoforms I, III and protein kinase-Ctheta in skeletal muscle fibres of normal and streptozotocin-induced diabetic rats with and without Ginkgo biloba extract treatment. Histochem J. 2001;33(4):213–9.
- 80. Qu H, Guo M, Chai H, Wang WT, Gao ZY, Shi DZ. Effects of Coenzyme Q10 on Statin-Induced Myopathy: An Updated Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2018a ;7(19):e009835–009835.
- 81. Qu H, Meng YY, Chai H, Liang F, Zhang JY, Gao ZY, et al The effect of statin treatment on circulating coenzyme Q10 concentrations: an updated meta-analysis of randomized controlled trials. Eur J Med Res. 2018b ;23(1):57.
- 82. Saini AS, Taliyan R, Sharma PL. Protective effect and mechanism of Ginkgo biloba extract-EGb 761 on STZ-induced diabetic cardiomyopathy in rats. Pharmacogn Mag. 2014;10(38):172–8.
- 83. Salami JA, Warraich H, Valero-Elizondo J, Spatz ES, Desai NR, Rana JS, et al National Trends in Statin Use and Expenditures in the US Adult Population From 2002 to 2013: Insights From the Medical Expenditure Panel Survey. JAMA Cardiol. 2017;2(1):56–65.
- 84. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117(3):399–412.
- 85. Schirris TJ, Renkema GH, Ritschel T, Voermans NC, Bilos A, van Engelen BG, et al Statin-Induced Myopathy Is Associated with Mitochondrial Complex III Inhibition. Cell Metab. 2015;22(3):399–407.
- 86. Sinzinger H, Wolfram R, Peskar BA. Muscular side effects of statins. J Cardiovasc Pharmacol. 2002;40(2):163–71.
- 87. Sirtori CR. The pharmacology of statins. Pharmacol Res. 2014;88:3–11.
- 88. Sirvent P, Mercier J, Lacampagne A. New insights into mechanisms of statin-associated myotoxicity. Curr Opin Pharmacol. 2008;8(3):333–8.
- 89. Smith JV, Luo Y. Studies on molecular mechanisms of Ginkgo biloba extract. Appl Microbiol Biotechnol. 2004;64(4):465–72.
- 90. Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, et al The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14(3):395–403.
- 91. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et alAmerican College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25Suppl 2):S1–45.
- 92. Suter A, Niemer W, Klopp R. A new ginkgo fresh plant extract increases microcirculation and radical scavenging activity in elderly patients. Adv Ther. 2011;28(12):1078–88.
- 93. Taylor BA, Lorson L, White CM, Thompson PD. A randomized trial of coenzyme Q10 in patients with confirmed statin myopathy. Atherosclerosis. 2015;238(2):329–35.
- 94. Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, et al The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008;45(1):18–31.
- 95. Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289(13):1681–90.
- 96. Tian G., J. Sawashita, H. Kubo, S.Y. Nishio, S. Hashimoto, N. Suzuki, H. Yoshimura, M. Tsuruoka, Y. Wang, Y. Liu, H. Luo, Z. Xu, M. Mori, M. Kitano, K. Hosoe, T. Takeda, S. Usami, K. Higuchi (2014) Ubiquinol-10 supplementation activates mitochondria functions to decelerate senescence in senescence-accelerated mice. Antioxidants & redox signaling 20(16): 2606-2620.
- 97. Tomasetti M, Alleva R, Solenghi MD, Littarru GP. Distribution of antioxidants among blood components and lipoproteins: significance of lipids/CoQ10 ratio as a possible marker of increased risk for atherosclerosis. Biofactors. 1999;9(2-4):231–40.
- 98. Tomaszewski M., K.M. Stepien, J. Tomaszewska, S.J. Czuczwar (2011) Statin-induced myopathies. Pharmacological reports : PR 63(4): 859-866.
- 99. Ucar M, Mjörndal T, Dahlqvist R. HMG-CoA reductase inhibitors and myotoxicity. Drug Saf. 2000;22(6):441–57.
- 100. Vaughan RA, Garcia-Smith R, Bisoffi M, Conn CA, Trujillo KA. Ubiquinol rescues simvastatin-suppression of mitochondrial content, function and metabolism: implications for statin-induced rhabdomyolysis. Eur J Pharmacol. 2013;711(1-3):1–9.
- 101. Vellas B, Coley N, Ousset PJ, Berrut G, Dartigues JF, Dubois B, et alGuidAge Study Group. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): a randomised placebo-controlled trial. Lancet Neurol. 2012;11(10):851–9.
- 102. Venkatesh S, Ramachandran A, Zachariah A, Oommen A. Mitochondrial ATP synthase inhibition and nitric oxide are involved in muscle weakness that occurs in acute exposure of rats to monocrotophos. Toxicol Mech Methods. 2009;19(3):239–45.
- 103. Waclawik AJ, Lindal S, Engel AG. Experimental lovastatin myopathy. J Neuropathol Exp Neurol. 1993;52(5):542–9.
- 104. Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, et al Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7(6):687–92.
- 105. Westwood FR, Bigley A, Randall K, Marsden AM, Scott RC. Statin-induced muscle necrosis in the rat: distribution, development, and fibre selectivity. Toxicol Pathol. 2005;33(2):246–57.
- 106. Wray CJ, Mammen JM, Hershko DD, Hasselgren PO. Sepsis upregulates the gene expression of multiple ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol. 2003;35(5):698–705.
- 107. Zayed AE, Saleh A, Gomaa AM, Abd-Elkareem M, Anwar MM, Hassanein KM, et al Protective Effect of Ginkgo biloba and Magnetized Water on Nephropathy in Induced Type 2 Diabetes in Rat. Oxid Med Cell Longev. 2018;2018:1785614.
- 108. Zheng R, Huang S, Zhu J, Lin W, Xu H, Zheng X. Leucine attenuates muscle atrophy and autophagosome formation by activating PI3K/AKT/mTOR signaling pathway in rotator cuff tears. Cell Tissue Res. 2019;378(1):113–25.
- 109. Zissimopoulos JM, Barthold D, Brinton RD, Joyce G. Sex and Race Differences in the Association Between Statin Use and the Incidence of Alzheimer Disease. JAMA Neurol. 2017;74(2):225–32.
