Introduction
Tissue engineering has emerged in the last decade as an important approach to regenerate an injured organ by combining cells, matrices, biologically active molecules, and physiological stimuli. Cardiac tissue engineering can be used to restore cardiac function after surgery, to replace heart valves, and to improve cardiac function after injury. In addition to controlling the biocompatibility and immunocompatibility of cell-seeded patches, it is important to develop methods to reduce the number of cells used for implantation and to identify scaffolds and pathways for cell differentiation [Di Felice, 2015; Di Felice et al., 2015a].
Recently, an important aspect of an engineered tissue has emerged: it can be used as an organoid, a small defined environment that is similar to the in vivo organ, has the same properties, is interactive, and is an important alternative to using animals in research laboratories [Voges et al., 2017; Varzideh et al., 2019].
In recent months, organoids consisting of 50% human pluripotent stem cell-derived cardiomyocytes and 50% non-myocytes (with a 4:2:1 ratio of human ventricular cardiac fibroblasts, human umbilical vein endothelial cells, and human adipose-derived mesenchymal stromal cells), have been proposed as useful cardiac models to study toxicology, cell-cell interactions, genetic alterations, and novel therapies for cardiovascular disease [Filippo Buono et al., 2020; Richards et al., 2020]. However, all of these cells are not true and endogenous cardiac tissue progenitor cells. One cell type that could be used to generate cardiac organoids may be the tyrosine protein kinase kit (c-Kit)-positive cardiac progenitor cell (CPC), a type of progenitor cell previously isolated from cardiac tissue. These cells were first identified in the adult rat heart by Beltrami et al. [2003] and used as cell suspension in the first clinical trial of cardiac tissue regeneration [Chimenti et al., 2011]. In 2009, our research group showed that c-Kit-positive cardiac cells isolated from an adult rat heart and cultured in a type I collagen gel were able to synthesize their own collagen fibers, organize vessels, form a capsule, differentiate into endothelial cells and cardiac fibroblasts, and differentiate to cardiomyocytes [Di Felice et al., 2009]. In 2015, our research group showed that porous silk fibroin scaffolds and electrospun meshes can be used in vitro to induce differentiation of adult rat CPCs, and that partially oriented scaffolds were more efficient than the other materials tested. We also observed that partially oriented scaffolds and collagen alone were able to activate Z-body formation to initiate myofibril assembly [Di Felice et al., 2015b].
Other 3-dimensional (3D) structures have been used in the past to stimulate CPC differentiation. In an interesting study, using an experimental myocardial infarction model and injecting CPCs, it was shown that the use of functionalized self-assembling peptide hydrogels with a peptide that mimics the Notch 1 ligand, Jagged1 (RJ), improved acute retention and cardiac function [Boopathy et al., 2014].
In the present study, we propose the use of c-Kit-positive CPCs as the main population of a cardiac cellularized silk fibroin scaffold. We isolated CPCs from adult rat hearts, confirmed the expression of 3 markers already used by our research group to identify CPC cells (c-Kit, Sca-1, and MDR1 [Di Felice et al., 2015b]) and titin as a cardiac marker. Then, we tested their ability to differentiate in 3D cultures with collagen I in vitro and implanted this mass of cells in the subcutaneous region of immunosuppressed animals (nude mice, severe combined immunodeficient [SCID] mice, and nude rats). In parallel, we also investigated the host response to silk fibroin scaffolds previously tested in vitro [Di Felice et al., 2015b] and their potential use in creating an in vivo model of a mass of cardiac cells. The 3 immunosuppressed animal models are most commonly used to test the immune response to allograft implantation and have been used to simulate immunosuppressive therapies. Due to a genetic mutation, the nude mouse and rat models lack a normal immune system and functional thymus gland. They have a suppressed immune system due to a reduced number of T cells. Nude mice are ideal for tumor and tissue studies [Pantelouris, 1968]. SCID mice have a genetic immunodeficiency affecting their B and T cells. Due to the lack of mature B and T lymphocytes, these mouse models are ideal for xenografting cells and tissues [Awwad et al., 1999].
Our work showed that although CPCs were able to express markers and structural proteins typical of cardiac tissue in vitro, in vivo they activated a CD3+ subset of T cells and induced their expansion (CD3+ T cells are also present in nude animals despite their compromised immune system). On the other hand, scaffolds alone also induced a host response based on activation of giant cells and release of interleukins, like interleukin-4 (IL-4) and interleukin-13 (IL-13). Among the tested scaffolds, the electrospun fibroin meshes (F-scaffolds) induced a lower response compared to the porous silk scaffolds that we had previously used in our experiments to promote cardiac CPC differentiation in vitro [Di Felice et al., 2015b].
Ultimately, the CPCs-F scaffold combination induced CD3+ lymphocyte activation. This unexpected response suggests that it is not possible to create an in vivo model of a mass of cardiac cells using CPCs or silk fibroin scaffolds.
Materials and Methods
Material Preparation
Fibroin-Water Solution
Bombyx mori cocoons (kindly provided by Socio Lario, Cassina Rizzardi, Como, Italy) were boiled for 1.5 h in an aqueous solution containing 1.1 g/L Na2CO3 (10 g of silk/L of solution) and then for another 1.5 h in a water bath containing 0.4 g/L Na2CO3. The cocoons were rinsed thoroughly with warm distilled water to extract the residual glue-like sericin proteins and then air-dried. The experiment optimization of the degumming process and its impact on the silk fibroin properties and the amount of sericin extracted or left in the fibroin solution has been recently discussed and published by the research group of Prof. Antonella Motta [Bucciarelli et al., 2021].
The fibroin-water solution was prepared by dissolving fibroin in an aqueous solution containing 9.3 M LiBr (10% w/v, Fluka Chemical, St. Louis, MO, USA) at 65°C for 2 h, followed by dialysis (3 days) against distilled water with a 3,500 Da MW-CO membrane (Slyde-A-Lyzer, Pierce, Rockford, IL, USA) to eliminate the salt. The resulting solution was concentrated by dialyzing against a polyethylene glycol (PEG)-water solution (25% w/v) for 5 h and filtered through a 160–250-μm filter (Duran Group, Wertheim/Main, Germany). The final concentration of silk fibroin in the aqueous solution was approximately 15% w/v, as determined with a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA).
Freeze-Dried Sponges
3D porous fibroin scaffolds were prepared using a freeze-drying technique with varied parameters to obtain different pore sizes and orientations. The aqueous fibroin solution was diluted to 5%, poured into polystyrene Petri dishes, frozen at −80°C, and freeze-dried (sample RP). Sponges with an oriented structure were also obtained using the 5% fibroin solution; however, the Petri dish was positioned vertically to induce a temperature gradient (sample O). All obtained samples were stabilized by treating with a methanol/water solution (80/20 v/v) for 10 min, rinsed several times with distilled water to eliminate the solvent, and freeze-dried again.
Electrospun Nets
The aqueous fibroin solution was frozen at −20°C, lyophilized at −50°C, and stored in a desiccator until use. A 15% w/v solution was prepared dissolving the freeze-dried fibroin in formic acid (98–99%). A 3-mL syringe with a metal needle was filled with the solution and mounted in a programmable syringe pump (Model 11 Plus, Harvard Apparatus, Holliston, MA, USA) set at a constant flow rate of 0.002 mL/min. A positive voltage (15 kV or 20 kV) was applied to the solution by the needle, while a rotational mandrel, which was covered with aluminum, was connected to the ground electrode. The deposition was performed at room temperature (RT) at constant rotational speed (3,250 rpm) for 8 h. The resulting non-woven nets were stabilized in methanol/water (80/20 v/v) for 10 min and washed in distilled water for 2 days to remove the residual solvent (sample F).
Sample Morphological Evaluation by Field Emission Scanning Electron Microscopy
All samples were examined in a dry state with a field emission scanning electron microscope (FE-SEM; Supra 40, Zeiss, Oberkochen, Germany) after coating with gold in a reduced argon atmosphere.
Animals
This study was conducted in strict accordance with the recommendations of the Italian Ministry of Health’s Guide to the Care and Use of Laboratory Animals. Every effort was made to minimize suffering. The experiments were performed before the entry into force of Decree Law no. 26/2014, in application of European Directive 2010/63/Eu.
Animal models used: Adult Sprague-Dawley rats (up to 8 months old, n = 10), athymic nude-Foxn1nu mice (nude mice, n = 39), CB-17/IcrHan®Hsd-Prkdcscid mice (SCID, n = 24), and athymic Hsd:RH-Foxn1rnu rats (nude rats, n = 24). All animals were purchased from Harlan Laboratories Inc. (Indianapolis, IN, USA) and were housed on a 12:12 h light:dark cycle, with water and food available ad libitum. Animals were euthanized 21 days after sample injection by cervical dislocation after sedation.
Experimental Groups
One week before inoculation, mice from the same animal model were randomly assigned to one of the experimental conditions: collagen I from rat tail (Coll), CPCs seeded in rat collagen I for 21 days (CPCs + coll), CPCs seeded in rat collagen I for 45 days (CPCs + coll-45), BD OPLA scaffolds alone (BD OPLA), RP-silk scaffold (RP), O-silk scaffold (O), fibroin nets (F), CPCs seeded on the surface of F-fibroin nets (CPC-F-scaffold). Each group consisted of 3 animals; there were 72 animals in total.
For the tumorigenicity test in vivo, 1 week before inoculation, athymic nude-Foxn1nu (nude) mice were randomly assigned to one of the following experimental conditions: Hep-2 cells (Hep-2), VERO cells (VERO), CPCs (CPCs). Each group was composed of 5 mice, with a total of 15 mice.
All groups are summarized in Table 1.
CPC Isolation and Culture
Adult Sprague-Dawley rats (up to 8 months old, n = 10) were anesthetized with vaporized isoflurane, 0.20 mg/kg intramuscular (IM) Zoletin 20, 0.25 mg/kg IM medetomidine, and 0.0025 mg/kg subcutaneous (SC) atropine, following a “non-recovery” procedure. The hearts were excised and were placed directly into a Falcon tube with 50 mL of Hank’s balanced salt solution (HBSS; Invitrogen, Life Technologies Corp., Carlsbad, CA, USA) with 50 U/mL collagenase II (Life Technologies Corp.) and 3 mmol/L CaCl2 to prevent blood coagulation and to allow for the rapid penetration of the collagenase solution directly into the coronary vessels. The atria were separated from the ventricles in a laminar flow hood. The ventricles were cut into 4 pieces, and these pieces (1 heart/tube) were placed into 20 mL fresh HBSS with 50 U/mL collagenase II and 3 mmol/L CaCl2. The solution was collected following a 10-min incubation period at 37°C in a rotating dry incubator, and pieces were left on the bottom of the 50-mL Falcon tube. Isolated cells were removed from the collagenase solution by centrifugation and put into fresh M-199 medium (BD Biosciences, Franklin Lakes, NJ, USA) supplemented with 20% fetal bovine serum (FBS; Biolife Italiana S.r.l., Milano, Italy), 3 mg/mL fungizone, 300 mg/mL streptomycin, and 300 U/mL penicillin (antibiotic-antimycotic 100×; cat. No. 15240-062, Invitrogen, Life Technologies Corp., Grand Island, NY, USA). Another 20 mL of HBSS with 50 U/mL collagenase II and 3 mmol/L CaCl2 was added to the 50-mL Falcon tube with the 4 pieces, which was incubated for another 10 min at 37°C. We then performed repeated centrifugations as previously described [Di Felice et al., 2009, 2015b]. Only the second cell fraction (enriched in c-Kit+/Sca-1+/MDR-1+ cells) was plated into 75-cm2 poly-D-lysine-coated BD flasks with fresh M-199 medium supplemented with 20% FBS. The first fraction was discarded. After allowing the cells to grow, cells were mildly harvested with a solution of 0.05% porcine trypsin and 0.02% EDTA (trypsin-EDTA solution 10×; T4174, Sigma-Aldrich, St Louis, MO, USA) in phosphate buffered saline (PBS) or HBSS for no more than 2 min; cells that did not detach from the plasticware were cardiac fibroblasts and were discarded. To maintain an undifferentiated state, cells were cultured at 600,000 cells/25 cm2 in M-199 supplemented with 20% FBS and harvested 2 days per week with a trypsin-EDTA solution (Sigma-Aldrich).
Cellularized Silk Fibroin Scaffold and Cardiac Cell Mass Formation Technique
Cells were cultured in a 50-μm-thick collagen I gel (rat tail collagen, 3 mg/mL, diluted in M199 medium; cat. No. 354236, BD Biosciences, Sparks, MD, USA) with F-scaffold (CPCs-F scaffold) or without (CPCs + coll) in the 0.4-µm pore size PET track-etched membrane 24 well format Cell Culture Insert (2 × 105 cells/insert; cat. No. 353095, Corning). Both CPCs + coll and CPCs-F scaffold were placed on a 0.4-µm pore size insert, covered with M-199/20% FBS medium and incubated for 21 days in a cell culture incubator with medium changed twice a week.
Flow Cytometry
Cells were detached from flasks, counted, and placed in FACS tubes (200,000 cells/sample). Cells were washed once with PBS and fixed with 4% paraformaldehyde in PBS. After washing, cells were blocked with incubation buffer (IB, PBS:M-199 supplemented with 10% FBS, 1:9 v/v), placed in the primary antibody solution in IB at RT (anti-c-Kit; KAP-TK005, Stressgen Bioreagents, Ann Arbor, MI, USA; diluted 1:200) for 45 min, rinsed twice in PBS, and then incubated in the secondary antibody solution (diluted 1:200 in IB for 45 min at RT). After rinsing with PBS, cells were analyzed using a FACSVerse Flow Cytometer (BD Biosciences).
Cellularized Silk Fibroin Scaffolds and Cardiac Cell Mass Microinjection
Each type of scaffold or mass of cardiac cells was injected with a 0.5-mm trocar in the dorsal SC region of the nude mice (n = 24), SCID mice (n = 24), and nude rats (n = 24), with 2 cellularized scaffolds or masses per animal, one for each side (see Table 1). The animals were kept in captivity for 21 or 45 days. After that period, they were sacrificed by displacement of the first cervical vertebra, and the bumps were excised, fixed in a solution of acetone:methanol:water (2:2:1 v/v) and dipped in paraffin. Finally, the embedded bumps were cut into 5-µm sections and stained with hematoxylin and eosin (H&E) or Masson’s trichrome staining.
Tumorigenicity Assays
In vitro
A tumorigenic control of CPCs for each donor rat (n = 10) was assessed in vitro and in vivo. CPCs, VERO cells (negative control), and Hep-2 cells (positive control) were cultured in vitro in different 75-cm2 flasks (BD Biosciences) as described in Furesz et al. [1989]. Later, each cell culture (1 × 105 cells) was inoculated in 6-well plates with a solid medium consisting of MEM and Agar Noble (Sigma-Aldrich) and incubated at 37°C. Cells were observed under an inverted microscope for 3 weeks. The number of colony forming units was counted and compared with the control (Hep-2) (data not shown).
In vivo
CPCs (1 × 106 per sample; n = 10), VERO cells (n = 10), and Hep-2 cells (n = 10) were inoculated in vivo by intracutaneous injection (1 mL insulin syringe). Each cell type was injected into the SC region of both sides of 5-week-old athymic nude-Foxn1nu mice (nude mice, n = 5 per group) in a volume of 1 mL culture medium (2 inoculations of the same cell samples per animal). Animals were observed for 21 days or until the formation of new nodules was detected in the injection area of Hep-2-injected mice. The mice were sacrificed after 21 days and the presence of neoplasms was evaluated. The experiment was considered valid if at least 4 of 5 mice inoculated with the positive control (Hep-2) cells produced neoplasms. Animal groups are summarized in Table 1.
Histological Analysis of Scaffolds or Constructs in vivo
H&E Staining
Paraffin sections were stained with H&E and used to assess the presence of scaffolds in the biopsies, scaffold integration, and appearance of the constructs. Biopsies or scaffolds containing cells were fixed in an acetone:methanol:water solution (2:2:1) for 12 h, washed in tap water, and dehydrated with ethanol at 30, 50, 70, 95, and 100% v/v. After dehydration, the tissue pieces were placed in xylol for 1 h and embedded in paraffin. The paraffin-embedded tissue samples were cut into 5-μm sections. The sections were deparaffinized with xylene for 10 min and hydrated with a decreasing ethanol gradient. They were stained with hematoxylin (Merck KGaA, Darmstadt, Germany) for 4 min, blocked in tap water for 15 min, treated with eosin (Merck KGaA) for 1 min, and rinsed in water. Sections were dehydrated and mounted with Canada balsam (Panreac Química S.L.U., Barcelona, Spain). Images were taken with a Leica DM5000 microscope (Leica Microsystems, Wetzlar, Germany).
Analysis of Fibrosis and Giant Cells Using Masson’s Trichrome Staining
Paraffin-embedded sections were stained with Masson’s trichrome staining kit (Bio-Optica, Milan, Italy) according to the manufacturer’s instructions to assess the extent of fibrosis around the implants and the presence of giant cells. The collagen, nuclei, and cell cytoplasm appeared blue, black, and red, respectively. Images were taken with a Leica DM5000 microscope (Leica Microsystems).
Immunohistochemistry and Immunocytochemistry
Immunohistochemistry and immunocytochemistry were performed with a biotin-free method using a specific probe to detect rabbit antibodies, followed by an HRP polymer that binds to both the probe and rabbit antibodies (MACH 1 Kit; Biocare Medical, Concord, CA, USA). For immunohistochemistry after deparaffinization and rehydration, tissue sections were incubated with a 3% hydrogen peroxide solution (Sigma-Aldrich) for 5 min. After incubation with the protein blocking agent Background Sniper (Biocare Medical) for 15 min, the tissue sections were incubated with an anti-CD3 antibody (rabbit polyclonal, AO452, diluted 1:100; DAKO Denmarks A/S, Glostrup, Denmark) for 1 h.
For immunocytochemistry, cells were fixed with paraformaldehyde and methanol on ice, washed in PBS and incubated with a 3% hydrogen peroxide solution (Sigma-Aldrich) for 5 min. After incubation with the protein blocking agent Background Sniper for 15 min, the fixed cells were incubated with an anti-titin antibody (SC-8724 Titin (C-20), diluted 1:100; Santa Cruz Biotechnology Inc., Dallas, USA) for 1 h.
The linked primary antibody was detected using the HRP-streptavidin peroxidase system according to the manufacturer’s instructions. Biocare’s Betazoid DAB was used as the chromogen. The sections were then washed in tap water and counterstained with hematoxylin for 1 min. VectaMount (Vector Laboratories Inc., Burlingame, CA, USA) was used as mounting medium.
Confocal Scanning Analysis
Cells cultured on poly-D-lysine (Sigma-Aldrich)-coated chamber slides were first fixed with 4% paraformaldehyde for 30 min and then with ice-cold methanol for 30 min. Antigen retrieval was performed with 10 mM citrate buffer (pH 6.0) containing 0.05% Tween 20 for 10 min. After 30 min incubation with 5% bovine serum albumin (Sigma-Aldrich), cells were incubated overnight at 4°C with primary antibodies (dilution 1:100; anti-CD11b [integrin α M], clone M-19, sc-6614; anti-IL-4, clone M-19, sc-1261; anti-IL-13, clone M-17, sc-1776; all Santa Cruz Biotechnology). The linked primary antibodies were detected with a FITC-conjugated secondary anti-goat antibody (Sigma-Aldrich) at a dilution of 1:50. Nuclei were stained with 1 μM TOTO-3 stain in PBS (T3604; Life Technologies) or stained with Hoechst for 5 min. Images were captured using a ZEISS LSM710 Exciter Laser Scanning Confocal Microscope.
Fluorescence in situ Hybridization
Paraffin-embedded sections were kept at 37°C overnight and then at 60°C for 10 min. The sections were deparaffinized in xylol at 60°C for 10 min, hydrated through a graded ethanol series, and immersed in citrate buffer containing 1% Tween 20 for 15 min for heat-induced target retrieval. Slides were cooled for 5 min at RT and placed in PBS containing 0.1% Tween (Sigma-Aldrich). To catalyze RNA degradation, slides were treated with 10 μg/μL RNase at 37°C for 1 h. Fluorescence in situ hybridization (FISH) was performed on the sections using a custom-made N-terminal Cy3-labeled peptide nucleic acid (PNA) probe that recognizes mouse centromeres but not rat centromeres (N-ATTCGTTGGAAACGGGA-C; Exiqon, Vedbaek, Denmark). The sequence was adopted from Vander Griend et al. [2009]. The PNA probe was used at a concentration of 6.4 pmol in hybridization buffer (50% formamide, 2× SSC, pH 7), and the sections were denatured at 75°C for 5 min, followed by hybridization at 60°C for 1 h. Sections were washed 3 times with 0.1× SSC at the hybridization temperature for 5 min and twice with 4× SSC containing 0.05% Tween 20 at 37°C, followed by washing in PBS at RT for 5 min. Nuclei were counterstained with a 1:1,000 dilution of TOTO-3 (Life Technologies) in PBS at RT. Sections were mounted with Vectashield (DAKO) for confocal analysis.
Statistical Analysis
All data were collected and analyzed for statistical significance. Mean ± standard deviation of the mean for each variable outcome were tabulated for each group and for each time-point of the experiments. Comparisons among groups and different time points were performed using one-way ANOVA test. All statistical analyses were performed using GraphPad PrismTM 4.0 software (GraphPad Software Inc., San Diego, CA, USA). The threshold of statistical significance was set at p < 0.05.
Results
Scaffold Characterization
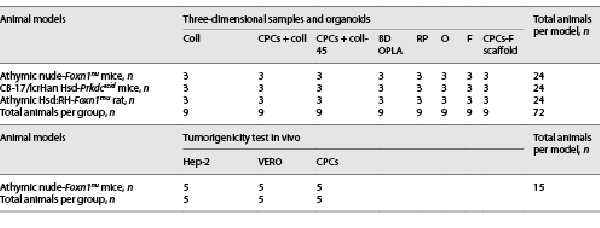
The morphological features of the silk fibroin constructs were observed using FE-SEM, as shown in Figure 1.
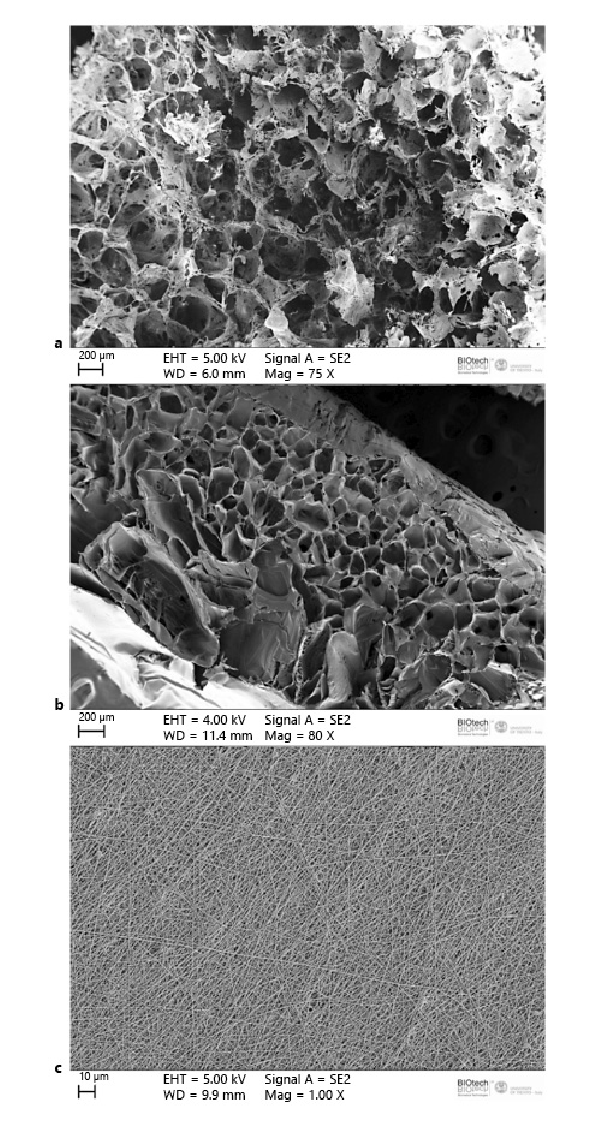
Fig. 1
FE-SEM micrographs of silk fibroin constructs. a Freeze-dried sponges with homogeneous pore distribution, sample RP. b Sponges with bimodal pore distribution, sample O. c Electrospun net, sample F.
The 3D porous structure obtained from the freeze-dried fibroin-water solution exhibited an interconnected homogeneous pore distribution (Fig. 1a). The sponges, on the other hand, exhibited a very distinct bimodal pore size distribution, which was achieved by inducing a temperature gradient (Fig. 1b). The fibers of the electrospun net were randomly distributed with a homogeneous fiber diameter size (Fig. 1c).
Cell Isolation, Characterization, and Cardiac Cell Mass Formation
We isolated immature cells from the heart of mature female donor rats (n = 10) using a differential adhesion method as previously described [Di Felice et al., 2009, 2015b]. Using poly-D-lysine and mild digestion with trypsin-EDTA solution, we obtained a sufficient amount of highly proliferative cells 2 weeks after tissue digestion. The isolated cells were then characterized by FACS analysis and were highly positive for c-Kit compared to the isotype control, as previously described [Di Felice et al., 2015b] (Fig. 2a). Even after multiple passages (n = 20 ± 5) in culture, cryopreservation, and thawing, all the cells were able to express the structural cardiac protein of the sarcomere, titin (Fig. 2b, c), and the stem cell markers c-Kit, Sca-1, and MDR-1 (Fig. 2d–f). All cells isolated from the donor rats (n = 10) expressed the same stem cell markers and structural proteins.
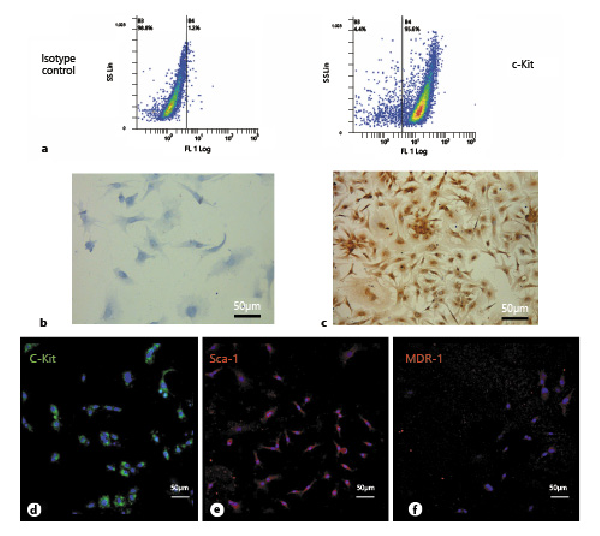
Fig. 2
a FACS analysis of isolated cardiac progenitor cells (CPCs) showing the percentage of cells expressing c-Kit 10 days after tissue digestion (n = 10). Non-immune isotype antibody was used as negative control. b Negative control of c. c Titin expression in CPCs in 2D cultures. d–f c-Kit, Sca-1, and MDR-1 expression analyzed by confocal microscopy. These images are representative of all experiments conducted on rats.
Two weeks after tissue digestion, cells were tested for tumorigenicity in vitro and in vivo. Seven to 10 days after the start of the in vitro tumorigenicity assay, the tumorigenic cells (Hep-2) began to proliferate and form multicellular aggregates, whereas the negative cells (VERO) and CPCs showed atrophy. One CPC sample for each donor rat was tested. Therefore, the tumorigenicity assay performed in vitro on the isolated CPCs was negative (data not shown).
The tumorigenicity assay in vivo was positive for the Hep-2 cells (positive control) and negative for the VERO (negative control) and CPCs. Solid tumors appeared in all nude mice injected with Hep-2 cells, whereas there was no evidence of tumors in the various organs analyzed from nude mice injected with VERO and CPCs (online suppl. Fig. 1; for all online suppl. material, see http://www.karger.com/doi/10.1159/000522568).
Having established that CPCs were not tumorigenic, we prepared and tested CPC-derived masses of cardiac cells for injection into immunosuppressed animals. c-Kit-positive CPCs were cultured in vitro in a collagen I gel for 21 days, as shown in Figure 3a, b. The CPCs formed a compact mass, well-organized, with newly developed small vessels (Fig. 3c, d; as previously described by Di Felice et al. [2009]) and were able to increase the expression of structural cardiac markers such as cardiac troponin T2 (Fig. 3e–g).
Fig. 3
a Diagram showing the cardiac progenitor cells (CPCs) in 3D culture. b CPCs cultured on the insert. c, d Paraffin-embedded sections of a CPC collagen organoid in vitro stained with H&E. e PCR analysis showing the GADPH expression in CPC collagen organoids. f PCR analysis of cardiac troponin T2 expression in CPC collagen organoid-like masses. g Cardiac troponin T2 expression normalized with GADPH. CN, negative control; 1, CPCs grown in flasks; 2, CPCs grown in collagen for 12 days; 3, CPCs grown in collagen for 16 days; 4, CPCs grown in collagen for 21 days. These images are representative of all experiments conducted on rats (n = 10).
Testing CPC-Collagen Masses of Cardiac Cells in vivo
To understand whether cardiac cellularized silk fibroin scaffolds composed of CPCs embedded in collagen I and grown in silk fibroin scaffolds, as previously described [Di Felice et al., 2015b], can be used to create a vascularized and functional mass of cells in vivo, we first tested the viability and differentiation of CPC-collagen masses in vivo, then the host response to biomaterials, and then the host response to the CPC-scaffold-collagen combination in immunosuppressed animals.
A collagen gel was used to implant the cells and minimize cell dispersion. A collagen I gel was chosen as the embedding medium because we have previously shown that it is a good substrate to induce cardiac differentiation of rat CPCs in vitro within 21 days [Di Felice et al., 2009]. A group of mice transplanted only with a collagen I gel without cells was included as a control. The gel alone and surgical manipulation did not induce an inflammatory response or attract immune cells to the engraftment site.
We injected CPC-collagen masses (CPCs + coll) into the SC region of nude mice (Fig. 4a–c). Samples were evaluated at 2 time points, 21 and 45 days, and the results were the same. Twenty-one days was the incubation time required for CPCs to differentiate to the cardiac phenotype in vitro [Di Felice et al., 2009, 2015b] and the time required to rule out the appearance of solid tumors. Forty-five days was also examined to allow complete differentiation of CPCs.
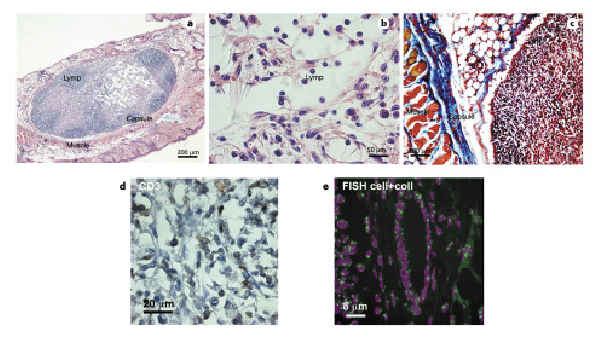
Fig. 4
Morphological analysis of in vivo reaction to cardiac progenitor cells (CPCs) and collagen I organoid-like masses, CD3 immunostaining, and FISH analysis. CPCs and collagen I organoid-like masses (2 mm2) were implanted subcutaneously into nude mice to evaluate immune response. After 45 days, biopsies were harvested, embedded in paraffin, and sections stained for further analysis. a–c H&E staining (a, b) and Masson’s trichrome staining (c) of CPC and collagen I organoid-like masses. Lymp, lymphocytes; Capsule, fibrous capsule of the foreign reaction; Muscle, muscle of the skin. d Infiltrate of CD3+ lymphocytes in CPC collagen organoid-like masses implanted in the subcutaneous region of nude mice. e FISH showing only mouse-positive cells in biopsy of CPC collagen organoid-like masses implanted in nude mice.
The masses grew and the cells proliferated, but at the same time induced a foreign body reaction, with nodule formation and an infiltrate of lymphocytes, as shown by H&E staining in Figure 4a, b.
Collagen remnants were still visible in the center of the encapsulated area (Fig. 4a, b, clear zone). The massive presence of lymphocytes was highlighted by Masson’s trichrome staining (Fig. 4c).
Furthermore, immunohistochemistry for CD3+ showed the presence of positive lymphocytes (Fig. 4d). Using FISH for mouse centromeres, we demonstrated that the implanted rat CPCs were undetectable in SCID mice after 21 and 45 days (Fig. 4e). Anatomopathological evaluation of all organs showed that they did not induce tumor formation (online suppl. Fig. 1).
These experiments suggest that the cells of rats were destroyed by the unfavorable immune response. As shown in Figure 4e, the vessels present in the biopsies were also of murine origin. The same infiltrate was present in nude rats when rat CPCs were implanted (online suppl. Fig. 2a).
Host Reaction to Biomaterials
Before investigating the fate of CPC silk fibroin masses of cells in vivo, we examined the host response to the biomaterials alone in nude mice, SCID mice, and nude rats. Skin biopsies were collected 45 days post-injection, fixed and embedded in paraffin for further analysis. The implanted BD (as a control), silk fibroin sponges, and electrospun nets were readily identifiable in the H&E-stained paraffin-embedded sections because of the size of the scaffolds, their morphology, and the intense staining of the tendons forming them (Fig. 5).
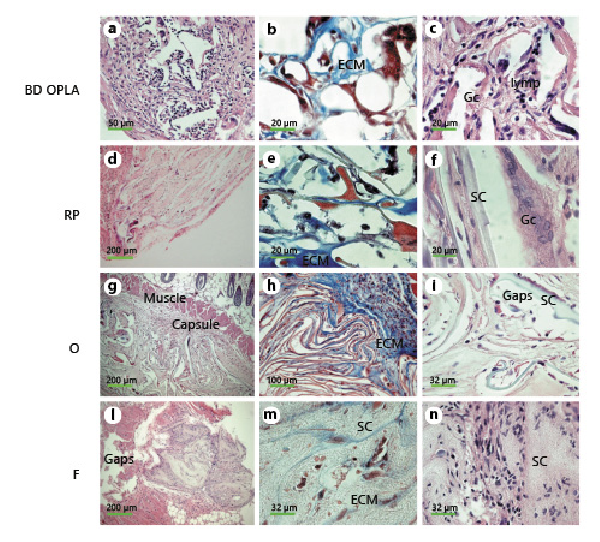
Fig. 5
Morphological analysis of in vivo reaction to biomaterials. Small pieces (2 mm2) of biomaterials alone were implanted subcutaneously into nude mice to evaluate immune response. After 45 days, biopsies were harvested, embedded in paraffin, and sections stained for further analysis. a, c, d, f, g, i, l, n H&E staining of BD (a, c), RP (d, f), O (g, i), F (l, n). b, e, h, m Masson’s trichrome staining of BD (b), RP (e), O (h), F (m). SC, scaffold; ECM, extracellular matrix; lymp, lymphocytes; Gc, giant cells; Capsule, fibrous capsule of the foreign reaction; Gaps, holes left by the implanted scaffold; Muscle, muscle of the skin.
Implanted BD, silk fibroin scaffolds, and meshes were examined in vivo after 45 days of incubation, and they did not appear to have undergone significant biodegradation (Fig. 5a, d, g, l). Fragments of BD, RP, and O scaffolds were encapsulated (Fig. 5a, d, g). The voids encompassed by the cords of the implanted scaffolds were filled with dermal fibroblasts, and the cords were covered with macrophages and giant cells (Fig. 5b, c, f, g, i). An amorphous extracellular matrix, cells of different types, thin bundles of collagen fibrils filled the interstitial spaces (Fig. 5b, e, h). No clusters or infiltrates of lymphocytes and/or plasma cells were detected. Giant cells were particularly evident in sections stained with both Masson’s trichrome and H&E (Fig. 5b, c, f, i). The appearance of F (Fig. 5l–n) was different from the porous scaffolds (Fig. 5d–i) and so was the induced host response. At 45 days post-injection, there was hardly any capsule left (Fig. 5l), and no macrophages were seen (Fig. 5m, n). Fibroblasts populated the surface of the F net, and no lymphocytes were present (Fig. 5m, n).
To confirm the presence of giant cells and the induction of a foreign body reaction, we assessed the expression levels of CD11b, IL-4, and IL-13 by immunofluorescence and confocal microscopy analysis (Fig. 6). CD11b, a surface marker of giant cells [Rodriguez et al., 2009], was expressed on the surface of macrophages attached to the tendons of the control scaffold BD, silk fibroin scaffolds RP and O.
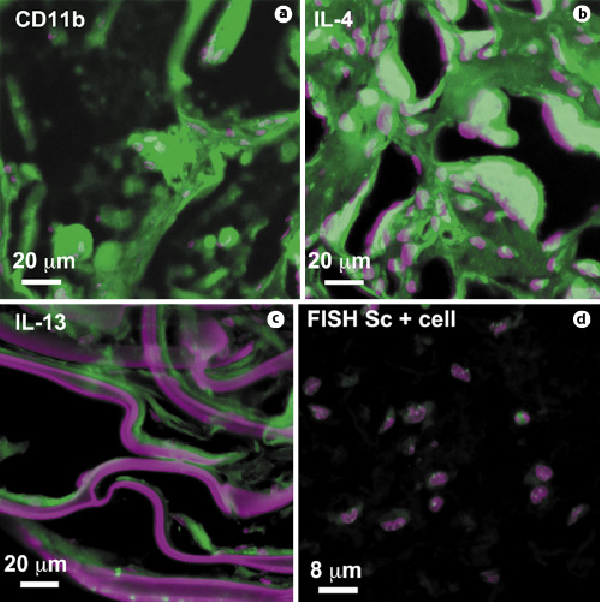
Fig. 6
Foreign body reaction to biomaterials. BD, RP-, O-, and F-scaffolds induced an IL-4/IL-13-mediated foreign body reaction with CD11b+ giant cells similar to that shown in a, b, and c, respectively. Nuclei were stained with Toto-3 (purple), and scaffolds also reacted with Toto-3, which appears purple. d FISH analysis showing only mouse-positive cells in biopsy of CPC-collagen cellularized silk F-scaffold. Only samples from 21 days are shown.
IL-4 and IL-13, two interleukins expressed during foreign body reaction that induce macrophage fusion on the surface of scaffolds [Higgins et al., 2009; Rodriguez et al., 2009], were detected in BD, RP, and O samples (Fig. 6b, c). In samples of skin biopsies with silk fibroin F nets, CD11b, IL-4, and IL-13 were barely expressed.
Fate of CPC-F Scaffolds
Because the F fibers were the least reactive, we implanted them along with the CPC and collagen (Sc + cell) in the subcutaneous area of nude mice. However, even though the F-scaffolds alone did not induce a foreign body reaction with giant cells, the CPCs were probably destroyed by the lymphocyte infiltrate, as shown by the FISH analysis (Fig. 6d).
Discussion
In the present study, we investigated the possibility of using CPCs, collagen, fibroin-based substrates, two 3D porous matrices, and an electrospun net to create a vascularized and functional in vivo model of a mass of cardiac cells. We first tested the viability and differentiation of CPC-collagen masses of cells in vivo, second the host response to biomaterials, and then the host response to the CPC scaffold-collagen combination in immunocompromised animals.
Scaffolds with 3 different geometries were prepared using a fibroin-water solution, including 2 sponges with different pore sizes and pore distributions and an electrospun net with randomly distributed fibers. Characterization of the morphological properties of the fibroin-based constructs showed that the pore size and pore distribution of the sponges were tuned according to the different protocols used to fabricate the scaffolds, which included freeze-drying and a temperature gradient in the quenching step. The results of the morphological analysis showed a homogeneous pore distribution and bipolar pore size with a well-oriented lamellar structure for the sponges. The electrospun net was found to contain randomly distributed fibers with a constant diameter. The pore size ranges and water absorption capacity of the scaffolds were previously measured and published [Font Tellado et al., 2017; Maniglio et al., 2018; Bucciarelli et al., 2019].
All porous scaffolds induced a foreign body reaction with giant cells and capsule formation. The electrospun net (F-scaffold) did not induce an evident foreign body reaction, but a small capsule was still visible. The foreign body reaction was confirmed by the presence of CD11b+ giant cells and the expression of the inflammatory interleukins, IL-4 and IL-13.
Silk fibroin can be isolated in sufficient quantities from the cocoons of the silkworm B. mori and is considered an adjustable and versatile commercially available biomaterial. The biocompatibility of silk fibroin can be improved by separating the immunogenic protein family, sericins, as we did in the present study, so that this biomaterial elicited only a moderate inflammatory response during subsequent implantation in rodents [Uebersax et al., 2013; Bucciarelli et al., 2021]. The biocompatibility of scaffolds has been shown to depend on a number of different parameters, including microstructure and architecture, both of which influence the inflammatory response. In addition, implantation of hybrid constructs, i.e., scaffolds pre-seeded with cells, can trigger an adaptive immune response towards the biological component and thus influence the host response to the implanted device [Franz et al., 2011].
The CPCs that we were able to isolate as primary cultures from adult rat hearts after a long collagenase digestion [Di Felice et al., 2009] did not induce tumor masses in the several organs analyzed, nor did they induce colony-forming units in vitro. We verified that the cells expressed the usual stem cell markers (c-Kit, Sca-1, and MDR-1) and some structural proteins typical of the heart in both 2D and 3D cultures (titin and cardiac troponin T2), as studied by Bernstein and Srivastava [2012]. In fact, we had already shown in a previous work that c-Kit-positive CPCs isolated by repeated treatments with collagenase and cultured in 3D under different conditions can differentiate and express many cardiac proteins in vivo [Di Felice et al., 2009, 2015b]. However, even though several research groups have isolated similar cardiac progenitor cells, no one has yet investigated their applicability as the main component of an in vivo model of a mass of cardiac cells. Because their immunogenicity has not been studied in vivo, we also examined the immune response induced by injection of the CPC-collagen masses, the host response to the CPC-scaffold-collagen combination in immunosuppressed animals.
Unfortunately, injection of the CPC-collagen masses induced a cell-mediated immune response with the formation of a capsule. The implanted cells were completely destroyed by the immune reaction.
Because the F-fibers were the least reactive scaffolds studied, we implanted them together with the CPCs and collagen (Sc + cell) into the subcutaneous area of nude mice, but even though the F-scaffolds alone did not induce a foreign body reaction with giant cells, the CPCs were destroyed by the lymphocyte infiltrate.
A foreign body reaction usually occurs when biomaterials alone are implanted for therapeutic clinical applications in tissue engineering. This reaction is the starting point for tissue regeneration in orthopedics because the foreign body reaction stimulates the regeneration of germinal/progenitor cells of the host tissue while degrading the scaffolds in a reasonable time frame [Anderson et al., 2008]. However, this phenomenon is not useful for stem cell therapy or in vivo modeling of vascular and functional masses of cardiac cells because, as shown here, the foreign body response destroys the cells very quickly after implantation, even when injected into immunosuppressed animals.
Organoids are structures that arise from the cells of a particular organ, or from a combination of different, partially differentiated tissues, or from progenitor cells of the organ itself that are capable of giving rise to the various tissues of the organ. They have gained importance in scientific research because they represent a valid alternative to animal experiments, and because they can be a valid tool for testing drugs, new molecules or even simulating the environment of a particular pathology. The use of organoids reduces the number of animals used for experiments.
Many organoids arise from mesodermal cells and are formed from a single mass of progenitor cells. When cells in the center of the mass are deprived of nutrients and oxygen, necrosis occurs, even in vitro. Our progenitor cells (CPCs) are naturally capable of giving rise to cells of endothelial origin, as we have shown previously [Di Felice et al., 2009], and are capable of forming vessels even in vitro within the collagen I gel. For this reason, we hoped that the same mass of cells could develop an adequate network of vessels in a more complex environment such as the subcutaneous region of immunocompromised mice. Vascular organoids are important both to ensure the supply of nutrients and oxygen to all cells in the mass and to provide a mesenchymal niche useful for maintaining organoid structure [Yu, 2021]. Previous studies have shown that induced human pluripotent stem cells are able to differentiate into a microvascular network, and this was integrated with the host vasculature to form a functional blood system when implanted into immunodeficient mice [Kusuma et al., 2013].
Here, we used FISH to show that the vasculature present in the implanted CPC masses was derived from the host and not from the donor cells.
In conclusion, CPCs are capable of expressing cardiac structural markers and organizing a vascular network in vitro, but when implanted (with or without fibroin nets) into the subcutaneous region of immunocompromised mice, they are likely to be destroyed by CD3+ lymphocytes, which are still active in this type of animals.
This study is important because it shows how multipotent cells, that can be isolated from the rat heart, can be used to at least attempt to create a functionalized mass of cardiac cells in vivo. Currently, it is very difficult to control the differentiation of stem cells of any origin in the layers of the heart wall. While the 4 cell types found in the heart are emerging, no one has yet been able to generate myocardium, endocardium, or epicardium and organize them well in between. Anyway, the use of cell masses with the characteristics of heart tissue is very important for the 3R principle and reduces the number of animals used for the experiments. The possibility of using masses of cells organized into tissues replaces the use of animals.
Statement of Ethics
This study was conducted in strict accordance with the recommendations of the Italian Ministry of Health’s Guide to the Care and Use of Laboratory Animals. The protocols of the experiments on animals were part of the Finalize Research project 2007 (Ricerca Finalizzata, 2007) funded by the Italian Ministry of Health with prot. no. RF-IZI-2007-634467. This study protocol was reviewed and approved by the Committee on the Ethics of Animal Experiments of the Istituto Zooprofilattico Sperimentale Sicilia, approval number [23466/08]. The experiments on animals were performed before the entry into force of Decree Law no. 26/2014, in application of European Directive 2010/63/Eu. The in vitro experiments shown in Figure 2 have been performed on cryopreserved cells obtained from the same animals in 2008.
Conflict of Interest Statement
No author has a conflict of interest.
Funding Sources
The study was sponsored by MINISTERO DELLA SALUTE (Ricerca Finalizzata, 2007; Grant No. RF-IZI-2007-634467).
Author Contributions
A.M. and C.M. provided silk and fibroin scaffolds; F.M., A.G., P.D.M., and G.C. provided animals and performed experiments on animals; R.B. and R.P. performed confocal analysis; F.G. and F.P. isolated cells from rat hearts and created masses of cardiac cells in vitro; V.D.F. wrote the paper, did data analysis and light microscopy analysis.
Data Availability Statement
The original data are available upon reasonable request. A preprint version of this article is available on Research Square.
References
- 1. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008 Apr;20(2):86–100.http://dx.doi.org/10.1016/j.smim.2007.11.004
- 2. Awwad JT, Sayegh RA, Tao XJ, Hassan T, Awwad ST, Isaacson K. The SCID mouse: an experimental model for endometriosis. Hum Reprod. 1999 Dec;14(12):3107–11.http://dx.doi.org/10.1093/humrep/14.12.3107
- 3. Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003 Sep;114(6):763–76.
- 4. Bernstein HS, Srivastava D. Stem cell therapy for cardiac disease. Pediatr Res. 2012 Apr;71(4 Pt 2):491–9.http://dx.doi.org/10.1038/pr.2011.61
- 5. Boopathy AV, Che PL, Somasuntharam I, Fiore VF, Cabigas EB, Ban K, et al. The modulation of cardiac progenitor cell function by hydrogel-dependent Notch1 activation. Biomaterials. 2014 Sep;35(28):8103–12.
- 6. Bucciarelli A, Muthukumar T, Kim JS, Kim WK, Quaranta A, Maniglio D, et al. Preparation and Statistical Characterization of Tunable Porous Sponge Scaffolds using UV Cross-linking of Methacrylate-Modified Silk Fibroin. ACS Biomater Sci Eng. 2019 Dec;5(12):6374–88.
- 7. Bucciarelli A, Greco G, Corridori I, Pugno NM, Motta A. A Design of Experiment Rational Optimization of the Degumming Process and Its Impact on the Silk Fibroin Properties. ACS Biomater Sci Eng. 2021 Apr 12;7(4):1374–93.http://dx.doi.org/10.1021/acsbiomaterials.0c01657
- 8. Chimenti I, Rizzitelli G, Gaetani R, Angelini F, Ionta V, Forte E, et al. Human cardiosphere-seeded gelatin and collagen scaffolds as cardiogenic engineered bioconstructs. Biomaterials. 2011 Dec;32(35):9271–81.
- 9. Di Felice V. Commentary on: "Tissue engineering: How to build a heart”. Front Physiol. 2015;6:84.
- 10. Di Felice V, Ardizzone NM, De Luca A, Marciano V, Gammazza AM, Macaluso F, et al. OPLA scaffold, collagen I, and horse serum induce an higher degree of myogenic differentiation of adult rat cardiac stem cells. J Cell Physiol. 2009 Dec;221(3):729–39.
- 11. Di Felice V, Forte G, Coletti D. Biomaterials and bioactive molecules to drive differentiation in striated muscle tissue engineering. Front Physiol. 2015a;6:52.http://dx.doi.org/10.3389/fphys.2015.00052
- 12. Di Felice V, Serradifalco C, Rizzuto L, De Luca A, Rappa F, Barone R, et al. Silk fibroin scaffolds enhance cell commitment of adult rat cardiac progenitor cells. J Tissue Eng Regen Med. 2015b Nov;9(11):E51–64.
- 13. Filippo Buono M, von Boehmer L, Strang J, Hoerstrup SP, Emmert MY, Nugraha B. Human Cardiac Organoids for Modeling Genetic Cardiomyopathy. Cells. 2020;9(7):1733.http://dx.doi.org/10.3390/cells9071733
- 14. Font Tellado S, Bonani W, Balmayor ER, Foehr P, Motta A, Migliaresi C, et al. Fabrication and Characterization of Biphasic Silk Fibroin Scaffolds for Tendon/Ligament-to-Bone Tissue Engineering. Tissue Eng Part A. 2017 Aug;23(15‐16):859–72.
- 15. Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011 Oct;32(28):6692–709.http://dx.doi.org/10.1016/j.biomaterials.2011.05.078
- 16. Furesz J, Fanok A, Contreras G, Becker B. Tumorigenicity testing of various cell substrates for production of biologicals. Dev Biol Stand. 1989;70:233–43.
- 17. Higgins DM, Basaraba RJ, Hohnbaum AC, Lee EJ, Grainger DW, Gonzalez-Juarrero M. Localized immunosuppressive environment in the foreign body response to implanted biomaterials. Am J Pathol. 2009 Jul;175(1):161–70.http://dx.doi.org/10.2353/ajpath.2009.080962
- 18. Kusuma S, Shen YI, Hanjaya-Putra D, Mali P, Cheng L, Gerecht S. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc Natl Acad Sci U S A. 2013 Jul;110(31):12601–6.http://dx.doi.org/10.1073/pnas.1306562110
- 19. Maniglio D, Bonani W, Migliaresi C, Motta A. Silk fibroin porous scaffolds by N2O foaming. J Biomater Sci Polym Ed. 2018 April;29(5):491–506.http://dx.doi.org/10.1080/09205063.2018.1423811
- 20. Pantelouris EM. Absence of thymus in a mouse mutant. Nature. 1968 Jan;217(5126):370–1.http://dx.doi.org/10.1038/217370a0
- 21. Richards DJ, Li Y, Kerr CM, Yao J, Beeson GC, Coyle RC, et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat Biomed Eng. 2020 April;4(4):446–62.
- 22. Rodriguez A, Macewan SR, Meyerson H, Kirk JT, Anderson JM. The foreign body reaction in T-cell-deficient mice. J Biomed Mater Res A. 2009 Jul;90(1):106–13.http://dx.doi.org/10.1002/jbm.a.32050
- 23. Uebersax L, Apfel T, Nuss KM, Vogt R, Kim HY, Meinel L, et al. Biocompatibility and osteoconduction of macroporous silk fibroin implants in cortical defects in sheep. Eur J Pharm Biopharm. 2013 Sep;85(1):107–18.
- 24. Vander Griend DJ, Konishi Y, De Marzo AM, Isaacs JT, Meeker AK. Dual-label centromere and telomere FISH identifies human, rat, and mouse cell contribution to multispecies recombinant urogenital sinus xenografts. Prostate. 2009 Oct;69(14):1557–64.http://dx.doi.org/10.1002/pros.21001
- 25. Varzideh F, Pahlavan S, Ansari H, Halvaei M, Kostin S, Feiz MS, et al. Human cardiomyocytes undergo enhanced maturation in embryonic stem cell-derived organoid transplants. Biomaterials. 2019 Feb;192:537–50.
- 26. Voges HK, Mills RJ, Elliott DA, Parton RG, Porrello ER, Hudson JE. Development of a human cardiac organoid injury model reveals innate regenerative potential. Development. 2017 Mar;144(6):1118–27.http://dx.doi.org/10.1242/dev.143966
- 27. Yu J. Vascularized Organoids: A More Complete Model. Int J Stem Cells. 2021 May;14(2):127–37.http://dx.doi.org/10.15283/ijsc20143