Introduction
There is a possible survival benefit of primary tumor resection (PTR) in synchronous metastatic colon cancer (mCC), which is currently being evaluated prospectively [-]. Results of retrospective studies are conflicting, with some studies indicating an association between PTR and improved overall survival (OS) [-]. Recently, the iPACS trial in which PTR followed by systemic therapy was compared to systemic therapy without prior PTR was prematurely terminated due to futility []. Although the authors of this randomized controlled trial (RCT) demonstrated that PTR does not seem to result in improved survival for the entire group of metastatic colorectal cancer (mCRC) patients, there might be subgroups that benefit from PTR.
With primary tumor sidedness in mCC being regarded as an important prognostic factor, the association between PTR and OS might be dependent on primary tumor sidedness []. It is already known that the benefit of certain systemic therapy regimens may depend on sidedness. For example, anti-EGFR antibodies are most effective in patients with left-sided RAS wild-type mCC []. Although some studies concerning the role of PTR have adjusted for tumor sidedness in their analyses, the interaction between PTR, OS, and primary tumor sidedness has not been reported previously in synchronous mCC patients treated with palliative systemic therapy. Therefore, our aim was to investigate whether the association between PTR and OS was dependent on primary tumor sidedness in patients with synchronous mCC, using data from 2 prospective RCTs.
Methods
Patient Selection
Data of patients with histologically proven synchronous mCC were retrieved from 2 phase 3 RCTs (CAIRO and CAIRO2). Inclusion and exclusion criteria of these studies have been published elsewhere [, ]. The CAIRO study compared sequential chemotherapy versus combination chemotherapy in mCRC. Sequential chemotherapy consisted of first-line treatment with capecitabine, second-line irinotecan, and third-line capecitabine plus oxaliplatin (CAPOX). Combination treatment consisted of first-line treatment with capecitabine plus irinotecan and second-line CAPOX. In the CAIRO2 study, mCRC patients were randomized between CAPOX + bevacizumab (CAPOX-B: arm A) and CAPOX-B + cetuximab (arm B). For the current analysis, we only included patients treated in arm A, as the addition of cetuximab to CAPOX-B resulted in a significantly decreased progression-free survival, and is currently not part of standard care []. Patients with rectal cancer were also excluded, due to the different tumor biology of rectal tumors and associated survival. All tumors proximal of the splenic flexure were considered right-sided colon cancer (RCC). OS was calculated using date of randomization as a starting point. Synchronous disease was defined as presence of metastases within 6 months after histological diagnosis of the primary tumor [].
Statistical Analysis
Baseline characteristics were compared using a χ2 test. The median age between the groups was compared using the Kruskal-Wallis test. We used mixed-effects Cox regression models to study the association between PTR, sidedness, and OS accounting for clustering of patients within studies. Age, treatment arm, performance status, number of affected organs by metastases, serum lactate dehydrogenase (LDH), and year of enrollment were added as fixed effects to adjust for confounding; the study was added as random effect. To investigate whether the association between PTR and OS depends on sidedness, we tested the statistical significance of the interaction between sidedness and PTR status []. The median survival of RCC and left-sided colon cancer (LCC) patients was adjusted for confounders by the use of inverse probability weights []. p values were derived from 2-tailed tests, and a p value below 0.05 was considered significant. R version 3.6.3 was used for statistical analysis.
Results
Patient Selection and Characteristics
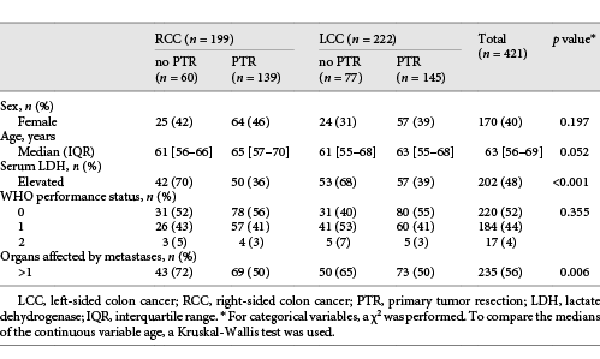
Of the 820 patients in CAIRO, 376 patients were excluded because of ineligibility, metachronous metastases, or unknown primary tumor location (Fig. 1). In the CAIRO2 study, 526 patients were excluded due to ineligibility, metachronous metastases, unknown date of metastatic disease diagnosis, unknown primary tumor location, or randomization into the CAPOX-B cetuximab study arm. Of the remaining 673 synchronous mCRC patients of both CAIRO and CAIRO2, 252 rectal cancer patients were excluded. Of the 421 selected patients from CAIRO (n = 279) and CAIRO2 (n = 142), 199 (47%) had RCC (Table 1). Within the RCC and LCC groups, respectively, 139 (70%) and 145 (65%) underwent PTR. In both RCC and LCC, patients who underwent PTR were more frequently female and older. In addition, they more often had only 1 organ affected by metastases, had a lower serum LDH and a WHO performance score of 0. Differences in baseline characteristics were statistically significant for serum LDH (p < 0.001) and number of affected organs by metastases (p = 0.006). The median number of days between date of PTR and date of randomization was 43 (interquartile range: 30–77). A pT4 tumor was observed in 78 (28%) of the patients who underwent PTR (see online suppl. Table 1; for all online suppl. material, see http://www.karger.com/doi/10.1159/000517477).
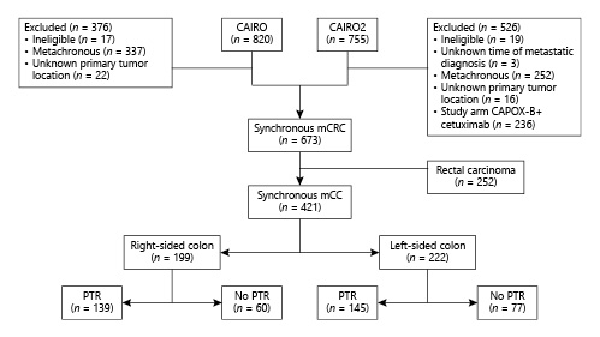
Fig. 1
Flowchart. mCC, metastatic colon cancer; PTR, primary tumor resection; mCRC, metastatic colorectal cancer; CAPOX-B, capecitabine, oxaliplatin, and bevacizumab.
Overall Survival
A total of 17 (4%) patients were still alive after a median follow-up duration of 73 months. A vast majority (404 patients, 96%) died during follow-up. The median OS adjusted for confounders in RCC versus LCC was 12.5 months (11.1–13.5) versus 19.5 months (95% CI 17.6–22.1) (HRRCC 1.71 [95% CI 1.39–2.10]). Unadjusted survival curves of patients with RCC and patients with LCC are displayed in Figure 2.
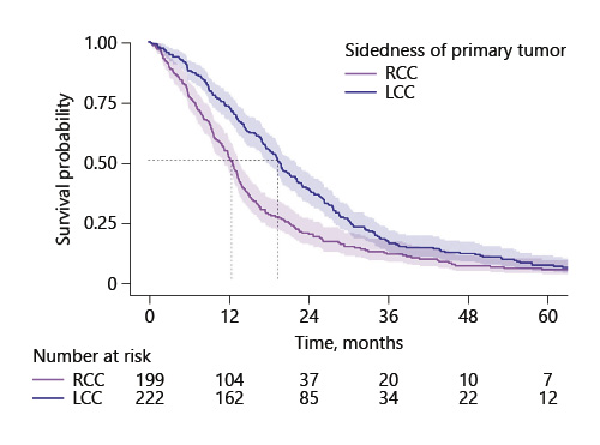
Fig. 2
Survival curves of patients with right-sided and left-sided mCC. RCC, right-sided colon cancer; LCC, left-sided colon cancer; mCC, metastatic colon cancer.
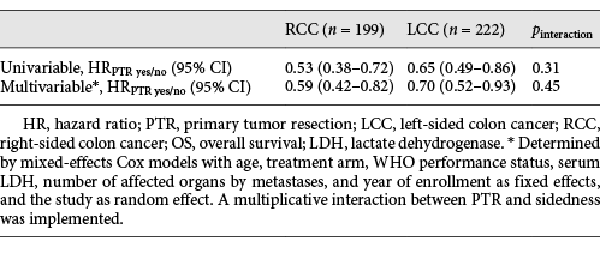
In both RCC and LCC, univariable analysis showed that PTR was associated with a significant improved OS compared to no PTR (Table 2: RCC: HRPTR yes/no 0.53 [0.38–0.72], LCC: HRPTR yes/no 0.65 [0.49–0.86]; pinteraction = 0.31) In multivariable analysis, PTR remained associated with improved survival, regardless of sidedness (RCC: HRPTR yes/no 0.59 [0.42–0.82], LCC: HRPTR yes/no 0.70 [0.52–0.93]; pinteraction = 0.45). Unadjusted survival curves are shown in online suppl. Figure 1.
Discussion
In the present studies, we found that the association between primary tumor sidedness and OS was not dependent on primary tumor location. To our knowledge, this is the first study in which the interaction between PTR and sidedness is investigated in patients participating in RCTs. Moreover, we observed a clinically relevant worse survival of patients with synchronous RCC than LCC.
In contrast to the results of our study, Zhang et al. [] found that PTR was associated with improved survival in RCC patients, and not LCC patients, with synchronous metastases. However, this was a retrospective single-center study with only a limited number of patients (n = 194) and only a quarter of their population was treated with targeted agents []. Kim et al. [] found in a single-center observational study that PTR was associated with improved survival in both RCC and LCC. However, they did not limit their selection to synchronous metastatic patients, did not correct for important prognostic factors, such as number of affected organs, and only a third of their population was treated with modern targeted therapy []. Data from our study are derived from 2 large, prospective multicenter phase 3 RCTs. The observation that PTR is associated with improved survival in RCTs has been described before, but none of these studies incorporated the possible interaction between sidedness and PTR in the analysis [].
The mechanism behind the observed survival difference between synchronous metastatic RCC and LCC is insufficiently understood but might be the result of several factors. First, molecular status differs per primary tumor location. Mutations in KRAS, BRAF, and PIK3CA are more likely to occur in RCC than in LCC [, ]. Furthermore, right-sided tumors are more frequently microsatellite-instable, CpG island methylator phenotype high, and are associated with the consensus molecular subtype 1. All 3 of these characteristics are associated with a worse survival in mCC [-]. In contrast, LCC is associated with the prognostically more favorable consensus molecular subtype 2 []. Second, the prognostically unfavorable mucinous and signet ring cell histology types are more prevalent in RCC []. Third, RCC more frequently metastasizes to the peritoneum, which is prognostically unfavorable []. Fourth, differences in microbiome and biofilm formation in RCC and LCC have been observed [, ]. All these aforementioned factors might be the result of the different embryologic origin of RCC and LCC. The caecum, ascending colon, hepatic flexure, and two-thirds of the transverse colon originate from the midgut. The distal one-third of the transverse colon, splenic flexure, descending colon, sigmoid, and rectum develop out of the hindgut [].
Several hypotheses have been formulated to explain why PTR might increase OS in synchronous mCC. PTR is assumed to prevent complications related to the primary tumor, such as acute bowel obstruction and perforation. These complications would necessitate emergency surgery, which is associated with a higher mortality risk. However, it has been argued that modern systemic therapy mitigates the risks of the occurrence of these complications and therefore makes prophylactic PTR obsolete []. Another reason why PTR might increase OS, is by a reduction of the tumor load. Tumor reduction might delay tumor growth, and it might also improve the response to chemotherapy []. Furthermore, it is hypothesized that stem cells present in the primary tumor are involved in metastases. These stem cells might be relatively resistant to radiation and chemotherapy []. Moreover, supporters of the “tumor self-seeding” theory point out that the circulating tumor cells derived from the primary tumor can colonize the primary from which they originated. This process might encourage angiogenesis and tumor growth []. Finally, resection might improve OS by reducing systemic inflammation. Systemic inflammation possibly reduces OS by inducing T-cell anergy and loss of cytotoxicity [].
Of the prospective trials in which PTR in synchronous mCC is evaluated, only results of the iPACS trial are currently available [, , ]. This trial showed no benefit of PTR for the entire group of mCRC patients. However, results of the other trials remain awaited, and there still might be subgroups of mCRC patients who do benefit from PTR. Several authors have tried to construct risk scores based on observational data to pinpoint subgroups [, ]. Age was the only common factor in the risk scores. The relatively small sample size of the iPACS trial (n = 160) impedes the identification of subgroups. Pooling of data from iPACS and the other awaited RCTs might enable definitive identification of possible subgroups.
Our study is limited by possible confounding by indication, because reasons for PTR and presence of symptoms of the primary tumor are unknown. Possible selection bias could have occurred, because only the patients who were sufficiently fit to receive systemic therapy after PTR were eligible for the trials. Moreover, our study is limited by sparse information on T stage, especially in patients treated with systemic therapy only, and the absence of data concerning invasion of adjacent organs. Although, we cannot rule out that PTR was avoided in challenging cases, such as pT4b tumors, the proportion of T4 tumors in our patient group (28%) that underwent PTR was comparable to the proportion of T4 tumors reported in the entire Dutch mCRC population (26%), including both patients treated with and without PTR []. After adjustment for important prognostic factors such as WHO performance status, number of affected organs by metastases, and LDH, PTR remained associated with improved survival. In addition, this retrospective analysis enabled us to study the interaction between PTR and sidedness in a population treated with modern systemic therapy and targeted agents.
Conclusion
In conclusion, we found that the association between PTR and OS is independent of the important prognostic factor primary tumor sidedness in synchronous mCC. Additional data from prospective RCTs, in which the effect of PTR is analyzed, remain necessary to confirm this observation and to evaluate if there are subgroups of mCC patients that benefit from PTR.
Acknowledgements
We thank Linda Mol, PhD, Netherlands Cancer Registry, for the data management of the CAIRO studies and her advice. She was not compensated.
Statement of Ethics
The study protocol was reviewed and approved by the Central Committee of Human-Related Research Arnhem – Nijmegen (approval numbers: CAIRO: 2002/019; CAIRO2: 2005/076) and by the local Ethics Committees of all participating centers. All participants gave written informed consent before study entry. The study was performed in accordance with the Declaration of Helsinki.
Conflict of Interest Statement
Prof. Dr. M. Koopman, Prof. Dr. J.H.W. de Wilt, and Mr. D.E.W. van der Kruijssen reported a grant of the Dutch Cancer Society (KWF) and an unrestricted grant of Roche for the CAIRO4 trial, in which the role of PTR is investigated. No other disclosures were reported.
Funding Sources
The authors declare no funding for this research.
Author Contributions
Mr. van der Kruijssen, Ms. K.L. van Rooijen, and Dr. S.A. Kurk had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The study concept and design: van der Kruijssen, van Rooijen, Kurk, and Koopman. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: van der Kruijssen, van Rooijen, and Koopman. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Elias, Kurk, and van der Kruijssen. Supervision: Koopman.
Data Availability Statement
The datasets generated and/or analyzed for this study are not publicly available.
References
- 1. ’t Lam-Boer J, Mol L, Verhoef C, de Haan AFJ, Yilmaz M, Punt CJA, et al. The CAIRO4 study: the role of surgery of the primary tumour with few or absent symptoms in patients with synchronous unresectable metastases of colorectal cancer: a randomized phase III study of the Dutch Colorectal Cancer Group (DCCG). BMC Cancer. 2014;14(1):741.
- 2. Rahbari NN, Lordick F, Fink C, Bork U, Stange A, Jäger D, et al. Resection of the primary tumour versus no resection prior to systemic therapy in patients with colon cancer and synchronous unresectable metastases (UICC stage IV): SYNCHRONOUS: a randomised controlled multicentre trial (ISRCTN30964555). BMC Cancer. 2012;12(1):142.http://dx.doi.org/10.1186/1471-2407-12-142.
- 3. Cotte E, Villeneuve L, Passot G, Boschetti G, Bin-Dorel S, Francois Y, et al. GRECCAR 8: impact on survival of the primary tumor resection in rectal cancer with unresectable synchronous metastasis: a randomized multicentre study. BMC Cancer. 2015;15(1):47.http://dx.doi.org/10.1186/s12885-015-1060-0.
- 4. Nitsche U, Stöß C, Stecher L, Wilhelm D, Friess H, Ceyhan GO. Meta-analysis of outcomes following resection of the primary tumour in patients presenting with metastatic colorectal cancer. Br J Surg. 2018;105:784–96.http://dx.doi.org/10.1002/bjs.10682.
- 5. van Rooijen KL, Shi Q, Goey KKH, Meyers J, Heinemann V, Diaz-Rubio E, et al. Prognostic value of primary tumour resection in synchronous metastatic colorectal cancer: individual patient data analysis of first-line randomised trials from the ARCAD database. Eur J Cancer. 2018;91(September 2016):99–106.http://dx.doi.org/10.1016/j.ejca.2017.12.014.
- 6. ’t Lam-Boer J, Van der Geest LG, Verhoef C, Elferink ME, Koopman M, de Wilt JHW. Palliative resection of the primary tumor is associated with improved overall survival in incurable stage IV colorectal cancer: a nationwide population-based propensity-score adjusted study in the Netherlands. Int J Cancer. 2016;139(9):2082–94.
- 7. Alawadi Z, Phatak UR, Hu CY, Bailey CE, You YN, Kao LS, et al. Comparative effectiveness of primary tumor resection in patients with stage IV colon cancer. Cancer. 2017;123(7):1124–33.http://dx.doi.org/10.1002/cncr.30230.
- 8. Kanemitsu Y, Shitara K, Mizusawa J, Hamaguchi T, Shida D, Komori K, et al. Primary tumor resection plus chemotherapy versus chemotherapy alone for colorectal cancer patients with asymptomatic, synchronous unresectable metastases (JCOG1007; iPACS): a randomized clinical trial. J Clin Oncol. 2021. [Epub ahead of print].https://doi.org/10.1200/JCO.20.02447.
- 9. Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98.http://dx.doi.org/10.1016/j.ejca.2016.10.007.
- 10. Koopman M, Antonini NF, Douma J, Wals J, Honkoop AH, Erdkamp FLG, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet. 2007 Jul;370(9582):135–42.
- 11. Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360(6):563–72.http://dx.doi.org/10.1056/NEJMoa0808268.
- 12. Mekenkamp LJ, Koopman M, Teerenstra S, van Krieken JH, Mol L, Nagtegaal ID, et al. Clinicopathological features and outcome in advanced colorectal cancer patients with synchronous vs metachronous metastases. Br J Cancer. 2010;103(2):159–64.http://dx.doi.org/10.1038/sj.bjc.6605737.
- 13. Brankovic M, Kardys I, Steyerberg EW, Lemeshow S, Markovic M, Rizopoulos D, et al. Understanding of interaction (subgroup) analysis in clinical trials. Eur J Clin Invest. 2019;49(8):e13145.http://dx.doi.org/10.1111/eci.13145.
- 14. Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45–9.http://dx.doi.org/10.1016/j.cmpb.2003.10.004.
- 15. Zhang RX, Ma WJ, Gu YT, Zhang TQ, Huang ZM, Lu ZH, et al. Primary tumor location as a predictor of the benefit of palliative resection for colorectal cancer with unresectable metastasis. World J Surg Oncol. 2017;15(1):138.http://wjso.biomedcentral.com/articles/10.1186/s12957-017-1198-0.
- 16. Kim JH, Jin S, Jeon MJ, Jung HY, Byun S, Jung K, et al. Survival benefit of palliative primary tumor resection based on tumor location in patients with metastatic colorectal cancer: a single-center retrospective study. Korean J Gastroenterol. 2020;76(1):17–27.http://dx.doi.org/10.4166/kjg.2020.76.1.17.
- 17. Venderbosch S, de Wilt JH, Teerenstra S, Loosveld OJ, van Bochove A, Sinnige HA, et al. Prognostic value of resection of primary tumor in patients with stage IV colorectal cancer: retrospective analysis of two randomized studies and a review of the literature. Ann Surg Oncol. 2011;18(12):3252–60.http://dx.doi.org/10.1245/s10434-011-1951-5.
- 18. Bylsma LC, Gillezeau C, Garawin TA, Kelsh MA, Fryzek JP, Sangaré L, et al. Prevalence of RAS and BRAF mutations in metastatic colorectal cancer patients by tumor sidedness: a systematic review and meta-analysis. Cancer Med. 2020;9(3):1044–57.http://dx.doi.org/10.1002/cam4.2747.
- 19. Nunes L, Aasebø K, Mathot L, Ljungström V, Edqvist PH, Sundström M, et al. Molecular characterization of a large unselected cohort of metastatic colorectal cancers in relation to primary tumor location, rare metastatic sites and prognosis. Acta Oncol. 2020;59(4):417–26.http://dx.doi.org/10.1080/0284186X.2019.1711169.
- 20. Zong L, Abe M, Ji J, Zhu WG, Yu D. Tracking the correlation between CpG island methylator phenotype and other molecular features and clinicopathological features in human colorectal cancers: a systematic review and meta-analysis. Clin Transl Gastroenterol. 2016;7:e151.http://dx.doi.org/10.1038/ctg.2016.14.
- 21. Chen K-H, Lin L-I, Tseng L-H, Lin Y-L, Liau J-Y, Tsai J-H, et al. The prognostic role of CpG island methylator phenotype in metastatic colorectal cancer. J Clin Oncol. 2018 Feb 1;36(4 Suppl):667.http://dx.doi.org/10.1200/jco.2018.36.4_suppl.667.
- 22. Stintzing S, Wirapati P, Lenz HJ, Neureiter D, Fischer von Weikersthal L, Decker T, et al. Consensus molecular subgroups (CMS) of colorectal cancer (CRC) and first-line efficacy of FOLFIRI plus cetuximab or bevacizumab in the FIRE3 (AIO KRK-0306) trial. Ann Oncol. 2019;30(11):1796–803.http://dx.doi.org/10.1093/annonc/mdz387.
- 23. Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–6.http://dx.doi.org/10.1038/nm.3967.
- 24. Hugen N, Van de Velde CJH, De Wilt JHW, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25(3):651–7.http://dx.doi.org/10.1093/annonc/mdt591.
- 25. Brouwer NPM, van der Kruijssen DEW, Hugen N, de Hingh IHJT, Nagtegaal ID, Verhoeven RHA, et al. The impact of primary tumor location in synchronous metastatic colorectal cancer: differences in metastatic sites and survival. Ann Surg Oncol. 2020;27(5):1580–8.http://dx.doi.org/10.1245/s10434-019-08100-5.
- 26. Flemer B, Lynch DB, Brown JM, Jeffery IB, Ryan FJ, Claesson MJ, et al. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 2017;66(4):633–43.http://dx.doi.org/10.1136/gutjnl-2015-309595.
- 27. Dejea CM, Wick EC, Hechenbleikner EM, White JR, Mark Welch JL, Rossetti BJ, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci U S A. 2014;111(51):18321–6.http://dx.doi.org/10.1073/pnas.1406199111.
- 28. Stintzing S, Tejpar S, Gibbs P, Thiebach L, Lenz HJ. Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes. Eur J Cancer. 2017;84:69–80.http://dx.doi.org/10.1016/j.ejca.2017.07.016.
- 29. Poultsides GA, Paty PB. Reassessing the need for primary tumor surgery in unresectable metastatic colorectal cancer: overview and perspective. Ther Adv Med Oncol. 2011;3(1):35–42.http://dx.doi.org/10.1177/1758834010386283.
- 30. Xu H, Xia Z, Jia X, Chen K, Li D, Dai Y, et al. Primary tumor resection is associated with improved survival in stage IV colorectal cancer: an instrumental variable analysis. Sci Rep. 2015;5(August):16516.http://dx.doi.org/10.1038/srep16516.
- 31. Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26(17):2813–20.http://dx.doi.org/10.1200/JCO.2008.16.3931.
- 32. Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, et al. Tumor self-seeding by circulating cancer cells. Cell. 2010;139(7):1315–26.http://dx.doi.org/10.1016/j.cell.2009.11.025.
- 33. Turner N, Tran B, Tran PV, Sinnathamby M, Wong HL, Jones I, et al. Primary tumor resection in patients with metastatic colorectal cancer is associated with reversal of systemic inflammation and improved survival. Clin Colorectal Cancer. 2015;14(3):185–91.http://dx.doi.org/10.1016/j.clcc.2015.02.004.
- 34. Dorajoo SR, Tan WJ, Koo SX, Tan WS, Chew MH, Tang CL, et al. A scoring model for predicting survival following primary tumour resection in stage IV colorectal cancer patients with unresectable metastasis. Int J Colorectal Dis. 2015;31:235–45.http://dx.doi.org/10.1007/s00384-015-2419-z.
- 35. Li ZM, Peng YF, Du CZ, Gu J. Colon cancer with unresectable synchronous metastases: the AAAP scoring system for predicting the outcome after primary tumour resection. Colorectal Dis. 2016;18(3):255–63.http://dx.doi.org/10.1111/codi.13123.
Dave E.W. van der Kruijssen and Karlijn L. van Rooijen are co-first authors.
Registration numbers at Clinicaltrials.gov: NCT00312000 and NCT00208546.