Learning objectives
Understand diversity, equity, and inclusion (DE&I) in the context of cardiovascular (CV) research and its impact on research findings, translation, and patient outcomes.
Identify strategies that have demonstrated effectiveness in improving the participation of underrepresented population in CV disease research.
Develop greater confidence to advocate for the application of strategies and practices presented in the paper to improve DE&I in CV research.
The problem
Cardiovascular disease (CVD) is a prominent contributor to global mortality and morbidity, impacting people across diverse demographics. Nevertheless, cardiovascular (CV) research, including clinical trials, has traditionally fallen short in demonstrating inclusive practices for diversity, equity, and inclusion (DE&I), notably apparent in the unrepresentative enrolment and poorly sustained engagement of underrepresented groups, including women and culturally and linguistically diverse populations. Poor DE&I in clinical trials has been well-documented since the early 1990s, and despite many leading research funding bodies developing guidelines and policies mandating the inclusion of women and culturally and linguistically diverse populations in government-funded research, representation of both groups in CV trials remains worse than other specialties., A recently published review of 153 landmark CV randomized controlled trials (RCTs) from 1986 to 2019 (including over 1.1 million participants) revealed that only 56% of RCTs reported information about participant race. The review also found that no significant improvement in inclusion of underrepresented racial and ethnic groups has occurred over time. Race can have significant pathophysiological implications in CVD therapy as evidenced in the landmark African–American Heart Failure Trial (A-HeFT), which demonstrated a different response to standard heart failure therapy in African–American populations.,
An audit of five clinical trials listed on the American College of Cardiology Top Clinical Trials for 2022 underscores the persistency of the problem in that the majority of participants was Caucasian males, none of the trials clearly reported gender data, and some trials failed to report participant race (see Supplementary material online, Table S1). Although the proportion of transgendered or gender-diverse individuals (i.e. non-binary or genderqueer), is thought to be small (0.3–0.5% of the world’s population), there is a growing need to understand how sex, gender, and gender dysphoria influence CV traits, ischaemic heart disease, and heart failure mechanisms. How we choose to collect this data will influence how well we can identify and elucidate these links. Factors underpinning the continued deficit with regard to female participation include persistent historical and systemic bias in attitudes around the female hormonal profile and reproductive risk and the concurrent contradictory belief that women would have similar responses to drugs as men. In addition, women make up only 10% of CV clinical trials leadership teams or are listed as first or last authors on clinical trial publications.
Diversity, equity, and inclusion deficits in CVD research can also impact guideline development, with guidelines inevitably based on the extrapolation of results obtained in unrepresentative patient populations, potentially exacerbating pre-existing health disparities and hindering the development of effective prevention and treatment strategies for all those affected by CVD.,,, In turn, the makeup of CVD guideline authorship teams reflects disparity in the inclusion of women as chairs or members of guideline development committees. The evidence points to the fact that where women do lead in research teams and in translation, DE&I in participant recruitment and retention, as well as participation in guideline development, improve.,
The persistent DE&I deficit in CVD research requires a ‘root and branch’ approach with leadership required at every level of the research ecosystem from trial governance to guideline development. This methods paper is aimed at clinician–researchers who may find themselves involved at various points in the CVD research cycle and collates evidence and strategies to improve DE&I, applicable at each stage.
Moving towards a solution: strategies to improve diversity, equity, and inclusion in cardiovascular disease research
As clinician–researchers, we are uniquely placed to advocate for and have an ethical and moral responsibility to design, conduct, and implement research that ensures evidence-based CV care is representative and accessible. Diversity, equity, and inclusion principles need to be incorporated throughout the research cycle, from conceptualization through to translation and knowledge exchange. To do this, it is important to become familiar with existing evidence-based strategies that focus on DE&I across the various phases of the research cycle (Box 1).
Box 1Definition of key DE&I terms in the context of cardiovascular disease research
Diversity is the representation of a wide range of people with different backgrounds, identities, and perspectives in a particular group—in this case, cardiovascular disease (CVD) research participants, research teams, and those involved in research translation. Dimensions may include race, ethnicity, gender, sexual orientation, age, disability, religion, and socioeconomic status.
Equity is about, justice, and equal opportunities. It is the recognition that different people may require different resources, support, or accommodations to achieve equitable CVD outcomes, ensuring that their identities do not dictate their access to opportunities in research participation.
Inclusion refers to the behaviours and practices that ensure all individuals and groups are valued. It is about making sure participants are representative of the population the treatment or intervention is intended for and that the study results are meaningful and applicable to the target population.
Underrepresentation happens when groups or individuals are not adequately or proportionately represented within a particular research setting. It often highlights the need for increased attention to the enhanced opportunities these groups may need to address imbalances and promote equitable participation.,
Co-design is a collaborative approach to cardiovascular research that involves including diverse stakeholders, and people affected by cardiovascular conditions, in designing the proposed research-based solutions. It can be used to address potential barriers that hinder effective research translation across underrepresented groups.
Culturally tailored communication involves adapting messages, materials, or strategies to effectively reach and resonate with specific groups. It involves recognizing the cultural norms, values, beliefs, and linguistic preferences of the target audience to ensure that research communication is culturally appropriate, sensitive, and relatable.
Decentralized clinical trials (DCTs) leverage remote data collection, allowing participants to complete study requirements from home while still being closely monitored. They can potentially address barriers to diversity, patient participation, and accessibility in the clinical trial recruitment and retention.
Targeted recruitment research refers to the deliberate and strategic efforts made to identify and enrol participants from specific demographic or population groups into research studies.
Community engagement involves proactive and ongoing collaboration with the communities being served throughout all phases of the research cycle. It requires bidirectional communication, with researchers listening to and learning with the community.
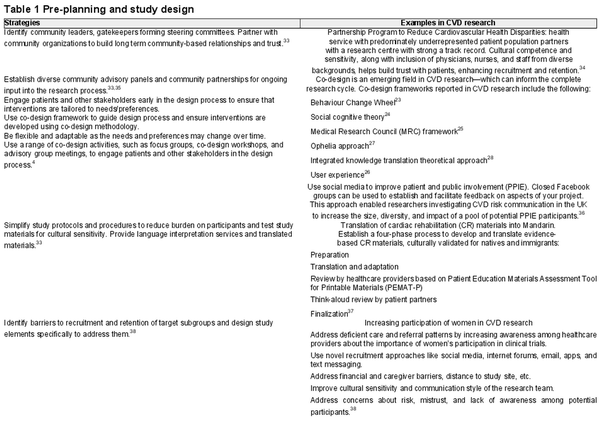
Pre-planning and study design
Ensuring meaningful and relevant research for underrepresented communities necessitates early involvement of diverse patients, community members, and stakeholders in formulating research questions. One effective approach to achieve this is through research co-design processes. Co-design emphasizes collaboration with the community and makes use of existing strengths and resources, fostering a sense of ownership. Various frameworks and examples have been published that focus on different aspects of the participatory process. By adopting a co-design framework, the community is actively engaged in the creation and development of new, innovative, and culturally tailored strategies that address identified problems. Working within an identified co-design conceptual framework that fits the aims of the study is important. For example, Nesbitt and colleagues utilized the user experience design framework (UX design) in the development of a web-based portal for cardiac rehabilitation. In a narrative review of previously published frameworks intended to facilitate lay participation in research, Greenhalgh and colleagues identified a total of 56 resources. Their conclusion is that a universal framework that applies across the board may be less valuable than customizing a variety of resources that can be merged and tailored to the specific research context.
Incorporating patient and community members as part of the research team yields numerous benefits. Firstly, it provides a valuable source of social support, allowing individuals to feel empowered and to actively participate in shaping the research agenda. Additionally, involving community members helps to build trust and establish rapport between researchers and the community, facilitating culturally sensitive engagement throughout the research process. The Australian Consumer Involvement and Engagement Toolkit, a recently published resource, offers practical guidance on authentic partnership with patients and community members in clinical research.
Early planning and budgeting are required to resource the development of inclusive study materials and build partnerships with external community organisations. This aspect of the research planning phase can be daunting for clinician–researchers, and it is useful to access tools and guidance such as the Consumer Involvement Cost Calculator provided by the Australian Clinical Trials Alliance. Clinician–researchers need to be able to advocate for sufficient funding to enable deployment of DE&I strategies and translation of findings back to target groups.
Table 1 provides DE&I strategies and evidenced-based examples for the pre-planning and study design phases.
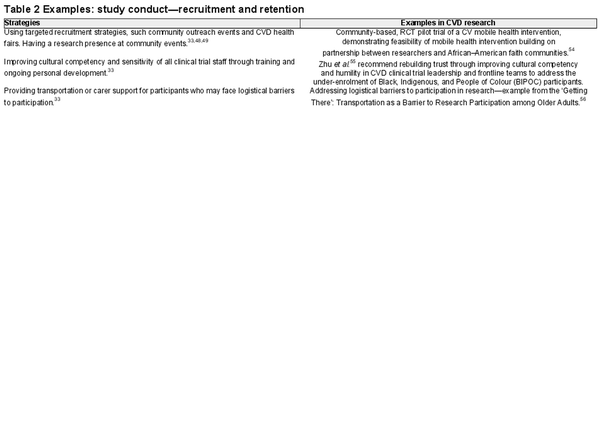
Study conduct: recruitment and retention
To ensure that underrepresented populations have an equal opportunity to participate in clinical research, researchers must carefully consider the eligibility criteria. Subjective eligibility criteria that rely on clinician judgement (e.g. a participant’s perceived ability to provide informed consent or comply with study procedures) that is likely to disproportionately affect people from underrepresented groups should be avoided. Instead, the use of objective assessments, such as diagnostic tests or biomarker measurements to determine eligibility, may be more appropriate in some circumstances. Cognitive ability can be objectively assessed using an appropriate tool, or a diagnosis of major cognitive impairment may be found in the medical record. But every exclusion criterion need to be scientifically justified and those included as participants should reflect the larger group of all those affected by the researched disease or condition. For example, cognitive impairment is highly prevalent in heart failure, yet many studies still exclude these patients., Including underrepresented groups may require concerted efforts from the research team to successfully recruit and safely retain representative cohorts.
Targeted recruitment strategies, such as partnering with community organisations or using social media platforms to reach underrepresented populations, have been successfully deployed in several studies., Ensuring culturally tailored communication strategies includes making sure that the language and visual materials developed in information documents and in interventions are culturally appropriate and relevant., Researchers can then use materials that are relevant and resonant with underrepresented populations to communicate the goals, procedures, and potential benefits and risks of the study. Approaching potential participants in places in which they are already active, particularly in settings that are familiar and comfortable to them, and providing incentives relevant to them and their community have also been found to encourage participation. Decentralized clinical trial methods that leverage digital technologies to reduce the need for participants to travel back to the study centre frequently could also be used to address barriers faced by some underrepresented groups.

Cultural sensitivity during recruitment could include considering the role of family. In Western culture, privacy and patient confidentiality often require that healthcare professionals (HCPs) talk with the patient alone, but in many cultures, the family is part of the decision-making processes. Asking consent for potential participant to take part in a research programme may be a ‘family business’ rather than a lone decision to be made by the potential participant. You will more often be directed to ‘ask my wife or my partner or my son or my daughter’ for consent. Some cultures are collectivist in orientation, and potential participants may be more receptive when the benefits of the research are not only for the individual but also for the family and the community at large. For example, some Asian people view self as an extension of significant others and as such they are all included in the discussion about important matters such as participating in a clinical trial.
Table 2 provides DE&I strategies and evidenced-based examples for the study recruitment and retention phases.
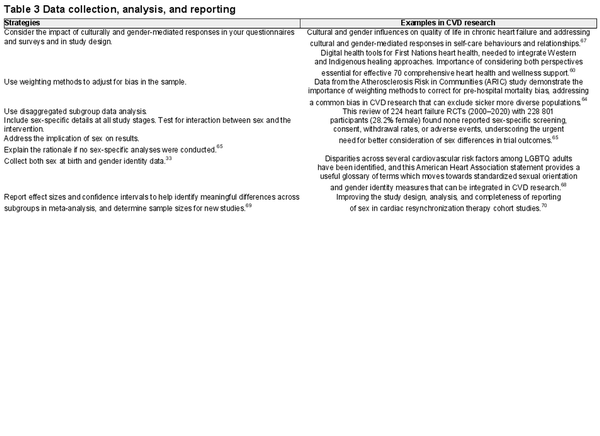
Data collection, analysis, and reporting
Ensuring participant safety and comfort during data collection is crucial. This involves providing DE&I and cultural awareness training to research staff and maintaining engagement with community members to build ongoing trust and mutual respect. Special attention is needed for populations with historical grievances, such as First Nations. Community voices and Indigenous knowledge should be prioritized and meaningful partnerships established., Using a framework such as community-based participatory research can be used to support knowledge sharing that prioritizes lived experience and places community voices at the centre of the partnership. Where appropriate, and under the leadership of community and/or First Nations research partners and elders, the incorporation of Indigenous research methodologies, such as Two-Eyed Seeing, Yarning, or Storytelling, can be considered for data collection.,
When working with culturally and linguistically diverse populations, translating study materials requires careful consideration. Colloquialisms and English terminologies may lack equivalence in other languages, emphasizing the need for briefings with translation experts and consultations with culturally congruent community members to ensure accurate interpretation during data analysis.
For quantitative data analysis, researchers should employ statistical techniques, including weighting methods to address sample biases and should include analysis of disaggregated data, to help identify disparities and patterns across subgroups. Considering the impact of multiple intersecting identities on health outcomes can require sophisticated statistical approaches to account for complex interactions between social identities and health outcomes. These might include multiple main effects, statistical interactions, and multilevel modelling, to explore the additive, multiplicative, and multilevel effects of intersecting identities on CV outcomes. This is particularly relevant when considering sex-specific and gender-based disparities in outcomes where intersecting social determinants may be particularly impactful. Embedding these methods early in the study design phase is also crucial.
Reporting results explicitly so that the demographic characteristics of your study participants are clearly outlined and variations in study outcomes across different demographic groups are highlighted is important. This should include recognition of intersecting identities (e.g. race, gender, and sexual orientation) and their influence on study outcomes where possible.
Table 3 provides DE&I strategies and evidenced-based examples data collection and analysis phases.
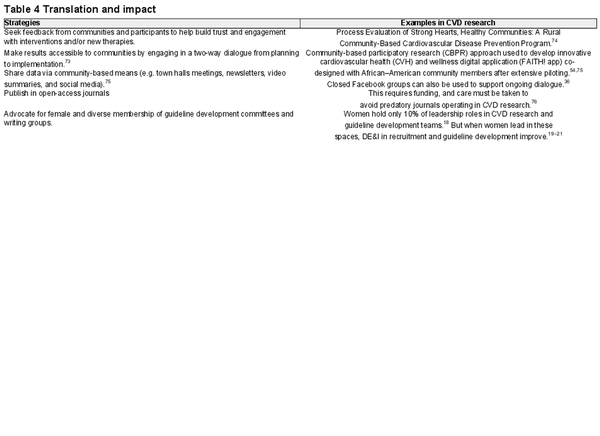
Translation and impact
The translation and implementation of research into practice is a critical part of the research process with the persistent 17-year gap between research and translation further entrenching DE&I deficits in CVD outcomes., To address this, a flexible research dissemination plan might include developing tailored presentations to participating community groups, establishing a point person to serve as a community liaison, and a continuing dialogue with community members after the study. The principles of co-design and community participation discussed earlier can accelerate community uptake of research findings through feedback and consultation processes. Communicating research findings back to key participants and community leaders helps builds trust, accelerates uptake of evidence-based research findings, and sets the scene for future collaborations. Fostering this dialogue, particularly with populations that are historically mistrustful of research, aids in both developing culturally relevant interventions and improving uptake of relevant research findings.
Table 4 provides DE&I strategies and evidenced-based examples for the reporting, translation, and impact phases.
Research team and research environment
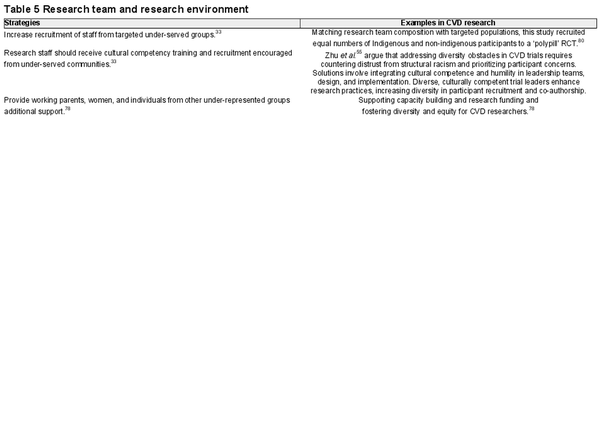
A key driver of diverse, equitable, and inclusive research is the makeup of the investigator team. Recent findings from a systematic bibliometric review highlight that trials led by women are more likely to enrol diverse participants and report race and ethnicity. But tackling the DE&I climate within a research team or institution requires an active stance from senior leadership and mentoring of staff from underrepresented groups, along with the promotion of ally behaviours amongst existing staff., As clinician–researchers, practical steps to promote diversity and inclusion in our workplaces include engaging in DE&I training, staying informed about research on organizational diversity and discrimination, endorsing ally behaviours (such as advocating for and attending DE&I events), reflecting on personal biases, and cultivating diverse social support.
Table 5 provides DE&I strategies and evidenced-based examples regarding the research team and environment.
Additional resources
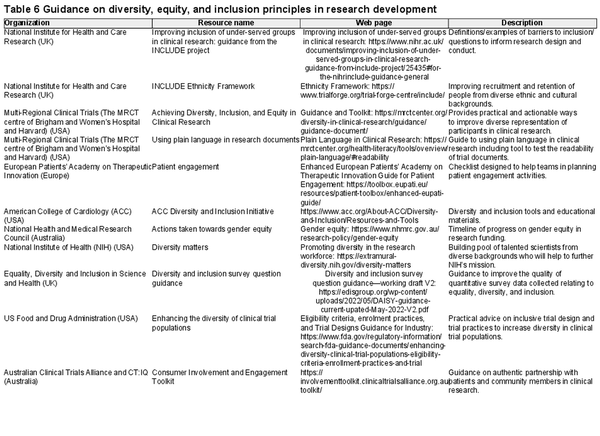
Table 6 provides a summary of available resources from international peak bodies and organisations that support a greater understanding and adoption of DE&I strategies in research. Box 2 provides a practical checklist researchers can use when designing and delivering inclusive research.
Box 2.Adapted from the National Institute for Health and Care Research, Guidance from the INCLUDE project (2020).
Checklist to Guide Research Teams in Designing and Delivering Inclusive Research:
What are the characteristics/demographics of the population which your research looks to serve?
How will your inclusion/exclusion criteria enable your trial population to match the population that you aim to serve?
Can you justify any difference between your projected trial population and the population you aim to serve?
How will your recruitment and retention methods engage with underrepresented groups?
What evidence have you that your intervention is feasible and accessible to a broad range of patients in the populations that your research seeks to serve?
Are your outcomes validated and relevant to a broad range of patients in the populations that your research seeks to serve?
Who are the underrepresented groups within your study catchment area? (e.g., geographical areas that the delivery team operates in)
What are the barriers to including these groups in research in our area?
What actions can we take to overcome those local barriers?
What tools, training and resources does your team need to implement these actions successfully?
Conclusion
Addressing persistent deficiencies in DE&I in CV research requires action across the entire research continuum, from the inception of study concepts to the dissemination of findings. In the pre-planning and study design phase, early engagement with patients and community stakeholders helps to ensure research objectives are aligned with real-world healthcare needs. During the execution of studies, meticulous attention to eligibility criteria, the inclusivity of study materials, and the implementation of targeted recruitment strategies, such as forging partnerships with community organizations and employing culturally tailored communication approaches, becomes pivotal. Finally, advocating for sufficient resources to implement DE&I practices, prioritizing funding for research initiatives explicitly dedicated to DE&I, and improving diversity in journal peer review processes and editorial boards will enhance the translation and impact of research outcomes. Underpinning success is also the cultivation of diverse and inclusive research teams and environments to support the effective recruitment and retention of underrepresented participants. In summary, the advancement of DE&I principles at every stage of the research cycle is a responsibility shared by researchers, institutions, and funding entities alike. This collective commitment is essential for substantial progress towards achieving equitable CVD health outcomes.
Acknowledgements
S.C.I. receives funding through a Heart Foundation Future Leader Fellowship by the Heart Foundation of Australia (Ref: 102821).
References
- 1. Versavel S, Subasinghe A, Johnson K, Golonski N, Muhlhausen J, Perry P, et al Diversity, equity, and inclusion in clinical trials: a practical guide from the perspective of a trial sponsor. Contemp Clin Trials 2023;126:107092.
- 2. Itchhaporia D. Paving the way for health equity in cardiology. J Am Coll Cardiol 2021;77:2613–2616.
- 3. Vilcant V, Ceron C, Verma G, Zeltser R, Makaryus AN. Inclusion of under-represented racial and ethnic groups in cardiovascular clinical trials. Heart Lung Circ 2022;31:1263–1268.
- 4. Tobb K, Kocher M, Bullock-Palmer RP. Underrepresentation of women in cardiovascular trials- it is time to shatter this glass ceiling. Am Heart J Plus 2022;13:100109.
- 5. Talevski J, Kulnik ST, Jessup RL, Falls R, Cvetanovska N, Beauchamp A. Use of co-design methodology in the development of cardiovascular disease secondary prevention interventions: a scoping review. Health Expect 2023;26:16–29.
- 6. Broadwin C, Azizi Z, Rodriguez F. Clinical trial technologies for improving equity and inclusion in cardiovascular clinical research. Cardiol Ther 2023;12:215–225.
- 7. Wu Y, Prasanna A, Miller HN, Ogungbe O, Peeler A, Juraschek SP, et al Female recruitment into cardiovascular disease trials. Am J Cardiol 2023;198:88–91.
- 8. Washington V, Franklin JB, Huang ES, Mega JL, Abernethy AP. Diversity, equity, and inclusion in clinical research: a path toward precision health for everyone. Clin Pharmacol Ther 2023;113:575–584.
- 9. Ortega RF, Yancy CW, Mehran R, Batchelor W. Overcoming lack of diversity in cardiovascular clinical trials. Circulation 2019;140:1690–1692.
- 10. Sardar MR, Badri M, Prince CT, Seltzer J, Kowey PR. Underrepresentation of women, elderly patients, and racial minorities in the randomized trials used for cardiovascular guidelines. JAMA Intern Med 2014;174:1868–1870.
- 11. Bhatt, D.L. Top Journal Scans and Clinical Trials, American College of Cardiology. 2022. https://www.acc.org/Latest-in-Cardiology/Articles/2022/12/15/01/2022-Top-Clinical-Trials-and-Journal-Scans (14 January 2024).
- 12. Steuernagel CR, Lam CSP, Greenhalgh T. Countering sex and gender bias in cardiovascular research requires more than equal recruitment and sex disaggregated analyses. BMJ 2023;382:e075031.
- 13. Filbey L, Zhu JW, D'Angelo F, Thabane L, Khan MS, Lewis E, et al Improving representativeness in trials: a call to action from the Global Cardiovascular Clinical Trialists Forum. Eur Heart J 2023;44:921–930.
- 14. Michos ED, Reddy TK, Gulati M, Brewer LC, Bond RM, Velarde GP, et al Improving the enrollment of women and racially/ethnically diverse populations in cardiovascular clinical trials: an ASPC practice statement. Am J Prev Cardiol 2021;8:100250.
- 15. Taylor AL, Wright JT. Importance of race/ethnicity in clinical trials. Circulation 2005;112:3654–3666.
- 16. Taylor AL, Ziesche S, Yancy C, Carson P, D'Agostino R Jr, Ferdinand K, et al Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004;351:2049–2057.
- 17. Regitz-Zagrosek V, Oertelt-Prigione S, Prescott E, Franconi F, Gerdts E, et alEUGenMed Cardiovascular Clinical Study Group Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J 2015;37:24–34.
- 18. Walsh MN. Gender diversity in cardiovascular clinical trial research begins at the top. J Am Coll Cardiol 2022;79:929–932.
- 19. Rai D, Kumar A, Waheed SH, Pandey R, Guerriero M, Kapoor A, et al Gender differences in international cardiology guideline authorship: a comparison of the US, Canadian, and European Cardiology Guidelines from 2006 to 2020. J Am Heart Assoc 2022;11:e024249.
- 20. Bhat V, Ozair A, Bellur S, Subhash NR, Kumar A, Majmundar M, et al Inequities in country- and gender-based authorship representation in cardiology-related Cochrane reviews. JACC Adv 2022;1:100140.
- 21. Wei S, Le N, Zhu JW, Breathett K, Greene SJ, Mamas MA, et al Factors associated with racial and ethnic diversity among heart failure trial participants: a systematic bibliometric review. Circ Heart Fail 2022;15:e008685.
- 22. Greenhalgh T, Hinton L, Finlay T, Macfarlane A, Fahy N, Clyde B, et al Frameworks for supporting patient and public involvement in research: systematic review and co-design pilot. Health Expect 2019;22:785–801.
- 23. Bonner C, Fajardo MA, Doust J, McCaffery K, Trevena L. Implementing cardiovascular disease prevention guidelines to translate evidence-based medicine and shared decision making into general practice: theory-based intervention development, qualitative piloting and quantitative feasibility. Implement Sci 2019;14:86.
- 24. Walsh DMJ, Moran K, Cornelissen V, Buys R, Claes J, Zampognaro P, et al The development and codesign of the PATHway intervention: a theory-driven eHealth platform for the self-management of cardiovascular disease. Transl Behav Med 2019;9:76–98.
- 25. Raynor DK, Ismail H, Blenkinsopp A, Fylan B, Armitage G, Silcock J. Experience-based co-design—adapting the method for a researcher-initiated study in a multi-site setting. Health Expect 2020;23:562–570.
- 26. Nesbitt K, Beleigoli A, Du H, Tirimacco R, Clark RA. Web-based cardiac rehabilitation: A co-design workshop. In: Maeder AJ, Higa C, van den Berg MEL, Gough C, editors. Telehealth Innovations in Remote Healthcare Services Delivery - Global Telehealth 2020: Global Telehealth 2020. Amsterdam: IOS Press. 2021. p. 96–105. (Studies in Health Technology and Informatics). doi: .
- 27. Aaby A, Simonsen CB, Ryom K, Maindal HT. Improving organizational health literacy responsiveness in cardiac rehabilitation using a co-design methodology: results from the Heart Skills Study. Int J Environ Res Public Health 2020;17:1015.
- 28. Ramage ER, Burke M, Galloway M, Graham ID, Janssen H, Marsden DL, et al Fit for purpose. Co-production of complex behavioural interventions. A practical guide and exemplar of co-producing a telehealth-delivered exercise intervention for people with stroke. Health Res Policy Syst 2022;20:2.
- 29. Chauhan A, Leefe J, Shé ÉN, Harrison R. Optimising co-design with ethnic minority consumers. Int J Equity Health 2021;20:240.
- 30. Sacristán JA, Aguarón A, Avendaño-Solá C, Garrido P, Carrión J, Gutiérrez A, et al Patient involvement in clinical research: why, when, and how. Patient Prefer Adherence 2016;10:631–640.
- 31. Coulter K, Ingram M, McClelland DJ, Lohr A. Positionality of community health workers on health intervention research teams: a scoping review. Front Public Health 2020;8:208.
- 32. Symons T, Bowden J, McKenzie A, Fallon-Ferguson J, Weekes L, Ansell J, et al Development of the Consumer Involvement Engagement Toolkit: a digital resource to build capacity for undertaking patient-centred clinical trials in Australia. Public Health Res Pract 2023;33:32122209.
- 33. Bodicoat DH, Routen AC, Willis A, Ekezie W, Gillies C, Lawson C, et al Promoting inclusion in clinical trials—a rapid review of the literature and recommendations for action. Trials 2021;22:880.
- 34. Ezeugwu CO, Laird A, Mullins CD, Saluja DS, Winston RA. Lessons learned from community-based minority health care serving system participation in an NIH clinical trial. J Natl Med Assoc 2011;103:839–844.
- 35. Jamal Z, Perkins A, Allen C, Evans R, Sturgess J, Snowdon C, et al Patient and public involvement prior to trial initiation: lessons learnt for rapid partnership in the COVID-19 era. Res Involv Engagem 2021;7:13.
- 36. Fedorowicz S, Riley V, Cowap L, Ellis NJ, Chambers R, Grogan S, et al Using social media for patient and public involvement and engagement in health research: the process and impact of a closed Facebook group. Health Expect 2022;25:2786–2795.
- 37. Liu X, Ghisi GLM, Meng S, Grace SL, Shi W, Zhang L, et al Establishing a process to translate and adapt health education materials for natives and immigrants: the case of Mandarin adaptations of cardiac rehabilitation education. Heart Lung 2021;50:794–817.
- 38. Cho L, Vest AR, O’Donoghue ML, Ogunniyi MO, Sarma AA, Denby KJ, et al Increasing participation of women in cardiovascular trials: JACC council perspectives. J Am Coll Cardiol 2021;78:737–751.
- 39. McMurray JJV, Jackson AM, Lam CSP, Redfield MM, Anand IS, Ge J, et al Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction. Circulation 2020;141:338–351.
- 40. Stefanini GG, Baber U, Windecker S, Morice M-C, Sartori S, Leon MB, et al Safety and efficacy of drug-eluting stents in women: a patient-level pooled analysis of randomised trials. Lancet 2013;382:1879–1888.
- 41. Batchelor W, Kandzari DE, Davis S, Tami L, Wang JC, Othman I, et al Outcomes in women and minorities compared with white men 1 year after everolimus-eluting stent implantation. JAMA Cardiology 2017;2:1303–1313.
- 42. Tan MH, Thomas M, MacEachern MP. Using registries to recruit subjects for clinical trials. Contemp Clin Trials 2015;41:31–38.
- 43. Fox KAA, Accetta G, Pieper KS, Bassand J-P, Camm AJ, Fitzmaurice DA, et al Why are outcomes different for registry patients enrolled prospectively and retrospectively? Insights from the global anticoagulant registry in the FIELD-Atrial Fibrillation (GARFIELD-AF). Eur Heart J Qual Care Clin Outcomes 2017;4:27–35.
- 44. Grecu M, Blomström-Lundqvist C, Kautzner J, Laroche C, Van Gelder IC, Jordaens L, et al In-hospital and 12-month follow-up outcome from the ESC-EORP EHRA atrial fibrillation ablation long-term registry: sex differences. Europace 2020;22:66–73.
- 45. Dawson S, Banister K, Biggs K, Cotton S, Devane D, Gardner H, et al Trial forge guidance 3: randomised trials and how to recruit and retain individuals from ethnic minority groups—practical guidance to support better practice. Trials 2022;23:672.
- 46. Nieuwkerk ACV, Delewi R, Wolters FJ, Muller M, Daemen M, Biessels GJ. Cognitive impairment in patients with cardiac disease: implications for clinical practice. Stroke 2023;54:2181–2191.
- 47. Gurwitz JH. The exclusion of older people from participation in cardiovascular trials. AMA J Ethics 2014;16:365–368.
- 48. Ramsey TM, Snyder JK, Lovato LC, Roumie CL, Glasser SP, Cosgrove NM, et al Recruitment strategies and challenges in a large intervention trial: Systolic Blood Pressure Intervention Trial. Clin Trials 2016;13:319–330.
- 49. Arigo D, Pagoto S, Carter-Harris L, Lillie SE, Nebeker C. Using social media for health research: methodological and ethical considerations for recruitment and intervention delivery. Digit Health 2018;4:2055207618771757.
- 50. Cortés YI, Duran M, Marginean V, Harris LK, Cazales A, Santiago L, et al Lessons learned in clinical research recruitment of midlife Latinas during COVID-19. Menopause 2022;29:883–888.
- 51. Cunningham-Erves J, Kusnoor SV, Villalta-Gil V, Stallings SC, Ichimura JS, Israel TL, et al Development and pilot implementation of guidelines for culturally tailored research recruitment materials for African Americans and Latinos. BMC Med Res Methodol 2022;22:248.
- 52. Langer SL, Castro FG, Chen ACC, Davis KC, Joseph RP, Kim W, et al Recruitment and retention of underrepresented and vulnerable populations to research. Public Health Nurs 2021;38:1102–1115.
- 53. Koydemir S, Essau CA, Hodes M, Gau SS-F, De Vries PJ. Chapter 5 - Anxiety and anxiety disorders in young people: A Cross-cultural perspective. In: Hodes M, Gau SS-F, De Vries PJ, editors. Understanding uniqueness and diversity in child and adolescent mental health. Online: Academic Press; 2018. p115–134.
- 54. Brewer LC, Jenkins S, Hayes SN, Kumbamu A, Jones C, Burke LE, et al Community-based, cluster-randomized pilot trial of a cardiovascular mobile health intervention: preliminary findings of the FAITH! trial. Circulation 2022;146:175–190.
- 55. Zhu JW, D'Angelo F, Miranda JJ, Yancy CW, Cupido B, Zannad F, et al Incorporating cultural competence and cultural humility in cardiovascular clinical trials to increase diversity among participants. J Am Coll Cardiol 2022;80:89–92.
- 56. Rigatti M, DeGurian AA, Albert SM. “Getting there”: transportation as a barrier to research participation among older adults. J Appl Gerontol 2022;41:1321–1328.
- 57. Ryder C, Mackean T, Coombs J, Williams H, Hunter K, Holland AJA, et al Indigenous research methodology—weaving a research interface. Inter J Soc Res Methodol 2020;23:255–267.
- 58. Simonds VW, Christopher S. Adapting Western research methods to indigenous ways of knowing. Am J Public Health 2013;103:2185–2192.
- 59. Thambinathan V, Kinsella EA. Decolonizing methodologies in qualitative research: creating spaces for transformative praxis. Int J Qual Methods 2021;20:160940692110147.
- 60. Wali S, Seidel J, Spence G, Innes L, Innes E, Simard A, et al Heart health begins with community: community-based research exploring innovative strategies to support First Nations heart health. CJC Open 2023;5:661–670.
- 61. Jones DA, Andiapen M, Van-Eijl TJA, Webb AJ, Antoniou S, Schilling RJ, et al The safety and efficacy of intracoronary nitrite infusion during acute myocardial infarction (NITRITE-AMI): study protocol of a randomised controlled trial. BMJ Open 2013;3:e002813.
- 62. Sylliboy JR, Latimer M, Marshall EA, MacLeod E. Communities take the lead: exploring indigenous health research practices through two-eyed seeing & kinship. Int J Circumpolar Health 2021;80:1929755.
- 63. Ozolins U, Hale S, Cheng X, Hyatt A, Schofield P. Translation and back-translation methodology in health research—a critique. Expert Rev Pharmacoecon Outcomes Res 2020;20:69–77.
- 64. Banack HR, Harper S, Kaufman JS. Accounting for selection bias in studies of acute cardiac events. Can J Cardiol 2018;34:709–716.
- 65. Au M, Whitelaw S, Khan MS, Mamas MA, Mbuagbaw L, Mulvagh SL, et al A systematic review of sex-specific reporting in heart failure clinical trials. JACC Adv 2022;1:100079.
- 66. Allana S, Ski CF, Thompson DR, Clark AM. Bringing intersectionality to cardiovascular health research in Canada. CJC Open 2021;3:S4–S8.
- 67. Surikova J, Payne A, Miller K-L, Ravaei A, Nolan RP. A cultural and gender-based approach to understanding patient adjustment to chronic heart failure. Health Qual Life Outcomes 2020;18:238.
- 68. Streed CG, Beach LB, Caceres BA, Dowshen NL, Moreau KL, Mukherjee M, et al Assessing and addressing cardiovascular health in people who are transgender and gender diverse: a scientific statement from the American Heart Association. Circulation 2021;144:e136–e148.
- 69. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 2013;4:863.
- 70. Dewidar O, Podinic I, Barbeau V, Patel D, Antequera A, Birnie D, et al Integrating sex and gender in studies of cardiac resynchronization therapy: a systematic review. ESC Heart Fail 2022;9:420–427.
- 71. Shalit A, Vallely L, Nguyen R, Bohren M, Wilson A, Homer CS, et al The representation of women on Australian Clinical Practice Guideline panels, 2010–2020. Med J Aust 2023;218:84–88.
- 72. Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med 2011;104:510–520.
- 73. McDavitt B, Bogart LM, Mutchler MG, Wagner GJ, Green HD, Lawrence SJ, et al Dissemination as dialogue: building trust and sharing research findings through community engagement. Prev Chronic Dis 2016;13:E38.
- 74. Sriram U, Sandreuter K, Graham M, Folta S, Pullyblank K, Paul L, et al Process evaluation of strong hearts, healthy communities: a rural community-based cardiovascular disease prevention program. J Nutr Educ Behav 2019;51:138–149.
- 75. Haynes N, Kaur A, Swain J, Joseph JJ, Brewer LC. Community-based participatory research to improve cardiovascular health among US racial and ethnic minority groups. Curr Epidemiol Rep 2022;9:212–221.
- 76. Vervoort D, Luc JGY, Sá MPBO, Etchill EW. Open access and article processing charges in cardiology and cardiac surgery journals: a cross-sectional analysis. Braz J Cardiovasc Surg 2021;36:453–460.
- 77. Capers Q, Johnson A, Berlacher K, Douglas PS. The urgent and ongoing need for diversity, inclusion, and equity in the cardiology workforce in the United States. J Am Heart Assoc 2021;10:e018893.
- 78. Chapman N, Thomas EE, Tan JTM, Inglis SC, Wu JHY, Climie RE, et al A roadmap of strategies to support cardiovascular researchers: from policy to practice. Nat Rev Cardiol 2022;19:765–777.
- 79. Ahmad AS, Sabat I, Trump-Steele R, King E. Evidence-based strategies for improving diversity and inclusion in undergraduate research labs. Front Psychol 2019;10:1305.
- 80. Selak V, Crengle S, Elley CR, Wadham A, Harwood M, Rafter N, et al Recruiting equal numbers of indigenous and non-indigenous participants to a ‘polypill’ randomized trial. Int J Equity Health 2013;12:44.
- 81. National Institute for Health and Care Research. NIHR (2020) improving inclusion of under-served groups in clinical research: guidance from the NIHR-INCLUDE project. UK: NIHR. UK: National Institute for Health and Care Research; 2020.