An estimated 4.1% of the Western population develops stable angina pectoris (SAP) during life, which is currently treated by optimal medical therapy, often followed by revascularization, performed percutaneously [i.e. percutaneous coronary intervention (PCI)] or surgically [i.e. coronary artery bypass grafting (CABG)]. However, recent studies found no superiority of revascularization in SAP patients without left-main stenosis on 1- and 2-year prognoses compared to optimal medical therapy., A potential alternative, cardiac rehabilitation (CR), demonstrated fewer symptoms and adverse advents in previous small randomized controlled trials, whilst a recent cohort study showed lower 18-month mortality, rehospitalization, and cardiovascular morbidity compared to PCI. More research is required to gain insight into clinical outcomes of CR, but also whether CR represents a cost-effective alternative to revascularization given the lower costs of CR, and large proportion of patients with SAP undergoing PCI., Therefore, we compared the effects of CR vs. PCI on major adverse cardiovascular events (MACE), new revascularizations, chest pain, and associated healthcare costs over a period of 24 months in patients with SAP.
We conducted a retrospective cohort study utilizing the database of a Dutch health insurance company [Coöperatie Volksgezondheidzorg (VGZ); >4.2 million individuals]. Patients treated with CR (SAP + CR) or PCI (SAP + PCI) within 6 months of diagnosis were eligible. Patients with both CR and PCI were excluded. Eligibility was determined using health insurance claims data, enrolling patients with claims on diagnosis-treatment combination ‘Stable angina pectoris’ (3200202) and treated with PCI or CR, identified with Dutch healthcare product codes (see Supplementary material online, S1A and B). Patients < 18 years, CABG as first treatment, unstable angina pectoris or myocardial infarction between SAP and treatment initiation, or CR without supervised exercise therapy were excluded. We followed the ‘Strengthening the Reporting of Observational studies in Epidemiology’ (STROBE)-reporting guideline and were exempted from informed consent by the Dutch Central Committee on Research Involving Human Subjects, because it involved retrospective analyses of an anonymized dataset.
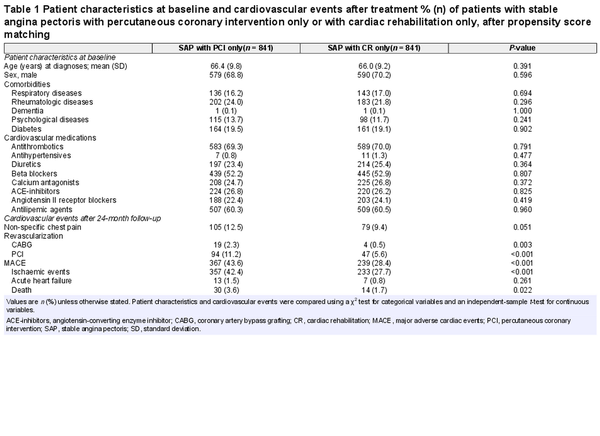
Data were collected from the moment of diagnosis, between 1 January 2013 and 30 June 2018, with 24-month follow-up, to refrain from the COVID period and use a fixed timeframe for cost analysis. Patient characteristics included age, sex, comorbidities, and cardiac medications, specified in Supplementary material online, S2. Cardiovascular events consisting of new revascularizations (elective/acute PCI/CABG), non-specific chest pain, and MACE (i.e. ischaemic events/acute heart failure/mortality) were collected using Dutch healthcare product codes (see Supplementary material online, S1C). Initial treatment (PCI/CR) and cardiovascular events within 24-month follow-up were linked to healthcare costs based on 2019, using ‘OpenDisData’ of the Dutch healthcare authority (see Supplementary material online, S3).
Analyses were performed using R Studio (2022.02.1) and various packages (see Supplementary material online, S4). Characteristics were presented as numbers (%) (categorical variables) or mean ± SD (continuous variables). To create comparable groups, we used 1:1 propensity score matching (PSM), using nearest-neighbour matching with a 0.1 pooled-SD calliper and logistic regression with the variables age, sex, respiratory diseases, dementia, diabetes, and (cardiac-related) medications. Patient characteristics and cardiovascular events were compared between groups with a χ2 test (categorical) or independent t-test (continuous). Kaplan–Meier test was used to assess survival, including log-rank test for comparing differences between groups. Hazard ratios (HR) were calculated using Cox-regression analysis. Costs were described as average, 95% confidence interval (CI) and compared with Mann–Whitney U tests. Significance was set at P < 0.05.
After exclusion of 1501 patients treated with PCI and CR, the cohort consisted of 4724 patients [SAP + PCI: n = 3883 (82.2%), SAP + CR: n = 841 (17.8%)]. Following PSM, 1682 (35.6%) patients were included in the analysis, all of whom completed 24-month follow-up and showed no differences between groups (Table 1).
Significantly fewer new PCIs (HR: 0.71, 95% CI: 0.79–1.01, Supplementary material online, S5) and CABGs (HR: 0.18, 95% CI: 0.04–0.81) were performed in SAP + CR compared to SAP + PCI. No statistical difference in non-specific chest pain prevalence was found (HR: 0.80, 95% CI: 0.60–1.08). SAP + CR had lower risks for MACE overall (HR: 0.54, 95% CI: 0.46–0.64, Table 1), including lower risk of ischaemic events (HR: 0.51, 95% CI: 0.43–0.61) and mortality (HR: 0.76, 95% CI: 0.40–1.45), and additionally better event-free survival compared to SAP + PCI (P < 0.001, Figure 1A).

Figure 1
Survival curve and average healthcare costs of patients with stable angina pectoris with treatment of percutaneous coronary intervention (PCI) or cardiac rehabilitation (CR). (A) Kaplan–Meier curve for overall event-free survival of MACE in a 24-month follow-up in patients with stable angina pectoris with treatment of PCI or a treatment of CR. (B) Average healthcare costs of both stable angina pectoris treatment and cardiovascular events within a 24-month follow-up after diagnosis in patients with stable angina pectoris with treatment of PCI or a treatment of CR, in euros, per patient.
Average treatment costs for PCI (€5686, 95% CI: €5530–€5841) were significantly higher compared to CR (€3520, 95% CI: €3436–€3604, P < 0.001). Per patient, cardiovascular event costs were significantly higher in SAP + PCI (€1700, 95% CI: €1459–€1940) compared with SAP + CR (€947, 95% CI: €724–€1170, P < 0.001), as well as total costs (SAP + PCI: €7385, 95% CI: €7067–€7704; SAP + CR: €4468, 95% CI: €4221–€4715, P < 0.001, Figure 1B), leading to €2918 (95% CI: €2517–€3319) higher costs for SAP + PCI per patient vs. SAP + CR.
In patients with SAP, we found that CR is associated with lower risks for MACE compared with PCI. This reinforces the association between CR and lower all-cause mortality, rehospitalization, and cardiovascular morbidity compared to revascularization., Importantly, these previous studies were either underpowered and performed in a different era (20 years ago) or contained significant variation. The inclusion of a large sample with real-world data provides support for superiority of CR to PCI in SAP patients leading to fewer MACE across 24-month follow-up. Regarding the expected a priori lower costs for CR combined with lower risks for MACE during follow-up, CR saved average costs of €2918 per individual. Since 15 369 elective PCIs were performed in the Netherlands in 2021, our observation could translate into savings up to €44 million/year in the Netherlands when CR replaces PCI as first-line treatment in patients with SAP.
Some limitations must be highlighted. Although we found no differences between PSM-matched groups, we cannot exclude potential presence of residual confounding by indication and selection bias, leading to distinct groups for disease severity and/or complaints. Despite extensive correction, some factors, like potential presence of left-main stenosis, could not be fully corrected, which may result in overestimating the benefit of CR on MACE risks. Furthermore, the healthcare cost-calculations may underestimate the actual cost savings and may have been conservative towards CR, as we focused on direct healthcare costs only. We did not account for other healthcare (e.g. ambulance) and/or socio-economic costs associated with poorer health status and/or loss of (work-related) productivity. The latter seems relevant as a previous meta-analysis reported better return-to-work following CR (66%) vs. usual care (58%).
In conclusion, CR has been linked to significantly fewer MACE and reduced healthcare costs compared to PCI across 24-month follow-up in patients with SAP. These observations have potential clinical and socio-economic impact, as it suggests that CR may be preferred as first-line treatment over PCI in patients with SAP.
Acknowledgements
The authors would like to thank VGZ for providing the data.
References
- 1. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al Heart Disease and Stroke Statistics—2021 update: a report from the American Heart Association. Circulation2021;143:e254–e743.
- 2. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O'Brien SM, Boden WE, et al Initial invasive or conservative strategy for stable coronary disease. N Engl J Med2020;382:1395–1407.
- 3. Stergiopoulos K, Boden WE, Hartigan P, Möbius-Winkler S, Hambrecht R, Hueb W, et al Percutaneous coronary intervention outcomes in patients with stable obstructive coronary artery disease and myocardial ischemia: a collaborative meta-analysis of contemporary randomized clinical trials. JAMA Intern Med2014;174:232–240.
- 4. Mobius-Winkler S, Uhlemann M, Adams V, Sandri M, Erbs S, Lenk K, et al Coronary collateral growth induced by physical exercise: results of the impact of intensive exercise training on coronary collateral circulation in patients with stable coronary artery disease (EXCITE) trial. Circulation2016;133:1438–1448; discussion 1448.
- 5. Hambrecht R, Walther C, Mobius-Winkler S, Gielen S, Linke A, Conradi K, et al Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation2004;109:1371–1378.
- 6. Buckley BJR, de Koning IA, Harrison SL, Fazio-Eynullayeva E, Underhill P, Kemps HMC, et al Exercise-based cardiac rehabilitation vs. percutaneous coronary intervention for chronic coronary syndrome: impact on morbidity and mortality. Eur J Prev Cardiol2022;29:1074–1080.
- 7. Fearon WF, Nishi T, De Bruyne B, Boothroyd DB, Barbato E, Tonino P, et al Clinical outcomes and cost-effectiveness of fractional flow reserve-guided percutaneous coronary intervention in patients with stable coronary artery disease: three-year follow-up of the FAME 2 trial (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation). Circulation2018;137:480–487.
- 8. Nederlandse Zorgauthoriteit (NZa). Zorgproductviewer. https://zorgproducten.nza.nl/ZorgproductViewer.aspx?psId=16&zpId=92481# (27 September 2023). 2023.
- 9. van Engen-Verheul M, de Vries H, Kemps H, Kraaijenhagen R, de Keizer N, Peek N. Cardiac rehabilitation uptake and its determinants in the Netherlands. Eur J Prev Cardiol2013;20:349–356.
- 10. Nederlandse Hart Registratie. Jaarcijfers coronairlijden. https://www.hartenvaatcijfers.nl/analysetool/pci-645cc (27 September 2023). 2023.
- 11. Nederlandse Zorgauthoriteit (NZa). Open data van de Nederlandse Zorgauthoriteit. https://www.opendisdata.nl/ (23 August 2023). 2023.
- 12. Sadeghi M, Rahiminam H, Amerizadeh A, Masoumi G, Heidari R, Shahabi J, et al Prevalence of return to work in cardiovascular patients after cardiac rehabilitation: a systematic review and meta-analysis. Curr Probl Cardiol2022;47:100876.