Introduction
Allergic immunotherapy is the only treatment modality to modify immune system for relieving allergic symptoms in patients with allergic rhinitis. Immunotherapy is a therapeutic method that induces desensitization to an allergic antigen and changes the allergic immune response. However, the subcutaneous immunotherapy (SCIT) is invasive, inconvenient, and can cause severe systemic side effects. In recent studies, sublingual immunotherapy (SLIT) has been reported as the first alternative to SCIT or pharmacotherapy. However, although SLIT has been shown to be effective in patients with allergy, there is no established treatment dose, duration of treatment, or parameters to identify treatment effects. And there were no reports about the comparison of clinical outcomes between the common used SLIT medications.
A multicenter SLIT study in children with allergic rhinitis (AR) has shown significantly improved clinical outcome in comparison with children treated with symptomatic drugs only. Sublingual immunotherapy for 2 years in patients with AR has shown lower symptom score, medication score, incidence of asthma, and new sensitization in comparison with controls. Symptoms of rhinitis and asthma in pediatric patients sensitization to HDM, at least 2 years follow-up after SLIT, were improved in both patients, and symptomatic medication was also decreased.
Recently, there are some reports that the effect of SLIT has appeared early at 6 months after starting the immunotherapy. Sublingual immunotherapy treatment with standardized extract of HDM for 1 year showed that total symptom scores (TSSs) and anti-allergic medication scores improved both in younger age-group and adult group, and the difference was not significant. Allergic symptoms significantly decreased after 1 year of SLIT treatment, and this effect was sustained after 2 or 3 years of treatment.
Allergoids are chemically modified allergens with natural allergen by carbamylation. The carbamylated monomeric allergoid tablets has reduced the immunoglobulin E (IgE) reactivity, yet maintaining the immunogenicity. Based on their small molecule size, they can be easily absorbed via the oral mucosa and suitable for SLIT. There was a comparison of biological activity of the sublingual allergens.
In Korea, we have used 3 different types of SLIT products. However, there were no reports about the clinical outcomes between the allergoid type and natural allergen type. In this study, we want to compare the clinical outcomes using 2 kinds of SLIT medication after 1 year treatment.
Patients and Methods
Study Design
Two hundred ninety-three allergic rhinitis patients for house dust mites (HDM) were enrolled for SLIT according to the general World Health Organization criteria. We have evaluated the patients’ characteristics, safety, and compliance in 293 patients who have started the SLIT. And then we have also analyzed the change in symptom score, medication score, satisfaction rate, and immunologic measurement in 84 patients who have continued the treatment over 1 year.
A written informed consent was obtained from all patients or their parents. The institutional review board of Gachon University Gil Medical Center (GIRBA No. 2324) approved the study protocol.
Patients
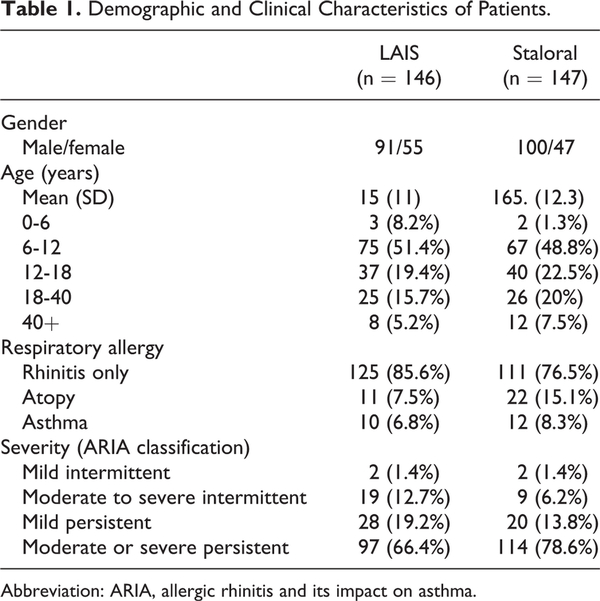
The patients with allergic rhinitis who had a positive skin prick test (SPT) with Dermatophagoides pteronyssinus and Dermatophagoides farinae were included in this study. Skin tests were performed using a panel of standardized allergen extracts (Allergopharma, Darmstadt, Germany). An HDM allergy was defined positive when the wheal diameters for D pteronyssinus and D farinae were equal to or greater than that of the positive control (histamine) on SPTs. The serum sIgE response to D pteronyssinus and D farinae was measured using (multiple allergen simultaneous tests and when the serum-specific IgE level was > 2+ for D pteronyssinus and D farinae, results were considered as positive. The patients who had history of asthma, atopic dermatitis, but who didn’t need regular medication were enrolled, while the patients with symptomatic asthma, atopic dermatitis that required regular medication such as oral steroid, steroid inhaler, or antihistamine, were excluded.
Sublingual Immunotherapy
Two hundred ninety-three allergic rhinitis patients for HDM were followed up with 2 kinds of SLIT according to the manufacture’s schedule. In 84 patients who underwent treatment over 1 year, 45 patients were administered with LAIS (Lofarma SpA, Milan, Italy) and 39 patients were administered with Staloral (Stallergenes, Antony, France). LAIS is the carbamylated monomeric allergoid tablet which contains a 50/50 mixture of D pteronyssinus and D farinae extracts and Staloral is composed with standardized extract of HDM D pteronyssinus 50% + D farinae 50%. During the treatment period, the patients were allowed to take rescue medication according to their needs.
Clinical Efficacy
Symptom Score
Patients were instructed to complete their diary cards of symptom scores throughout 1 year. The symptom scores were checked by 5 symptoms, such as rhinorrhea, sneezing, nasal obstruction, itching, and eye discomfort. Each symptoms were recorded on a 4-point scale (0 = no symptom, 1 = mild, 2 = moderate, and 3 = severe), and TSSs are defined as the sum of the scores of the 5 symptoms. Based on this score, we compared the scores at the starting and at the end of treatment. We also compared the symptom improvement rate between 2 medications, much improvement (75% reduction), improvement (50%-75% reduction), little improvement (25%-50% reduction), and similar (0%-25% reduction) according to the improvement rate.
Medication Score
The patients recorded the weekly medication score on the diary card depending on the types of medication (1: antihistamine, 2: intranasal corticosteroid). Total medication scores were defined as the sum of monthly medication use and we compared the total sum of the first month at the beginning and the last month at the end of 1 year. We also compared the improvement rate according to the frequency of medication use: decrease (more than 50% reduction), similar (25%-50% reduction), and no change (0%-25%).
Subjective Improvement and Satisfaction by Questionnaire
The patients were asked about their subjective improvement rate according to a questionnaire classified into 3 categories: much improvement (over 50%), little improvement (25%-50%), and similar (0%-25%). Based on this score change, we compared the subjective improvement rate before starting and at the end of treatment. The patient satisfaction rate were also assessed by questionnaire classified into 3 categories: dissatisfaction (0%-25%), a little satisfaction (25%-50%), and much satisfaction (over 50%) according to their satisfaction rate.
Immunological Measurements
We measured the serum level of specific IgE, and immunoglobulin G4 (IgG4) antibodies for D pteronyssinus and D farinae by ImmunoCAP® (Thermo Scientific, Uppsala, Sweden) at start of treatment and the end of the study.
Assessment of Compliance and Adverse Side Effects
The compliance rate for SLIT after starting the SLIT was monitored for 1 year. The patients who were dropped out during the treatment period were followed up and searched the reasons for discontinuing the SLIT. We have also analyzed the causes of stopping the medication. The participants recorded the adverse reactions after taking the SLIT during the whole treatment period.
Statistical Analysis
SPSS 16.0 (2007; SPSS Inc, Chicago, Illinois) was used. Wilcoxon signed-rank test was used for analysis. A P value <.05 were deemed to indicate statistical significance.
Results
Patients Characteristics
The demographic and clinical characteristics of these 293 patients who took part in this study are summarized in Table 1. There were no specific difference in age, sex distribution, combined respiratory allergy, and type of allergic rhinitis and its impact on asthma (ARIA) in 2 treatment groups. The 2 populations are comparable in study design. In the analysis of the age at which treatment was started, 6 to 12 years old group was the most common. Patient distribution according to ARIA classification showed moderate to severe persistent group accounted for the majority. Analysis of the associated allergic diseases showed that 85.6% in LAIS and 76.5% in Staloral of the patients had no associated allergic diseases (Table 1). Of 293 patients, a total of 84 patients (LAIS: n = 45, Staloral: n = 39) who have treated for at least 1 year were enrolled for the further analysis (Figure 1).
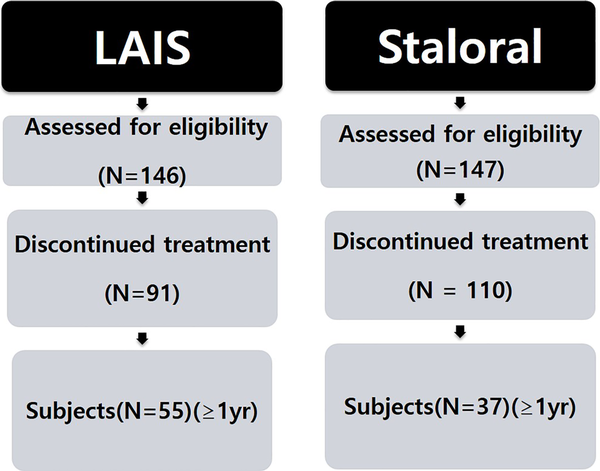
Figure 1
Of 294 patients, a total of 84 patients (LAIS: n = 45, Staroral: n = 39) who have treated for at least 1 year were enrolled for the further analysis.
Clinical Symptoms Score
The TSSs at the end were significantly improved compared to the initial score, respectively, in both treatment groups (P < .05). There was no significant difference in improving rate between the groups (Figure 2A). The TSS decreased by 51% (5.97 ± 0.31 vs 2.80 ± 0.20) in the LAIS group (Figure 2B) and 44% (7.68 ± 0.33 vs. 4.10 ± 0.28) in the Staloral group (Figure 2C). The percentage of patients showing a beneficial effect (improvement plus much improvement) in symptom score was 68.9% (31/45 patients) versus 53.9% (21/39 patient) in LAIS group and Staloral treatment group, respectively (Figure 2D).
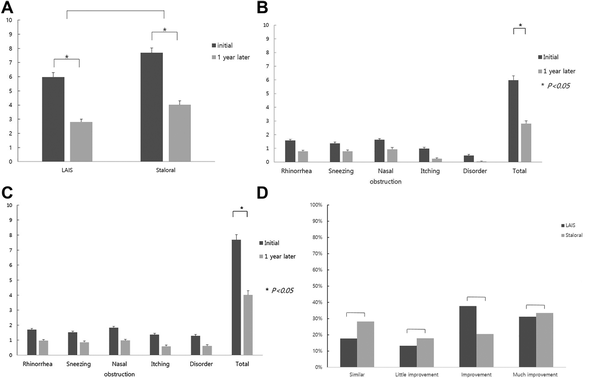
Figure 2
Symptom score change over 1 year (LAIS = 45, Staroral = 39). A, The total symptom scores (TSSs) at the end were significantly different compared to the start score in both groups. B, Symptom score change in LAIS treatment group. C, Symptom score change in Staloral treatment group. D, Symptom improvement rate between 2 medications. Improvement rate of sublingual immunotherapy (SLIT). Much improvement (75% reduction), improvement (50%-75% reduction), little improvement (25%-50% reduction), similar (0%-25% reduction) according to the improvement rate.
Medication Score
The total medication score was significantly decreased in both treatment groups compared to initial scores (P < .05). The medication score in Staloral group was more decreased 60% (1.03 ± 0.11 to 0.41 ± 0.07) compared to LAIS group 50.8% (1.18 ± 0.08 to 0.58 ± 0.12) at 1 year. However, there is no statistical difference between 2 groups (Figure 3A). Additionally, the percentage of patients showing a decrease in medication score was 82.2% (37/45 patient) in LAIS group versus 64.1% (25/39 patients) in Staloral group, respectively. However, there is no statistical difference between 2 groups (Figure 3B).
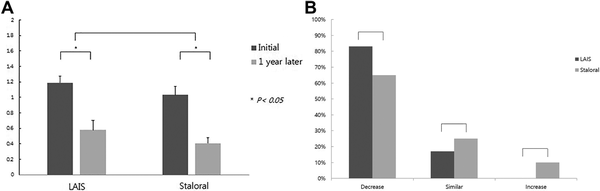
Figure 3
Medication frequency change over 1 year (LAIS = 45 vs Staroral = 39). A, The total medication score was significantly decreased in both treatment groups compared to initial scores (P < .05). B, The medication score in Staroral group was more decreased than LAIS group.
Subjective Symptom Improvement and Satisfaction
The percentage of patients showing a much improvement in subjective improvement score was 44.4% (20/45 patient) in LAIS group versus 46.1% (18/39 patients) in Staloral group, respectively, at the end of treatment. There was no significant difference between 2 groups (Figure 4A).
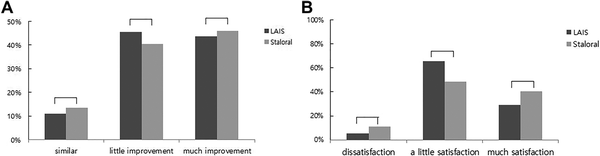
Figure 4
Subjective improvement and satisfaction by questionnaire at 1 year (LAIS = 45 vs Staroral = 39). A, Subjective improvement test by questionnaire shows no significant difference between 2 groups. B, Satisfaction by questionnaire at 1 year shows no significant difference between 2 groups. Satisfaction rate by questionnaire shows no significant difference between 2 groups.
The percentage of patients showing much satisfied in satisfaction rate was 29% (13/45 patient) in LAIS group versus 40% (16/39 patients) in Staloral group, respectively, at the end of treatment. There was no significant difference between 2 groups. The LAIS treatment group showed a significant difference (65% vs 49%) in the little satisfaction (25%-50%) group (Figure 4B).
Immunologic Parameters
The total serum level of specific IgE, for D pteronyssinus and D farinae at the end was not significantly changed compared to the initial level, respectively, in both treatment groups (Figure 5A). However, the serum IgG4 level for D pteronyssinus and D farinae of Staloral group (0.30 ± 0.05 vs 0.92 ± 0.25 and 0.30 ± 0.05 vs 1.21 ± 0.28) was significantly increased compared to LAIS group (0.25 ± 0.03 vs 0.23 ± 0.02 and 0.30 ± 0.05 vs 0.25 ± 0.03; Figure 5B).
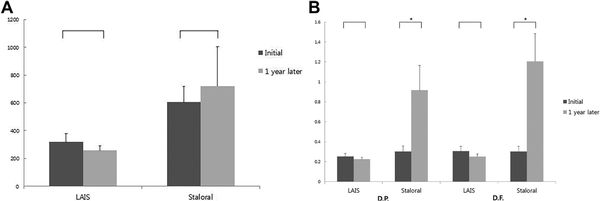
Figure 5
Measurement of immunoglobulin E (IgE), immunoglobulin G4 (IgG4) levels after 1 year treatment (LAIS = 45 vs Staloral = 39). A, IgE, (B) IgG4 for Dermatophagoides pteronyssinus and Dermatophagoides farinae.
Safety
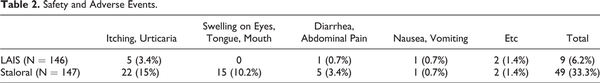
The incidence of adverse events during treatment was 6.2% in LAIS and 33.3% in Staloral, there was a significant difference between 2 groups (P < .05). The most common adverse events in both groups are itching and urticaria. All side effects were mild such as itching, swelling of the eyes, tongue and mouth, mild abdominal pain, and vomiting, and there were no serious side effects such as systemic anaphylaxis (Table 2).
Compliance
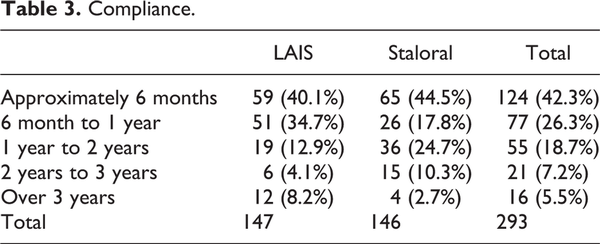
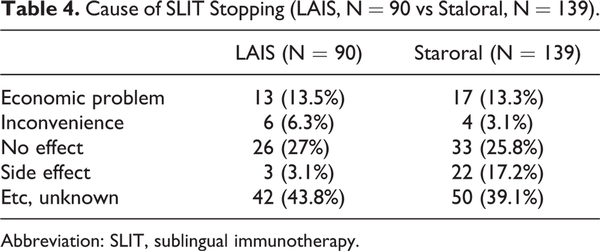
The maintenance rate over 1 year is 37.7% in LAIS and 25.1% in Staloral treatment groups. There was no significant difference between 2 groups (Table 3). The causes of stopping the treatment were no effect, economic problem, inconvenience, and side effect (Table 4).
Discussion
Our study has shown that SLIT has improved the allergic symptom over 50% after 1 year treatment in both treatment groups. And the SLIT could decrease the medication frequency if they can use with the pharmacotherapy. The early effect of immunotherapy might be very attractive in decreasing the pharmacotherapy burden. This result has shown the similar tendency with the previous study. Previous studies have shown similar effects in the 14- to 24-week treatment group.
In this study, there was no significant difference in symptom score change between the treatment groups. However, the percentage of patients showing a beneficial effect (improvement plus much improvement) in symptom score was greater in Staloral group.
The medication score was more decreased in Staloral group compared to LAIS group. These results suggest that the clinical outcomes in symptom improvement and medication frequency have shown more effective tendency in Staloral group than LAIS group in our study.
Sublingual immunotherapy with carbamylated monomeric allergoid tablets (LAIS) for 1-year treatment has significantly reduced all clinical parameters. Notably, the group treated with the higher dose showed significantly better clinical and immunological results. SLIT with LAIS showed 34% improvement in symptom score in comparison to the placebo group in grass pollen allergy and 22% in dust mite-induced allergic rhinoconjunctivitis. A clinical trial with 3 different doses of allergoid in mite allergic patients did not differ in clinical efficacy, safety, and effects on immunologic parameters. All the 3 doses showed performed significantly better results than pharmacotherapy alone.
Sublingual immunotherapy using Staloral 300 for 1 year in 186 HDM-induced allergic rhinitis patients showed that all the evaluated items, 12-month values were significantly lower than those at 6 months and baseline. Similarly, 6-month values were significantly lower than those at baseline. They suggest that the SLIT effect was shown in early 6 months after starting the SLIT. Symptoms of rhinitis and asthma were improved in 64.6% and 64.3% of patients, respectively, in 736 pediatric patients sensitization to HDM; at least 2-year follow-up after SLIT (Staloral 300 for 1 year) initiation. Sublingual immunotherapy using Staloral 300 for 3 years in elderly patients with a HDM allergy generated a significant clinical improvement (44% decrease in TSS, 51% decrease in the total medication score) in the active group compared with the placebo group.
In contrary to the positive results of SLIT, HDM SLIT in allergic rhinitis children for 3 years (Pangram in SLIT) does not prevent new sensitization and improvement in bronchial hyperresponsiveness. Sublingual immunotherapy for 2 years in rhinitis caused by HDM was safe, but there was a lack of consistent clinical benefit compared to placebo. No significant difference was observed between the active and placebo groups in total symptom and medication scores in the number of patients with high levels of indoor allergenic load. In US trial, there were no differences in symptom–medication scores according to dose. High-dose, increased serum D farina-specific IgG4 levels improved the bronchial threshold to allergen challenge.
In this study, the percentage of patients showing a much satisfaction was significantly increased in Staloral group compared to LAIS group. These data were correlated with the more symptom improvement rate in Staloral group. We think that the higher concentration of allergen in Staloral might correlate with this result. The majority of patients overall (85.1%) were perceived by the physicians to be satisfied or very satisfied with their treatment. In the study of measurement of HDM allergenic potency between 3 SLIT reagents, the allergen potencies of different HDM SLIT reagents are markedly different. The concentration of group 1 major allergens in Staloral was 33- to 44.5-fold higher than others; the concentration of group 2 major allergen was also 8.9- to 10.5-fold higher in Staloral than others.
The immunologic data in this study have also shown that the IgG4 was more 3 times increase in Staloral group compared to LAIS. After 1 year of SLIT, mite-specific IgE and IgG4 titers increased by 1.5-fold and 4-fold, respectively, in the active, but not in the placebo group and SLIT does not induce any IgE neosensitization to allergens in a 2-year period. Specific IgE (D pteronyssinus and D farinae) increased significantly in the active treatment group after 12 and 24 months, while no change was observed in the placebo group. Specific IgG4 levels were not significantly modified in either group.
In this study, adverse effects were reported in 6.2% of LAIS group and 33.3% of Staloral group. So the LAIS might be safer than Staloral. It might be the difference in potency of allergen and the absorption rate between the 2 methods. LAIS treatment in 198 patients has reported the side effects in 7.5% of patients and 0.52 per 1000 doses. Most of them were self-limiting and medical intervention was needed in 6 patients.
In our study, the maintenance rate over 1 year is 37.7% in LAIS and 25.1% in Staloral treatment group. The compliance was better in LAIS group. This might be the better and easier intake method of LAIS tablet compared to drop method. Our average compliance was very lower compared to other studies. Compliance was good in 86.5% of patients and SLIT was effective in 83.8%. Compliance was deemed to be good or very good by the physicians in 86.5% of patients overall. The dropout rate was 19.5% (32/164 patients) during the first month, 34% (56/164 patients) after 6 months, and 41% (68/164 patients) after 1 year of SLIT. The 3-year compliance rate was approximately 40% (65/164 patients). The most common causes of dropout during the first month of SLIT were high cost and inconvenience. In our study, the reasons for discontinuation of the SLIT were reported as economic reasons, inconvenience, no effect, and side effects. We think that the main cause of discontinuation is cost-effectiveness compared to other treatments (pharmacotherapy or SCIT). In Korea, the SLIT medication is very expensive compared to other medications, and the patients have a tendency to change their medication methods if they feel that their symptoms were improved after starting the SLIT. So we need to design to start the SLIT medication in the patients who have more needs and willingness for SLIT.
In conclusion, allergy immunotherapy is a very recommendable treatment option for the treatment of HDM-sensitized allergy patients. Studies involving more patients will need to investigate the appropriate treatment capacity and duration of treatment.
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The author(s) received no financial support for the research, authorship, and/or publication of this article.
Tae Kyu Kang
https://orcid.org/0000-0002-7274-0476
References
- 1. Lee S, Nolte H, Benninger MS. Clinical considerations in the use of sublingual immunotherapy for allergic rhinitis. Am J Rhinol Allergy. 2015;29(2):106–114.
- 2. Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;1(45):1572–1574.
- 3. Linkov G, Toskala E. Sublingual immunotherapy: what we can learn from the European experience. Curr Opin Otolaryngol Head Neck Surg. 2014;22(3):208–210.
- 4. Calderon MA, Casale TB, Nelson HS, Demoly P. An evidence-based analysis of house dust mite allergen immunotherapy: a call for more rigorous clinical studies. J Allergy Clin Immunol. 2013;132(6):1322–1336.
- 5. Acquistapace F, Agostinis F, Castella V, et al. Efficacy of sublingual specific immunotherapy in intermittent and persistent allergic rhinitis in children: an observational case-control study on 171 patients. The EFESO-children multicenter trial. Pediatr Allergy Immunol. 2009;20(7):660–664.
- 6. Milani M, Pecora S, Burastero S. Observational study of sublingual specific immunotherapy in persistent and intermittent allergic rhinitis: the EFESO trial. Curr Med Res Opin. 2008;24(9):2719–2724.
- 7. Trebuchon F, Lhéritier-Barrand M, David M, Demoly P. Characteristics and management of sublingual allergen immunotherapy in children with allergic rhinitis and asthma induced by house dust mite allergens. Clin Transl Allergy. 2014;4:15.
- 8. Cingi C, Bayar Muluk N, Ulusoy S, et al. Efficacy of sublingual immunotherapy for house dust mite allergic rhinitis. Eur Arch Otorhinolaryngol. 2015;272(11):3341–3346.
- 9. Han DH, Choi YS, Lee JE, et al. Clinical efficacy of sublingual immunotherapy in pediatric patients with allergic rhinitis sensitized to house dust mites: comparison to adult patients. Acta Otolaryngol. 2012;132(suppl 1):S88–S93.
- 10. Kim SH, Mun SJ, Han DH, Kim JW, Kim DY, Rhee CS. Three-year follow up results of sublingual Immunotherapy in patients with allergic rhinitis sensitized to house dust mites. Allergy Asthma Immunol Res. 2015;7(2):118–123.
- 11. Mösges R, Ritter B, Kayoko G, Allekotte S. Carbamylated monomeric allergoids as a therapeutic option for sublingual immunotherapy of dust mite- and grass pollen-induced allergic rhinoconjunctivitis: a systematic review of published trials with a meta-analysis of treatment using Lais® tablets. Acta Dermatovenerol Alp Pannonica Adriat. 2010;19(3):3–10.
- 12. Mösges R, Pasch N, Schlierenkämper U, Lehmacher W. Comparison of the biological activity of the most common sublingual allergen solutions made by two European manufacturers. Int Arch Allergy Immunol. 2006;139(4):325–329.
- 13. Wang DH, Chen L, Cheng L, et al. Fast onset of action of sublingual immunotherapy in house dust mite-induced allergic rhinitis: a multicenter, randomized, double-blind, placebo-controlled trial. Laryngoscope. 2013;123(6):1334–1340.
- 14. Di Gioacchino M, Cavallucci E, Ballone E, et al. Dose-dependent clinical and immunological efficacy of sublingual immunotherapy with mite monomeric allergoid. Int J Immunopathol Pharmacol. 2012;25(3):671–679.
- 15. Marogna M, Colombo F, Cerra C, et al. The clinical efficacy of a sublingual monomeric allergoid at different maintenance doses: a randomized controlled trial. Int J Immunopathol Pharmacol. 2010;23(3):937–945.
- 16. Bozek A, Ignasiak B, Filipowska B, Jarzab J. House dust mite sublingual immunotherapy: a double-blind, placebo controlled study in elderly patients with allergic rhinitis. Clin Exp Allergy. 2013;43(2):242–248.
- 17. Lim JH, Kim JY, Han DH, et al. Sublingual immunotherapy (SLIT) for house dust mites does not prevent new allergen sensitization and bronchial hyper-responsiveness in allergic rhinitis children. PLoS One. 2017;12(8):e0182295.
- 18. Guez S, Vatrinet C, Fadel R, André C. House-dust-mite sublingual-swallow immunotherapy (SLIT) in perennial rhinitis: a double-blind, placebo-controlled study. Allergy. 2000;55(4):369–375.
- 19. Bush RK, Swenson C, Fahlberg B, et al. House dust mite sublingual immunotherapy: results of a US trial. J Allergy Clin Immunol. 2011;127(4):974–981.
- 20. Park KH, Son M, Choi SY, et al. In vitro evaluation of allergen potencies of commercial house dust mite sublingual immunotherapy reagents. Allergy Asthma Immunol Res. 2015;7(2):124–129.
- 21. Baron-Bodo V, Batard T, Nguyen H, et al. Absence of IgE desensitization in house dust mite allergic patients following sublingual immunotherapy. Clin Exp Allergy. 2012;42(10):1510–1518.
- 22. Lombardi C, Gargioni S, Melchiorre A, et al. Safety of sublingual immunotherapy with monomeric allergoid in adults: multicenter post-marketing surveillance study. Allergy. 2001;56(10):989–992.