Introduction
Hearing Loss in the Elderly Individual
The average life expectancy in the United Kingdom is currently 79.2 years for a newborn baby boy and 82.9 years for a girl. It has been estimated that, by 2040, nearly 15% of the population will be over 75 years old in the United Kingdom. The Office for Budget Responsibility predict that total public spending will increase by nearly £80 billion over the next approximately 50 years principally due to the ageing population. There are drives to support the ageing population to lead longer and healthier working lives and to address barriers that may limit later life learning.
Hearing impairment is a very common problem among the elderly patients and is the third most common disability within the United States among the population older than 65 years. The prevalence of hearing loss (HL) in individuals aged 70 to 79 years is 55%, rising to 81% in those over 80 years, including 7.5% with severe-to-profound HL.
The implications of HL in elderly patients extend beyond simply auditory perception. With advancing age, HL that has not been appropriately rehabilitated can have severely damaging effects on general health outcomes including cognition, mental health, and quality of life. , A prospective case-control study demonstrated widespread cognitive impairment in elderly patients with severe hearing impairment compared to a matched control group.
A large cohort study by Lin et al found HL to be independently associated with lower baseline cognitive scores and, over a 6-year follow-up, result in an accelerated annual rate of cognitive decline. Hearing rehabilitation with hearing aids and cochlear implants (CI) has been shown to improve quality of life including cognitive skills and social performance score in elderly patients with a wide range of HL. - A recent study demonstrated over 97% of patients had a significant improvement in cognition after only 18 months of appropriate auditory rehabilitation.
In 2017, there were around 45 000 CIs implanted worldwide and the total number of CI users worldwide is close to 500 000. The number of people with CI is ever increasing, and within the United Kingdom, recent changes in the National Institute for Health and Care Excellence (NICE) criteria for CI candidacy has been estimated to result in a 70% increase in the number of CI recipients within 5 years.
While the efficacy of CI in rehabilitation of severe-to-profound HL is well established, careful consideration should be given to the balance between the potential impact of any associated comorbidities and the perceived long-term benefit of CI in elderly individuals, as well as the potential risks of surgery.
In this study, we evaluated the audiological, communication, and quality of life outcomes in elderly patients who received CI in our center.
Patients and Methods
A retrospective analysis was performed on all patients who received a unilateral CI aged ≥70 years between January 01, 2008 and May 31, 2017 at St. Thomas’s Hearing Implant Centre, London, United Kingdom. Preoperative assessment included as a minimum pure tone audiometry, speech perception using the Bamford-Kowal-Bench (BKB) sentence test (70 dB SPL sound intensity) in quiet conditions (BKBq) and imaging. The decision to offer CI was based on the audiological profile as outlined in the NICE guidance at the time of implantation. Due to the gradual evolution in clinical practice at our center, some patients also had BKB in noise (BKBn) at +10 dB signal-to-noise ratio and the Arthur Boothroyd (AB) word list (phonemes) as additional speech perception testing. The patient’s ability to use the telephone with familiar speakers was also recorded.
All patients included in this study had a minimum of 12 months of follow-up. Outcome measures included immediate and delayed surgical complications, postoperative speech perception scores, the ability to use the telephone with familiar speakers and patient-reported outcome measure (PROM) using the Glasgow Benefit Inventory (GBI) questionnaire after at least 12 months of device use. The cohort was subdivided into 2 age groups: those aged between 70 and 79 years and those aged ≥80 years at the time of implantation.
The Shapiro-Wilk test of normality demonstrated the patient cohort could not be assumed to be normally distributed. The Mann-Whitney U Test was used to compare median values of independent un-paired data while the Wilcoxon signed-rank test compared dependent paired data. McNemar test was used to compare paired nominal data and the χ2 test for unpaired nominal data. Statistically significant difference was considered to be P < .05. All statistical analyses were performed with SPSS version 23.0 (IBM Cord., Armonk, New York).
Results
Demographics
Over the 10-year study period, 64 patients aged ≥70 years received a unilateral CI (31 females, 33 males). The median age at implantation was 76.4 ± 5.3 years (mean 77.0, range 70.0-90.1). The median duration of post-implantation follow-up was 32.2 ± 28.0 months (mean 43.0, range 12.0-124.4). The subdivision of age groups is shown in Figure 1. The increase in the number of patients implanted older than the age of 70 years between January 01, 2008 and June 30, 2017 is illustrated in Figure 2.
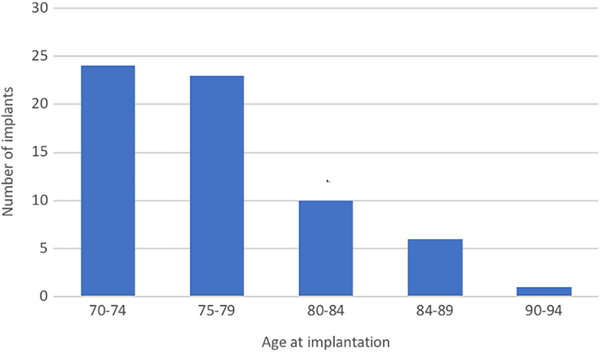
Figure 1
The subdivision of age groups at time of cochlear implantation.
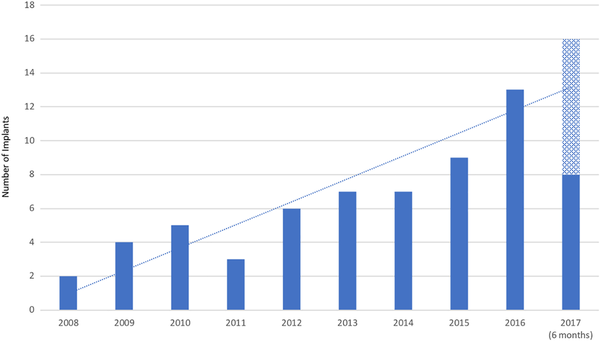
Figure 2
The number of patients per year older than 70 years receiving a cochlear implant over the last decade. The trend includes a projection of implants to be performed in the final year.
Over 75% (49/64) of patients had significant systemic comorbidities at the time of their CI surgery. The most common comorbidity was hypertension, noted in nearly 30% of patients. Other documented comorbidities included ischemic heart disease, diabetes mellitus, osteoporosis, and previous malignancy.
Surgery and Complications
Devices from 3 different CI manufacturers were used, with no statistically significant difference in device choice between the 2 age groups, 70 and 79 years and ≥80 years (χ2 = 2.4, P = 0.3). In 75% of the cases, there were no immediate postoperative complications. The most common complaint was early postoperative dizziness, reported in 20%. Persistent dizziness was unusual, with only 1 patient requiring formal vestibular rehabilitation. There were no increased rates of traumatic falls, fractures, or reduced mobility status.
Two (3%) patients required revision surgery. Of these, one patient had successful reimplantation following electrode extrusion. The other patient, who had initially derived great benefit from CI after implantation at 89 years old, developed an electrical device failure after 6 years and underwent explantation/reimplantation at the age of 95 years. After an initial uncomplicated recovery, he developed repeated wound breakdown with implant extrusion and subsequently permanent explantation.
Two (3%) patients became nonusers due to inadequate audiological benefit. Of note, there had been no audiological, medical, or radiological concerns about potential poor outcomes during the preoperative CI assessment, nor surgical complications for either patient (aged 73.3 and 80.0 years at the time of implantation). Two (3%) patients died of unrelated causes during the study period, at 33.1- and 33.6-months post-implantation.
Audiological Outcomes
There were statistically significant improvements in the median scores across all 3 measures of speech perception following implantation (P < 0.05, Wilcoxon signed-rank test; Figure 3). Speech perception scores improved postoperatively in 96.7% (58/60), with maximal benefit noted after just 3 months in 87.5%. In 2 patients, there was no objective improvement postoperatively (BKBq scores 0%-0% and 49%-44%, respectively); one patient had partial electrode insertion due to difficult anatomy. Despite the lack of improvement in objective measures, both patients still reported subjective overall benefit and continue to use their device consistently.
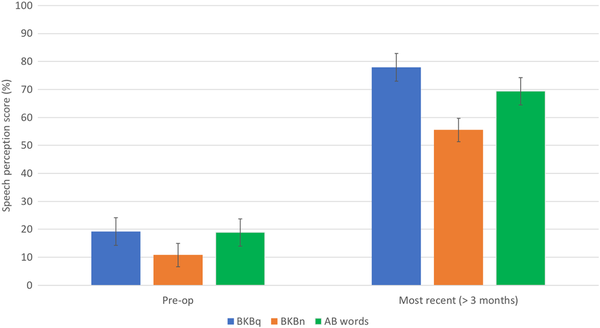
Figure 3
Pre- and postoperative speech perceptions scores (P < .05, Wilcoxon signed rank test).
There was no significant difference between the 70 and 79 and ≥80s age subgroups in the most recent BKBq, BKBn, and AB words scores (P = 0.28, P = 0.11, and P = 0.39, respectively, Mann-Whitney U test).
Telephone
Data relating to telephone use pre- and post-CI was documented in 87.5% (56/64). Of these, only 8.9% (5/56) had been able to communicate using the telephone to some extent preoperatively, with a median time of 48 months since last use (mean 88.3, range: 1.2-943.2). Following implantation, 67.9% (38/56) were able to use the telephone. This increase was statistically significant (P < 0.05, McNemar test). The younger age subgroup was more likely to be able to achieve this (χ2 = 5.3, P < 0.05). Only 1 patient who had been able to use the telephone preoperatively was no longer able to do so following implantation. This was also 1 of the 2 patients who saw no objective improvement in speech perception postoperatively.
Glasgow Benefit Inventory
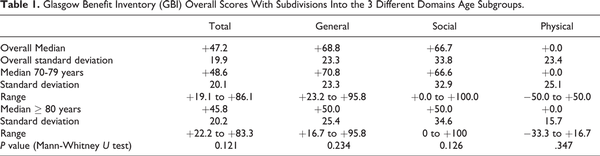
Glasgow Benefit Inventory, first described in 1996, is a validated PROM which is utilized postoperatively to assess the impact of the intervention on a patient. It consists of 18 questions and a 5-point Likert scale. Complete responses were received in 68.8% (44/64), and the overall results from GBI and each subdomain were calculated. As shown in Table 1, the results showed a significant positive impact on patients’ lives and their health status, notably within the “general” and “social” domains in both age subgroups. The median GBI score in our study cohort was 47.2 ± 19.9 (mean 55.1, range 19.1-86.1). The most significant benefit was noted within the “general” and “social” domains. The median score in the 70- to 79-year-old group was 48.6 ± 20.1 (mean 56.8, range 19.1-86.1) and 45.8 ± 20.2 (mean 45.6, range 22.2-83.3) in the ≥80-year-old group. Positive benefit was observed in both age groups with no statistically significant difference in scores between the 2 age groups in the overall score or the differing domains.
Discussion
Cochlear Implants Outcomes in the Elderly Individuals
The appropriateness of CI for hearing rehabilitation in the elderly patients has been a subject of interest for many years. The negative effect of HL in the elderly patients is well-documented, and hearing rehabilitation with CI has been suggested to play a role in limiting the age-related decline in health-related outcome in such patients. -, A recent study has shown significant improvements not only in auditory perception but also cognitive function following hearing rehabilitation with CI. Patients with impaired baseline cognitive function should, however, receive additional cognitive rehabilitation where appropriate to improve long-term outcomes.
We demonstrated improved speech perception (BKBq, BKBn, and AB words) in both age subgroups, suggesting that age alone is not a predictor of audiometric outcomes. Our experience is comparable to recent studies on CI outcomes in elderly recipients with postlingual deafness showing no significant correlation with age of implantation. ,, It is likely that, rather than age alone, more clearly established prognostic indicators such as duration of unaided deafness should receive more consideration during candidacy assessment.
The ability to use the telephone was assessed, in view of its importance in daily life and our increasing reliance on it as a communication tool for social interactions. In our study, 67.9% of patients were able to achieve this following implantation, compared to 8.9% preoperatively. Although improvements were observed in both age subgroups, older patients had a reduced chance of telephone use following CI. With ever-improving technology, notably Bluetooth-compatible wireless hearing assistance technology, it is hoped that an increasing number of CI users will have easier access to telephone use.
Adverse Events
One aspect of CI in elderly patients is the perception of increased surgical risks, both related to general anesthesia and specific to CI. Perioperative anesthetic complications of CI surgery in the supposedly high-risk groups such as the elderly patients still tend to be low, as shown in this study, with no complications directly attributable to general anesthesia, despite over 75% having significant systemic comorbidities. There were no unexpected admissions to an intensive care or high dependency unit postoperatively.
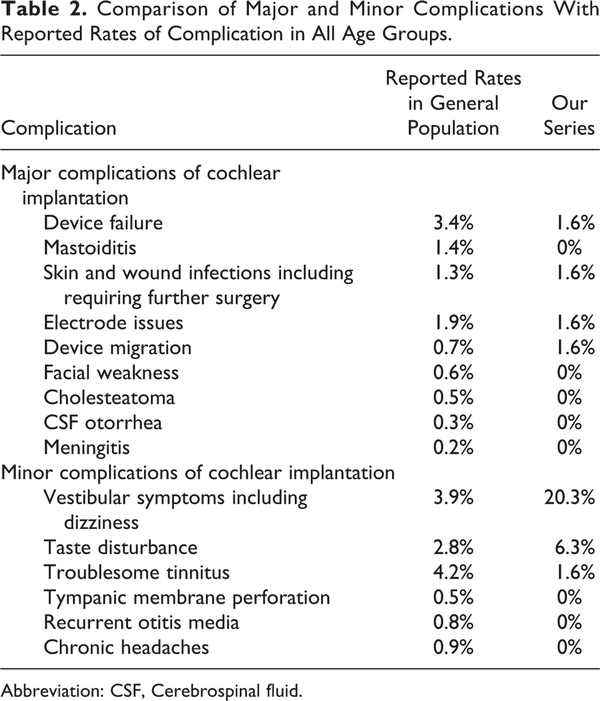
Cochlear implants surgery is widely regarded as a safe operation. Complication rates are classified into minor and major depending on their severity, as illustrated in Table 2. The rate of major complications in our series was comparable to the reported literature, with 75% of patients having no postoperative complications at all. In a review article which analyzed over 20 000 patients, the overall delayed complication rate was found to be 5.7%, with fewer complications in the adult cohort than the pediatric group. Both major and minor surgical complication rates in the elderly patients have been reported to be similar to other adult age groups.
Dizziness and balance disturbance affected 20% of our study cohort, in keeping with the previously reported rates of 14.9% to 28.6%. The vast majority saw spontaneous improvement with only 1 (1.6%) patient requiring further management. Postoperative imbalance is a concern in a group of patients already at increased risk of falls. With appropriate counseling and postoperative support, we noted no increased risk of falls or associated traumatic injuries in patients undergoing CI surgery. Elderly patients who may receive bilateral CI should be considered for formal preoperative vestibular assessment in view of the impact of potential bilateral vestibular hypofunction postoperatively and may require inpatient physiotherapy prior to discharge. Another complication that is more common among the elderly patients is taste disturbance, found in 6.3% of the study cohort; this is also comparable to 7% to 8% reported in other studies. The incidences of both vestibular dysfunction and taste disturbance are increased in the elderly population compared to the average figures of 3.9% and 2.8% across all age groups, and potential candidates should be counseled carefully, especially about postoperative balance disturbance, with appropriate measures put in place preoperatively where necessary.
We noted a long-term device use rate of 96.9%, with a median follow-up of 32.2 months (mean 43.0, range: 12-124.4). In a study by Choi et al, the long-term use of CI in patients aged older than 60 years was found to be greater than 80% more than 10 years after implantation. Interestingly, the authors also found an increased risk of nonuse with increasing age of the patient at time of implantation. Our cohort included 2 nonusers, one in each age subgroup, as described earlier.
Glasgow Benefit Inventory
Patient-reported outcome measures allowed us to assess the impact of CI on patient’s quality of life. A recent systematic review evaluating the utilization of GBI in otolaryngology demonstrated consistency of data in meta-analyses of studies on CI, supporting its use as a PROM tool in studies on CI outcomes.
There was no statistically significant difference between the 2 age groups in the overall GBI scores as well as within each of the 3 domains. In addition, there was no patient with a negative overall GBI score, although 18.2% had a negative score within the “physical health” domain. The overall benefit within the “physical health” domain was also considerably less, compared to the “general” and “social” subscale scores. This is not unexpected as receiving a CI would be in anticipation of reducing the amount of social support required and improving their general and social functions rather than physical health. Our positive findings are in keeping with the literature showing statistically significant improvement in PROMs after cochlear implantation. Our median GBI score of +47.2 suggests a very strong improvement in quality of life after CI surgery; although a direct comparison is not possible, it is of interest that the GBI score following cataract surgery has been reported to be +23.2.
Conclusion
Cochlear implantation is a safe and well-tolerated procedure in elderly patients. Significant improvements are observed in audiological performance including telephone use and in patient-reported outcomes, although report of improved telephone use appears to be less pronounced in those over 80 years of age.
Authors' Note Dan Jiang and Irumee Pai are also affiliated with King's College London, London, United Kingdom.
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Statistics of N. National life tables, UK: 2014 to 2016. 2017; https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2014to2016. Accessed November 12, 2019.
- 2. Science GOV. Future of an ageing population. 2016; https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/535187/gs-16-10-future-of-an-ageing-population.pdf. Accessed November 12, 2019.
- 3. Wan HE, Larsen LJ. Older Americans with a disability: 2008–2012. In: Reports ACS, ed: U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau. Washington, DC: 2015.
- 4. Goman AM, Lin FR. Prevalence of hearing loss by severity in the United States. Am j public health. 2016;106(10):1820–1822.
- 5. Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch neurol. 2011;68(2):214–220.
- 6. Rutherford BR, Brewster K, Golub JS, Kim AH, Roose SP. Sensation and psychiatry: linking age-related hearing loss to late-life depression and cognitive decline. Am J Psychiatry. 2017;175(3):215–224.
- 7. Huber M, Roesch S, Pletzer B, Lukaschyk J, Lesinski-Schiedat A, Illg A. Cognition in older adults with severe to profound sensorineural hearing loss compared to peers with normal hearing for age. Int j audiol. 2019:1–9. [Epub ahead of print].
- 8. Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA int med. 2013;173(4):293–299.
- 9. Knopke S, Gräbel S, Ruhrmann UF, Mazurek B, Szczepek AJ, Olze H. Impact of cochlear implantation on quality of life and mental comorbidity in patients aged 80 years. Laryngoscope. 2016;126(12):2811–2816.
- 10. Mosnier I, Vanier A, Bonnard D, et al. Long-term cognitive prognosis of profoundly deaf older adults after hearing rehabilitation using cochlear implants. J Am Geriatr Soc. 2018;66(8):1553–1561.
- 11. Volter C, Gotze L, Dazert S, Falkenstein M, Thomas JP. Can cochlear implantation improve neurocognition in the aging population? Clin interv aging. 2018;13:701–712.
- 12. Sarant J, Harris D, Busby P, et al. The effect of hearing aid use on cognition in older adults: can we delay decline or even improve cognitive function? J clin med. 2020;9(1):pii: E254.
- 13. Technavio. Global Cochlear Implants Market 2018-2022 Ireland, North Atlantic: 2018.
- 14. (National Institute for Health and Care Excellence) TNIfHaCE. Cochlear implants for children and adults with severe to profound deafness. In: London, UK:2019.
- 15. Robinson K, Gatehouse S, Browning GG. Measuring patient benefit from otorhinolaryngological surgery and therapy. Ann otol, rhinol laryngol. 1996;105(6):415–422.
- 16. Sorrentino T, Donati G, Nassif N, Pasini S, Redaelli de Zinis LO. Cognitive function and quality of life in older adult patients with cochlear implants. Int j audiol. 2019:1–7. [Epub ahead of print].
- 17. Cosetti MK, Pinkston JB, Flores JM, et al. Neurocognitive testing and cochlear implantation: insights into performance in older adults. Clin interv aging. 2016;11:603–613.
- 18. Wong DJ, Moran M, O’Leary SJ. Outcomes after cochlear implantation in the very elderly. Otol neurotol. 2016;37(1):46–51.
- 19. Iza LG, Martinez Z, Ugarte A, Fernandez M, Altuna X. Cochlear implantation in the elderly: outcomes, long-term evolution, and predictive factors. European Archives of Otorhinolaryngology. 2018;275(4):913–922.
- 20. Wolfe J, Morais Duke M, Schafer E, Cire G, Menapace C, O’Neill L. Evaluation of a wireless audio streaming accessory to improve mobile telephone performance of cochlear implant users. Intl j audiol. 2016;55(2):75–82.
- 21. Terry B, Kelt RE, Jeyakumar A. Delayed complications after cochlear implantation. JAMA otolaryngol head neck surg. 2015;141(11):1012–1017.
- 22. Choi JS, Contrera KJ, Betz JF, Blake CR, Niparko JK, Lin FR. Long-term use of cochlear implants in older adults: results from a large consecutive case series. Otology neurotol. 2014;35(5):815–820.
- 23. Hendry J, Chin A, Swan IR, Akeroyd MA, Browning GG. The Glasgow benefit inventory: a systematic review of the use and value of an otorhinolaryngological generic patient-recorded outcome measure. Clin otolaryngol. 2016;41(3):259–275.
- 24. Katta M, Udani P, Heemraz BS, Lee CN, Hammond CJ, Mahroo OA. Exploring correlations between change in visual acuity following routine cataract surgery and improvement in quality of life assessed with the Glasgow benefit inventory. Eye (London, England). 2018;32(9):1549–1550.