Introduction
Obstructive sleep apnea (OSA) is characterized by repeated episodes of upper airway obstruction, hypoxemia, and fragmented sleep. Continuous positive airway pressure (CPAP) has traditionally been the preferred treatment modality for OSA, although adherence is a well-known limiting factor. Hypoglossal nerve stimulation (HNS) first gained Food and Drug Administration (FDA) approval in 2014 as an alternative treatment option; however, little is known regarding the clinical characteristics of those presenting for possible HNS treatment for OSA.
In the general population, moderate-to-severe sleep apnea (apnea–hypopnea index [AHI] ≥ 15) is suspected to occur at alarming high prevalence rates of 49.7% of men and 23.7% of women. Sleep apnea is more common in older adults. This increased prevalence along with sleep apnea’s role in exacerbating many age-related disorders including cardiovascular disease, stroke, heart failure, and cognitive deficits highlights the importance of assessing and treating sleep apnea, especially in older adults.
Multiple strategies exist for the management and treatment of OSA symptoms. Currently, CPAP is one of the most successful and commonly employed strategies. Continuous positive airway pressure adherence is an important component of the overall therapy. Continuous positive airway pressure adherence generally translates into improved sleep quality, increased daytime alertness, reduced daytime sleepiness, and improved overall mood/attitude. Medically, treatment adherence often translates into decreases in blood pressure and improvements in cardiovascular abnormalities, such as atrial fibrillation and congestive heart failure. Continuous positive airway pressure adherence is often defined as utilization of therapy for an average of 4 hours a night for at least 70% of the nights. Studies have shown a wide range of CPAP compliance, ranging between 29% and 83% compliance. Given the low adherence, alternative OSA treatments are needed.
Hypoglossal nerve stimulation is an FDA-approved implantable neurostimulation system (Inspire Medical Systems) used to treat select CPAP-intolerant patients with OSA by stimulating the distal branches of the hypoglossal nerve. The stimulation leads to selective activation of the genioglossus muscle, resulting in airway enlargement at the level of the palate and tongue base. - Large multicenter trials have demonstrated the efficacy of this therapy showing improvement in AHI and quality-of-life measures. Furthermore, this therapy has a durable response with studies showing stable outcomes at 60 months postprocedure. Although patients undergoing HNS are intolerant of CPAP, adherence, even among this difficult group, is excellent with an average of 6.5 hours of use per night . Despite this, some individuals struggle to maintain good adherence due to a variety of issues including stimulation discomfort, insomnia, and cognitive impairment limiting proper use of the device. Currently, the clinical characteristics of patients seeking HNS therapy are unknown. Additionally, the effect of HNS on daytime functioning is largely unknown. To work toward answering these important questions, it is necessary to first establish baseline characteristics for this population.
The aim of the present investigation was to begin to fill this void by characterizing the functioning of older adults seeking consultation for HNS for the treatment of OSA. Specifically, we aimed to determine the clinical characteristics of these patients across the domains of insomnia symptoms, physical functioning, cognitive abilities, depression and anxiety symptoms, and anger. We hypothesized that the current patient sample would display higher rates of dysfunction across these domains compared to the general population.
Patients and Methods
Procedures
All patients seen for HNS consultation who were older than 50 years were presented with a questionnaire packet that assessed insomnia symptoms, sleep-related impairment, cognitive functioning, depression and anxiety symptoms, and anger. The local institutional review board deemed this activity to be exempt. The following measures were collected from each study participant.
Demographics
Information regarding participant age, race, sex, and severity of sleep apnea was abstracted from electronic medical records.
Insomnia Severity Index
Insomnia symptoms were measured using the Insomnia Severity Index (ISI). The ISI is a 7-item self-report measure that assesses the nature, severity, and impact of insomnia over the past month. These 7 items are scored utilizing a 5-point Likert scale from 0 (none) to 4 (very severe) to form a total score for insomnia symptomatology. The ISI has demonstrated high reliability (Cronbach α = 0.91) in clinical samples.
Patient-Reported Outcomes Measurement Information System (PROMIS) Physical Function
Self-reported capability was measured using the Patient-Reported Outcomes Measurement Information System (PROMIS) Physical Function v2.0 4-item short form. This measure assesses the ability to perform activities of daily living (ADL) and instrumental ADL, but not whether the activity was actually performed. These items are scored on a Likert scale from 1 (unable to do) to 5 (without any difficulty) to form a total score of physical function. The PROMIS Physical Function v2.0 4-item short form has demonstrated acceptable reliability (ie, Cronbach α = 0.92) in a large scale sample of varied race and age-groups.
PROMIS Sleep-Related Impairment
Alertness, sleepiness, tiredness, and functional impairments associated with sleep problems during waking hours were measured using the PROMIS Sleep-Related Impairment v1.0 4-item short form. The 4 items on this measure are scored on a Likert scale from 1 (not at all) to 5 (very much) to form a total score of sleep-related impairment. The reliability of the PROMIS SRI short form is acceptable (Cronbach α = 0.90).
PROMIS Cognitive Functioning Abilities
Cognitive deficits were measured using the PROMIS Cognitive Function Abilities v2.0 6-item short form. The items on this measure assess different facets of cognitive functions, such as mental acuity, thought processes, concentration, and memory. The 6 items on this measure are scored on a Likert scale from 1 (not at all) to 5 (very much) to form a total score of cognitive functioning. The PROMIS Cognitive Functioning Abilities has demonstrated good reliability (ie, Cronbach α = 0.95). ,
PROMIS Depression
Depressive symptoms were measured using the PROMIS Depression v1.0 4-item short form. This measure assesses self-reported negative mood, self-worth, social cognition, and decreased levels of positive affect. It is important to note this measure strictly addresses depressed mood and not somatic symptoms associated with depression. The 4 items on this measure are scored on a Likert scale from 1 (never) to 5 (always) to form a total score of depressed mood. The PROMIS depression short form demonstrated acceptable reliability, Cronbach α of >0.90, in a large racially/ethnically diverse sample.
PROMIS Anxiety
Emotional distress and anxiety were measured using the PROMIS Anxiety v1.0 4-item short form. This measure assesses self-reported fear, anxiety, hyperarousal, and somatic symptoms associated with anxiety. The 4 items on this measure are scored on a Likert scale from 1 (never) to 5 (always) to form a total score of anxiety. The PROMIS anxiety short form has demonstrated Cronbach α of 0.91 across numerous demographic characteristics.
PROMIS Anger
Self-reported facets of anger were measured using the PROMIS Anger v1.1 5-item short form. This measure assesses angry mood, negative interpersonal cognitions, and ability to control ones’ anger. The 5 items on this measure are scored on a Likert scale from 1 (never) to 5 (always) to form a total score of anger. PROMIS anger scale has demonstrated a Cronbach α of 0.96.
Data Analyses
Sample characteristics were described through means and frequency distributions. Scores on all PROMIS measures were converted to t scores using published PROMIS procedures that align raw scores with their equivalent t scores. These t scores can be used to assign clinical characteristics by thresholds based on published normative data that were developed through large scale calibration testing data. ,
Results
The final sample included 113 individuals older than 50 years (Mage = 63.30, standard deviation [SD] = 7.87) who were seeking consultation for HNS treatment for OSA. On average, the sample had severe OSA, as noted by an AHI (or respiratory event index if AHI was unavailable) of 33.91 (SD = 20.63).
In terms of clinical characteristics, the sample reported elevated levels of insomnia symptoms, as indicated by the ISI. The average ISI score was 12.63 (SD = 6.70). Notably, when using a cut point of greater than or equal to 10, nearly 88% of the sample scored at or above this level. In terms of PROMIS-rated sleep-related impairment, the sample average t score was 54.83 (SD = 11.65). Although approximately 20% of the general population scores at or above a t score of 60 on this measure, approximately 36% of the current sample scored at these elevated levels.
Regarding physical function, average t score of the sample was 46.73 (SD = 8.92). Although 20% of the general population will score at or below a t score of 40 on this measure, roughly 30% of the current sample scored at these elevated levels. In terms of PROMIS-rated cognitive function, average t score of the sample was 44.14 (SD = 11.12). Although 20% of the general population will score at or below a t score of 40 on this measure, approximately 38% of the current sample scored at these elevated levels.
In terms of PROMIS-rated depressive symptoms and anxiety symptoms, the sample average t score was 51.48 (SD = 10.30) and 49.70 (SD = 9.35), respectively. Again, approximately 20% of the general population score at or above a t score of 60 on these measures; however, 28% and 19% of the current sample scored at these elevated levels for depressive symptoms and anxiety symptoms, respectively. Lastly, in terms of PROMIS-rated anger symptoms, the sample average t score was 50.24 (SD = 11.87). Although approximately 20% of the general population score at or above a t score of 60 on this measure, approximately 17% of the current sample scored at these elevated levels. Refer to Figure 1 for detailed graphical representation of functioning across biopsychosocial domains in older adults seeking HNS consultation.
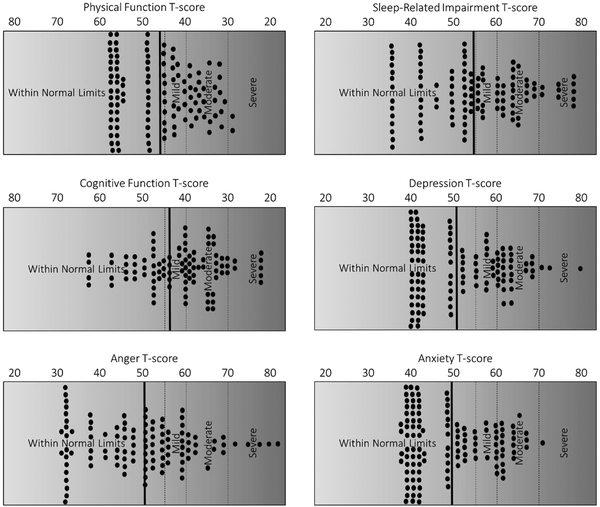
Figure 1
Clinical characteristics of older individuals seeking consultation for HNS treatment for obstructive sleep apnea. Notes: solid dots represent individual data points. Bold vertical line indicates mean level of functioning. Vertical dashed lines demarcate various suggested clinical cut points.
Discussion
In a sample of older adults seeking HNS for the treatment of OSA, we found rates of insomnia, sleep-related impairment, and depression symptoms much higher than those observed in the general population. We also observed self-reported physical and cognitive functioning that were significantly lower than the general population. However, anxiety symptoms and anger were consistent with levels from the general population.
The current study demonstrated that older patients seeking HNS treatment had elevated levels of disturbed sleep and mental health problems. Comorbid sleep disorders and psychological comorbidities have recently been identified as key factors in successful patient selection. The present study found 88% of patients reported elevated insomnia symptoms. This is higher than earlier studies examining the coexistence of OSA and insomnia, which reported comorbidity rates of 39% to 58% in a general cohort of patients with OSA. Elevated ISI scores such as seen in this study have previously been linked to poorer PAP adherence. A study of veterans undergoing HNS found that 56.6% met diagnostic criteria for comorbid insomnia and OSA. In contrast to what may be expected based on the PAP literature, compliance among the comorbid insomnia and OSA group was not statistically different than the OSA only group (5.6 vs 6.4 hours). Meleca and Kominsky examined 60 patients undergoing HNS and found that 19 required awake endoscopy due to issues of stimulation discomfort, awakening due to stimulation, or lack of benefit. The need for awake endoscopy was much more likely in those with comorbid insomnia and OSA (52.6%) versus those with OSA only (2.4%). Cumulatively, these data suggest the importance of considering comorbid insomnia in individuals seeking HNS therapy for the treatment of OSA. Whether such associations remain in older, nonveterans is yet to be determined.
In line with previous studies, the current sample had elevated depressive symptomology. Previous investigations have also reported elevated depressive symptomology in samples undergoing HNS therapy. Meta-analysis has revealed a positive impact of PAP on depression. Our findings of the high prevalence of moderate to severe cognitive complaints align with previous findings that older individuals with untreated moderate-severe OSA also demonstrate high rates of neurocognitive impairment. Importantly, treatment of OSA with PAP has shown positive gains in cognitive functioning for older adults. Importantly, the effects of HSN on depression or cognitive outcomes are not yet know.
HNS therapy has been shown to be a well-tolerated and effective modality for both improving quality of life and AHI in patients with OSA. However, the demographic and clinical characteristics of individuals seeking HNS are poorly categorized at this time. Based on our clinical data, it appears as though older adults seeking HNS therapy are characterized by a significant burden of insomnia and neurocognitive dysfunction. Understanding each patient’s biopsychosocial profile allows a better sense of the challenges that may be encountered in the care pathway before and after HNS, but such information is, unfortunately, largely missed in the traditional history and physical performed as part of a surgical workup. This study highlights the extent to which OSA, generally, and treatment resistant OSA, specifically, are associated with psychosocial burden in those without dramatic clinical characteristics.
The aim of our study was to determine the clinical characteristics of individuals seeking HNS for the treatment of OSA. Although our results were based on validated self-reported questionnaires, there is an inherent potential for recall bias in survey data. Our sample size was relatively large for a preliminary study of this kind, although further investigations will need to draw on a large cohort for more thorough analyses, longitudinal data collections, more diverse samples in terms of age and race/ethnicity, and examination of HNS therapy effects. Future studies will be well-suited to investigate whether HNS treatment normalizes any of the elevations in insomnia, mood, or cognitive functioning observed in the present sample.
This study sought to establish preliminary clinical, psychological, and social characteristics in older patients seeking HNS therapy for the treatment of OSA through administration and examination of several validated NIH PROMIS measures. The current findings indicate that older patients seeking HNS treatment tend to report elevated insomnia symptoms, sleep-related disturbances, depressive symptoms, and cognitive deficits. Routine clinical evaluation of patients seeking HNS therapy should include brief assessment of these important facets of daytime functioning to allow for future investigation of predictors of HNS compliance as well as potential HNS therapy effects.
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number K23AG049955 (PI: Dzierzewski).
Joseph M. Dzierzewski
https://orcid.org/0000-0002-9788-8813
Nima Vahidi
https://orcid.org/0000-0002-7250-9020
Ryan Nord
https://orcid.org/0000-0001-6415-8948
References
- 1. Rundo JV. Obstructive sleep apnea basics. Cleve Clin J Med. 2019;86(9 suppl 1):2–9. doi:10.3949/ccjm.86.s1.02
- 2. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi:10.1016/S2213-2600(15)00043-0
- 3. Rodriguez JC, Dzierzewski JM, Alessi CA. Sleep problems in the elderly. Med Clin North Am. 2015;99(2):431–439. doi:10.1016/j.mcna.2014.11.013
- 4. Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes Of Health). Circulation. 2008;118(10):1080–1111. doi:10.1161/CIRCULATIONAHA.107.189375
- 5. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi:10.1056/NEJMoa043104
- 6. Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. doi:10.1161/CIRCULATIONAHA.109.901801
- 7. Olaithe M, Bucks RS, Hillman DR, Eastwood PR. Cognitive deficits in obstructive sleep apnea: insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med Rev. 2018;38:39–49. doi:10.1016/j.smrv.2017.03.005
- 8. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. doi:10.1513/pats.200708-119MG
- 9. Rose MW. Positive airway pressure adherence: problems and interventions. Sleep Med Clin. 2006;1(4):533–539. doi:10.1016/j.jsmc.2006.10.005
- 10. Certal VF, Zaghi S, Riaz M, et al. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2015;125(5):1254–1264. doi:10.1002/lary.25032
- 11. Gillespie MB, Soose RJ, Woodson BT, et al. Upper airway stimulation for obstructive sleep apnea: patient-reported outcomes after 48 months of follow-up. Otolaryngol Head Neck Surg. 2017;156(4):765–771. doi:10.1177/0194599817691491
- 12. Soose RJ, Woodson BT, Gillespie MB, et al. Upper airway stimulation for obstructive sleep apnea: self-reported outcomes at 24 months. J Clin Sleep Med. 2016;12(1):43–48. doi:10.5664/jcsm.5390
- 13. Strollo PJ, Gillespie MB, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: durability of the treatment effect at 18 months. Sleep. 2015;38(10):1593–1598. doi:10.5665/sleep.5054
- 14. Strollo PJ, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139–149. doi:10.1056/NEJMoa1308659
- 15. Woodson BT, Soose RJ, Gillespie MB, et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181–188. doi:10.1177/0194599815616618
- 16. Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol Respir Environ Exerc Physiol. 1978;44(6):931–938. doi:10.1152/jappl.1978.44.6.931
- 17. Eastwood PR, Barnes M, Walsh JH, et al. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep. 2011;34(11):1479–1486. doi:10.5665/sleep.1380
- 18. Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307.
- 19. Morin CM, Belleville G, Bélanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi:10.1093/sleep/34.5.601
- 20. Fries JF, Cella D, Rose M, Krishnan E, Bruce B. Progress in assessing physical function in arthritis: PROMIS short forms and computerized adaptive testing. J Rheumatol. 2009;36(9):2061–2066. doi:10.3899/jrheum.090358
- 21. Jensen RE, Potosky AL, Reeve BB, et al. Validation of the PROMIS physical function measures in a diverse U.S. population-based cohort of cancer patients. Qual Life Res. 2015;24(10):2333–2344. doi:10.1007/s11136-015-0992-9
- 22. Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33(6):781–792. doi:10.1093/sleep/33.6.781
- 23. Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMISTM sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2011;10(1):6–24. doi:10.1080/15402002.2012.636266
- 24. Saffer BY, Lanting SC, Koehle MS, Klonsky ED, Iverson GL. Assessing cognitive impairment using PROMIS(®) applied cognition-abilities scales in a medical outpatient sample. Psychiatry Res. 2015;226(1):169–172. doi:10.1016/j.psychres.2014.12.043
- 25. Becker H, Stuifbergen A, Lee H, Kullberg V. Reliability and validity of PROMIS Cognitive abilities and cognitive concerns scales among people with multiple sclerosis. Int J MS Care. 2014;16(1):1–8. doi:10.7224/1537-2073.2012-047
- 26. Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the patient-reported outcomes measurement information system (PROMIS®): depression, anxiety, and anger. Assessment. 2011;18(3):263–283. doi:10.1177/1073191111411667
- 27. Teresi JA, Ocepek-Welikson K, Kleinman M, Ramirez M, Kim G. Psychometric properties and performance of the patient reported outcomes measurement information system® (PROMIS®) depression short forms in ethnically diverse groups. Psychol Test Assess Model. 2016;58(1):141–181.
- 28. Teresi JA, Ocepek-Welikson K, Kleinman M, Ramirez M, Kim G. Measurement equivalence of the patient reported outcomes measurement information system® (PROMIS®) anxiety short forms in ethnically diverse groups. Psychol Test Assess Model. 2016;58(1):183–219.
- 29. Cella D, Riley W, Stone A, et al. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi:10.1016/j.jclinepi.2010.04.011
- 30. Rothrock NE, Hays RD, Spritzer K, Yount SE, Riley W, Cella D. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the patient-reported outcomes measurement information system (PROMIS). J Clin Epidemiol. 2010;63(11):1195–1204. doi:10.1016/j.jclinepi.2010.04.012
- 31. Baptista PM, Costantino A, Moffa A, Rinaldi V, Casale M. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: patient selection and new perspectives. Nat Sci Sleep. 2020;12:151–159. doi:10.2147/NSS.S221542
- 32. Luyster FS, Buysse DJ, Strollo PJ. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med. 2010;6(2):196–204.
- 33. Wallace DM, Vargas SS, Schwartz SJ, Aloia MS, Shafazand S. Determinants of continuous positive airway pressure adherence in a sleep clinic cohort of South Florida Hispanic veterans. Sleep Breath. 2013;17(1):351–363. doi:10.1007/s11325-012-0702-6
- 34. Dhanda Patil R, Hong MP, Ishman SL. Hypoglossal nerve stimulation in veterans with comorbid insomnia and sleep apnea. Otolaryngol Head Neck Surg. 2021;164(6):1345–1353. doi:10.1177/0194599820982638
- 35. Meleca JB, Kominsky AH. Reconfiguration of upper airway stimulation devices utilizing awake endoscopy. The Laryngoscope. 2020;130:2494–2498. doi:10.1002/lary.28569
- 36. Kezirian EJ, Goding GS, Malhotra A, et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res. 2014;23(1):77–83. doi:10.1111/jsr.12079
- 37. Gupta MA, Simpson FC, Lyons DCA. The effect of treating obstructive sleep apnea with positive airway pressure on depression and other subjective symptoms: a systematic review and meta-analysis. Sleep Med Rev. 2016;28:55–68. doi:10.1016/j.smrv.2015.07.002
- 38. Dzierzewski JM, Dautovich N, Ravyts S. Sleep and cognition in the older adult. Sleep Med Rev. 2018;13(1):93–106. doi:10.1016/j.jsmc.2017.09.009
