Introduction
Nivolumab is an immune checkpoint inhibitor (ICI) and an anti-programmed death-1 (PD-1) monoclonal antibody. In the Checkmate 141 multi-regional phase III trial, the nivolumab group demonstrated statistically significantly longer overall survival (OS) than the group receiving a single agent of the investigator’s choice (methotrexate, docetaxel, cetuximab) for platinum-refractory, recurrent or metastatic head and neck squamous cell carcinoma. Currently, National Comprehensive Cancer Network (NCCN) guidelines recommend nivolumab as a subsequent-line category 1 therapy for platinum-refractory, recurrent or metastatic head and neck cancer, and the occurrence of tracheobronchial chondritis as an immune-related adverse event (irAE) associated with nivolumab and other ICIs is extremely rare. Herein, we report about a case in which tracheobronchial chondritis developed during the use of nivolumab for local recurrence of hypopharyngeal squamous cell carcinoma.
Case report
Clinical presentation
A 71-year-old man with a chief complaint of right neck swelling was referred to our head and neck oncology department for a detailed examination after first perceiving right neck swelling one month ago. He had previously undergone surgery for gallstones. He had smoked 40 cigarettes/day from 20 to 50 years of age. He consumed 2 cans of beer and 2 glasses of shochu per day. Neck palpation during the initial examination revealed multiple, fixed, hard, and painless lymphadenopathy in the patient’s right neck. Flexible fiberoptic laryngoscopy revealed a tumor with an irregular surface in the posterior hypopharyngeal wall. Biopsy of the hypopharyngeal tumor led to a diagnosis of squamous cell carcinoma. Contrast-enhanced computed tomography (CT) showed a densely stained tumor in the posterior hypopharyngeal wall. The right neck showed multiple lymphadenopathy from the superior deep jugular nodes to the inferior deep jugular nodes, while the left neck showed localized lymphadenopathy. Lymphadenopathy in the right neck presented with extranodal extension and completely surrounded the right carotid artery. 18-Fluorodeoxyglucose (FDG)-positron emission tomography (PET) combined with CT showed FDG uptake in the primary hypopharyngeal tumor and cervical lymph node metastasis, with no FDG uptake indicative of distant metastasis. The patient was diagnosed with T2N3bM0 stage IVB hypopharyngeal squamous cell carcinoma.
Because of right cervical lymph node metastasis having surrounded the right carotid artery, the carcinoma was judged to be inoperable. Definitive therapy was performed with concurrent cisplatin plus radiotherapy (cisplatin: three courses 80 mg/m2 doses; radiotherapy: total dose, 70 Gy). Complete response was observed in the primary hypopharyngeal tumor and cervical lymph node metastases on the CT scan taken 2 months and FDG-PET scan taken 3 months post-chemoradiotherapy. Five months after concluding the therapy, the patient presented with left cervical lymph node metastasis, prompting us to perform left neck dissection (level II−V). Five months later, right cervical lymph node metastasis occurred, prompting us to perform right neck dissection (level II−V). Two months after that, the patient experienced local recurrence in the hypopharynx. We had already performed concurrent cisplatin plus radiotherapy as well as bilateral neck dissection and had deemed salvage surgery to be impractical. Moreover, owing to the observation of left cervical lymph node metastasis five months after concurrent cisplatin plus radiotherapy, we deemed the carcinoma to be platinum refractory. Therefore, we elected to use nivolumab as a first-line chemotherapy and began to administer nivolumab (240 mg/body) once every two weeks.
Flexible fiberoptic laryngoscopy and CT after 9 cycles of nivolumab indicated complete response (CR). After 10 cycles of nivolumab, the patient developed cough and sputum. He had no fever, and his SpO2 (blood oxygen saturation) level was 97% on room air. In a blood sample, although his white blood count (WBC, 5060/μL) and KL-6 (sialylated glycoprotein Krebs von den Lungen-6; 141 U/mL) levels were normal, his C-reactive protein (CRP) levels were elevated at 10.11 mg/dL. Contrast-enhanced CT of the neck (Figure 1a, b) did not show interstitial lung disease but did demonstrate edematous thickening around the trachea and bilateral bronchi and elevated amounts of adjacent subcutaneous fat tissue. We suspected tracheobronchitis and administered oral Garenoxacin mesilate hydrate (Toyama and Taisho Pharmaceutical Co., Ltd., Tokyo, Japan) for one week. The patient’s symptoms did not improve, and his CRP levels remained at approximately 10 mg/dL (with no improvement). His procalcitonin levels were negative, thus ruling out bacterial infection. 18-FDG-PET/CT (Figure 1c-e) demonstrated diffuse FDG uptake in the trachea and bilateral bronchi. The standardized maximum uptake value was 7.5. When bronchoscopy was performed, redness and swelling throughout the bronchi were observed, though the subsegmental bronchi could not be observed owing to stenosis. Biopsy of the reddened portion of the bronchi demonstrated pathological findings consistent with an irAE (Figure 2a-f). Lymphocyte-based inflammatory cell infiltration was observed, primarily with T cells, and there was no B cell infiltration. CD4+ T cell and CD8+ T cell infiltration was observed. A blood sample was positive for anti-type II collagen antibodies. We diagnosed the patient with tracheobronchial chondritis as an irAE associated with anti-PD-1 antibody drugs and initiated treatment with prednisolone (60 mg, 1 mg/kg). After four days of prednisolone treatment, the patient’s cough and sputum disappeared. In addition, his CPR level (which had been ≥10 mg/dL for roughly one month) began to decrease. At one week, we reduced the prednisolone dose to 40 mg. CT performed two weeks after prednisolone was initiated (Figure 1f, g) revealed that tracheobronchial thickening had disappeared. We planned to consider whether to resume nivolumab after tapering and terminating prednisolone.
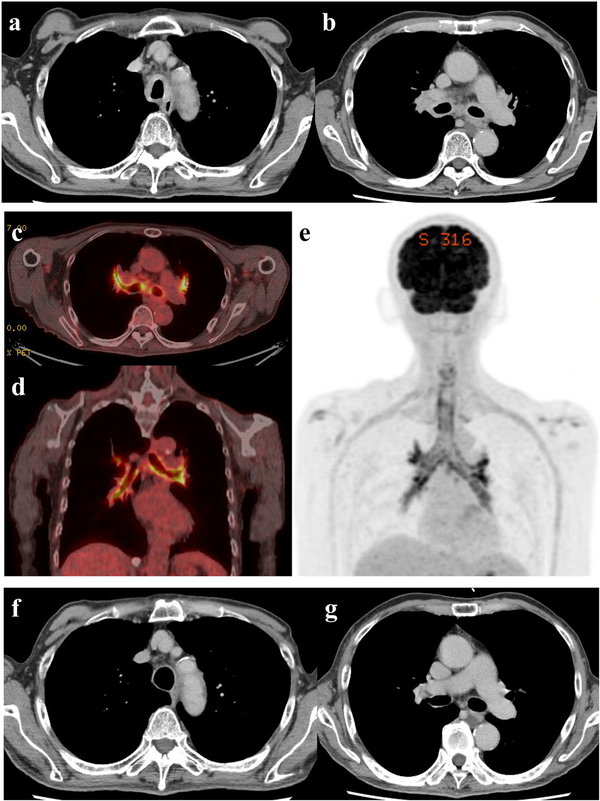
Figure 1
Imaging findings. (a, b) Axial contrast-enhanced computed tomography (CT) for suspected tracheal chondritis. (a) A CT image demonstrating thickening of the tracheal wall with edematous adjacent fat tissue. (b) A CT image demonstrating thickening of the wall of the bilateral main bronchi with edematous adjacent fat tissue. (c-e) 18-Fluorodeoxyglucose positron emission tomography (18-FDG-PET)/CT for suspected tracheal chondritis. (c) An axial 18-FDG-PET/CT image demonstrating obvious FDG uptake into the wall of the bilateral main bronchi and the lobar bronchi. (d) A coronal 18-FDG-PET/CT image demonstrating obvious FDG uptake into the wall of bilateral main bronchi and the lobar bronchi. (e) A maximum intensity projection image demonstrating FDG uptake into the wall of the trachea, the bilateral main bronchi, and the lobar bronchi. Multiple dots on the wall were consistent with inflammatory chondroid tissue. (f, g) Axial contrast-enhanced CT after therapeutic intervention for an immune-related adverse event (irAE). (f) An axial CT image demonstrating the thinned wall of the trachea compared with that of the trachea at the presentation of the irAE, accompanied with an adjacent fat density decrease. (g) An axial CT image demonstrating the thinned wall of the bilateral main bronchi.
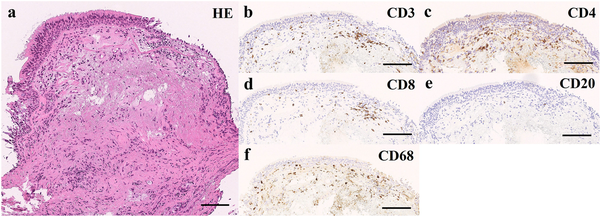
Figure 2
Microscopic observation of the bronchial tissue. (a-f) A bronchi biopsy was performed, and the sample was analyzed by (a) hematoxylin-eosin (HE) staining and (b-e) immunohistochemical detection using (b) anti-CD3, (c) anti-CD4, (d) anti-CD8, (e) anti-CD20, and (f) anti-CD68 antibodies (scale bar, 100 mm). Shedding and erosion of the mucosal epithelium were observed. Edema and desmoplasia were observed in the interstitial tissue directly below the mucosa. CD3+ T cell and CD68+ histiocyte inflammatory cell infiltration were likewise observed. We confirmed CD4+ T cell and CD8+ T cell infiltration, with almost no CD20+ B-cell infiltration.
Discussion
Because of evidence from the CheckMate 141 and KEYNOTE-048 studies, nivolumab and pembrolizumab have become extremely common as first-line or second-line treatment for recurrent or metastatic head and neck cancers. irAEs may occur with the use of ICIs. There are consensus-backed diagnoses and therapies for common irAEs. However, there is not enough information on the diagnosis of and treatment for very rare irAEs, such as reported herein.
Among patients who receive nivolumab for recurrent or metastatic head and neck cancer, OS is more favorable among patients who manifest irAEs., In a study conducted by Okamoto et al., OS was more favorable among patients who developed irAEs during treatment with nivolumab. A study by Matsuo et al. also found that among patients treated with nivolumab, OS, progression-free survival, and overall response rate were more favorable among patients with irAEs. These results demonstrate that irAEs should be well controlled so that ICIs can be continued over the long term.
irAEs with ICIs are more unpredictable than adverse events with cytotoxic anticancer or molecular targeted drugs. What matters is how irAEs are detected. Our patient manifested tracheobronchial chondritis, an extremely rare irAE, after CR was achieved with nivolumab. The tremendous rarity of irAEs initially hindered differential diagnosis. In CT, we observed tracheobronchial thickening and a contrast effect in the tracheobronchial wall. In 18-FDG-PET/CT, we observed high uptake of FDG throughout the trachea and bronchi, particularly in the tracheal cartilage, which served as evidence of tracheobronchial chondritis. Bronchoscopy revealed diffuse redness in the trachea and bronchi, while pathologic examination of a resected specimen revealed findings consistent with an irAE. The pathologic examination revealed inflammatory cell infiltration consisting primarily of lymphocytes and T cells but did not show B-cell infiltration. CD4+ T cell and CD8+ T cell infiltration was observed. We also observed CD68+ macrophages. Although the histopathological manifestation of tracheobronchial chondritis as an irAE is not extremely well characterized, the above findings are similar to those observed in ICI-related lung injury (reported in a previous study) and were histopathologically consistent with ICI-associated histology. Unlike the histology of bronchial asthma, we did not observe basement membrane thickening, marked eosinophilic infiltration, or bronchial gland hypertrophy. In addition, the patient did not respond whatsoever to initial treatment with antibiotics, whereas steroid therapy was remarkably effective. These findings were also indicative of an irAE.
We could find only 2 reports written in English of cases reporting tracheobronchial chondritis as an irAE., Both of these case reports were from Japan. More specifically, the cases reported by Kuba et al and by Asoh et al. were reports of hypopharyngeal squamous cell carcinoma and esophageal carcinoma, respectively. Nivolumab was used as an ICI in both cases. As in our case, both patients in the above case reports demonstrated elevated CRP levels with the development of tracheobronchial chondritis. In these reports, CRP levels were 10.81 mg/dL and 15.19 mg/dL, respectively. When we initiated steroid therapy, the patient’s symptoms improved and his CRP levels decreased. We note that these CRP levels were suggestive of tracheobronchial chondritis. We adjusted his prednisolone dose in consideration of various factors, including his CRP levels. In both of the above cited case reports, diagnoses were based on CT, bronchoscopy, and biopsy-based pathological findings. Secondarily, both patients were confirmed to be anti-type II collagen antibody-positive. Ogimoto et al. reported a case of relapsing polychondritis in a patient treated with nivolumab for mandibular cancer. In that report, FDG-PET/CT was performed and FDG uptake was observed in tracheobronchial cartilage and nasal septum certilage. Compared to the report by Ogimoto et al., the present case did not show as much FDG accumulation in the nasal septum cartilage but showed more profound FDG accumulation in the tracheobronchial cartilage. The novelty of this report is that we were able to present an image depicting the clear accumulation of FDG, focusing on the tracheal cartilage (Figures 1c-e).
In recent years, ICIs have played a crucial role in pharmacotherapy for cancer. Although ICIs are currently used primarily for recurrent or metastatic cancer, they are likely to be used in various situations (including induction chemotherapy) moving forward. Based on our findings and those of prior case reports, tracheobronchial chondritis, although infrequent, must be kept in mind as a potential irAE (as in the present case).
Conclusion
Our patient developed tracheobronchial chondritis as an irAE during the use of nivolumab for recurrent hypopharyngeal squamous cell carcinoma. This diagnosis was reached based on CT, 18-FDG-PET/CT, bronchoscopy, and pathologic examination findings. High-dose steroids improved the patient’s symptoms and imaging findings. Tracheobronchial chondritis must be recognized and considered a potential irAE when administering ICI.
Declaration of conflicting interests The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: K. Tsukahara has received grant support from Ono Pharmaceutical Co., Ltd and Bristol-Myers Squibb. The other authors have no actual or potential conflicts of interest or financial relationships to disclose.
Funding The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval Our institution does not require ethical approval for reporting individual cases.
Informed consent Written informed consent was obtained from the patient for his anonymized information to be published in this article.
References
- 1. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67.
- 2. NCCN Clinical Practice Guidelines in Oncology (Head and Neck Cancer). Pennsylvania: National Comprehensive Cancer Network (NCCN). 2022. Available at: https://www.nccn.org
- 3. Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet (London, England). 2019;394:1915–1928.
- 4. Okamoto I, Sato H, Kondo T, et al. Efficacy and safety of nivolumab in 100 patients with recurrent or metastatic head and neck cancer - a retrospective multicentre study. Acta Oto-Laryngologica. 2019;139:918–925.
- 5. Matsuo M, Yasumatsu R, Masuda M, et al. Relationship between immune-related adverse events and the long-term outcomes in recurrent/metastatic head and neck squamous cell carcinoma treated with nivolumab. Oral Oncology. 2020;101:104525.
- 6. Ibraheim H, Perucha E, Powell N. Pathology of immune-mediated tissue lesions following treatment with immune checkpoint inhibitors. Rheumatology (Oxford, England). 2019;58(Suppl 7):vii17–28.
- 7. Kuba K, Nakahira M, Inoue H, et al. Nivolumab-related tracheobronchial chondritis: extremely rare manifestation of an immune-related adverse effect. Head & Neck 2020;42:E43–8.
- 8. Asoh T, Yanagihara T, Tanaka R, et al. Tracheobronchial chondritis associated with immune checkpoint blockade. Internal Medicine 2021;60:2517–2518.
- 9. Ogimoto T, Yoshida H, Mizuta M, et al. Relapsing polychondritis after treatment with PD-1 blockade. Invest New Drugs. 2021. Online ahead of print.
