Introduction
Over the last 10 years, robot-assisted surgery gained importance in head and neck surgery. Transoral robotic surgery (TORS) has proven to be an effective tool for resection of head and neck tumors. - In this field, 3 approved robotic surgery systems are available: The Intuitive da Vinci system, the Medrobotics Flex Robotic Systems for TORS, and the Medineering Robotic Endoscope Guiding System. Originally designed for robotic endoscope guiding in endoscopic sinus and skull base surgery, the Medineering Robotic Endoscope Guiding System is approved for robotic assistance in all ENT procedures, including transoral endoscopic surgery and endoscopic ear surgery. In this study, we used the system for endoscopic orbital decompressions in patients with Graves’ orbitopathy (GO). Graves’ orbitopathy is the most common extrathyroidal complication of Graves’ disease. Graves’ disease is an autoimmune disease affecting extrathyroidal tissues such as orbital tissue and leading to the following clinical symptoms: lid retraction, impaired eye movement due an extraocular muscle fibrosis, and hypertrophy as well as proptosis due to increased intraorbital fat tissue and soft tissue inflammation. - In rare cases, one of the major risks of GO is permanent blindness due to optic nerve compression. One of the most common side effects after orbital decompression is esotropia, which has to be corrected surgically by performing a tendon elongation. Additionally, other treatment options for GO include steroids and orbital irradiation in order to reduce inflammation of the orbital tissue. More recently, targeted therapies for GO such as treatment with monoclonal antibodies by blocking the function of insulin growth factor receptor 1 have turned out to be effective in patients with mild or moderate GO. , In cases of insufficient lid closing or diplopia, orbital decompression is a surgical option to alleviate the conditions. , In patients with optic nerve compression, corneal ulcus, or increased intraocular pressure, the surgical orbital decompression is used as an emergency procedure as well.
Decompression surgery in GO can be performed by means of diverse approaches and techniques as reported in the literature: Procedures include orbital wall removal, removal of intraorbital fat tissue, advancement or augmentation of the orbital frame, and combined techniques. As a consequence of orbital wall removal, hyperplastic orbital tissue expands into the newly created space, thus leading to proptosis reduction and consecutive reduction in GO symptoms. The amount and number of orbital walls removed correlates with degree of GO reduction and is tailored to the patients’ needs; hence, removal of up to 4 orbital walls is reported in the literature. Because of a lower incidence of postoperative-onset diplopia, a balanced decompression method (removal of the medial and lateral orbital wall) is currently considered as the preferred technique of choice. For low morbidity outcomes, minimally invasive approaches are favorable. In our clinic, we prefer a transnasal endoscopic approach to the medial orbital walls and a transcutaneous endoscopic lateral approach to access the lateral wall via minimal invasive incision next to the lateral cantus. Overall, this technique provides good functional as well as aesthetic outcomes.
In transnasal and skull base endoscopic surgery, the endoscope is commonly maneuvered by one hand thus limiting the surgeon’s ability for operation. The Medineering Robotic Endoscope Guiding System was developed to overcome this disadvantage. Friedrich et al already evaluated the robotic arm in advanced transnasal surgery in fresh frozen cadavers and verified the applicability. In a single surgeon procedure, the system allows a “2-hand technique” and with 2 surgeons operating a “4-hand” technique is possible without an additional hand holding the endoscope. The objective of this study was to evaluate the feasibility and patients’ safety by using a robotic endoscope guiding system in 8 patients with GO undergoing endoscopic orbital decompression.
Material and Methods
Medineering Robotic Endoscope Guiding System
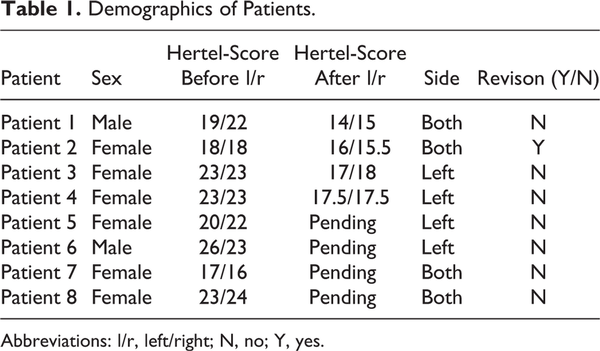
The system is a compact articulated robotic arm for the application of holding and maneuvering a rigid endoscope during transnasal interventions (Figure 1). The Medineering robotic arm received a CE mark from the European Commission to position passive adapters and robots in ear, nose, and throat surgery, neurosurgery, and spinal surgery. The positioning arm has 7 joints, so it can be driven in almost every position during surgery (Figure 1). It is fixed directly to the operating table, and its movements are controlled by a foot pedal during surgery (Figure 2).
Figure 1
The Medineering Robotic Endoscope Guiding System. The slim and flexible positioning arm is fixed directly to the operating table on the left-hand side of the picture and connected to a control unit. The system is ready-to-use within 10 minutes. Hence, it fits perfectly in every surgical workplace and workflow.

Figure 2
The food pedal of the Medineering Robotic Endoscope System is shown in Figure 2. The usage is smooth and intuitive.
Patients
The study was approved by the local ethics committee of the Medical Faculty of the University Duisburg-Essen and was performed according to Declaration of Helsinki. We performed balanced orbital decompression in 8 patients using the Medineering endoscopic guiding system. All patients underwent routine ophthalmological examination before and after surgery, including visual acuity, exophthalmometry (Hertel-Score), orthoptic evaluation, slit lamp, and fundoscopy. Moreover, all patients received a routine rhinoscopy before and after surgery, performed with a 30°, 4 mm diameter endoscope. Patients’ demographics are shown in Table 1. One patient underwent revision surgery after an incomplete orbital decompression in another department. Bilateral orbital decompression was applied in 5 of 8 cases, 3 patients underwent unilateral surgery. Patient 7 and 8 underwent emergency decompressions because of threatening loss of sight.
Surgical Technique
All surgeries were performed under general anesthesia by one surgeon. After nasal decongestion, the tip of the rigid endoscope attached to the robotic guiding system was manually placed in the nasal vestibule. The endoscope was equipped with a 4K camera, connected to a 55′ monitor (Olympus Visera 4k UHD CLV-5400 HD camera with LMD X55OS 4K display, Sony; Olympus, Tokyo, Japan). During surgery, the endoscope attached to the robotic arm was moved and repositioned using a foot pedal.
The surgeon was able to use standard surgical instruments and suction devices with both hands simultaneously during the intervention (Figure 2). The uncinate process and the ethmoid were removed completely while preserving the medial turbinate. The ostium of the maxillary sinus was widely enlarged to avoid postoperative chronic sinus disease. The anterior skull base, the ostia of the frontal and the sphenoid sinus, the lamina papyracea, and the medial as well as inferior orbital wall were exposed. The lamina papyracea was fractured and removed for exposing the periorbit. The periorbital membrane was incised and partially resected from posterior to anterior thus allowing the orbital content to prolapse into the ethmoid cavity. For resection of the lateral wall, a 10-mm incision next to the lateral cantus was performed. The periosteum was dissected and a bony triangle of the lateral wall was removed using piezosurgery. Next, the rigid endoscope attached to the robotic arm was placed in the surgical field thus allowing endoscopic 4-hand surgery via a 4K monitor (Figure 3). The deep lateral orbital wall was removed with a high speed drill. After resecting the lateral periorbit, orbital fat could be removed. The lateral orbital rim was reconstructed by replanting the anterior part of the temporarily resected bone using microplates. All surgical maneuvers were performed using the robotic endoscope holder.
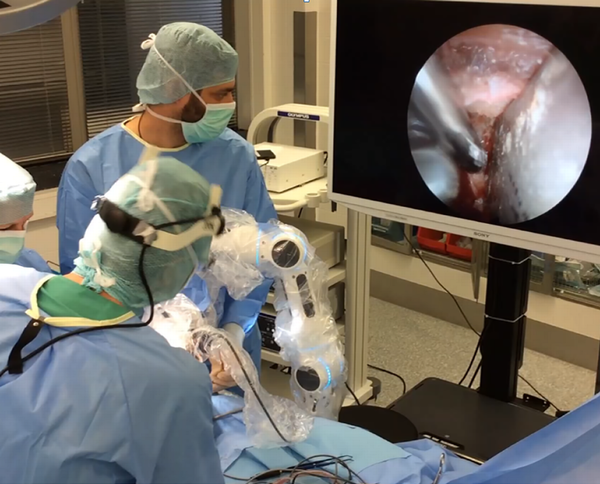
Figure 3
The lateral orbital decompression. While one surgeon assists by opening the cavity with 2 hooks, the operating surgeon has the possibility to use his 2 hands for surgery while the Medineering robotic arm guides the endoscope.
Results
Handling of the Medineering Robotic Endoscope Guiding System
In the present study, the endoscope could be placed easily by the Medineering Robotic Endoscope Guiding System in the nasal cavity. Tool motion and control were comfortably and precisely performed by using the foot pedal. Movements with the attached endoscope were feasible, thus allowing 2- to 4-hand technique. As compared to surgery without the Medineering endoscopic guiding system, the setup time was longer (less than 10 minutes) while the operating time was not extended. Intraoperatively, there were no adverse events (AE) or serious AEs. It is recommended to use an endoscope with irrigation channel.
Patients Outcome
Postoperatively, 4 of the patients offered normal Hertel readings (≤18 mm) after 3 months, while the results of the other patients are pending. Patients 7 and 8 showed a returning visual acuity immediately after decompression. Allover, visual acuity was obtained in all patients. None of the patients developed acute sinusitis, synechia, or needed further treatment, while especially synechia is a common side effect after endoscopic skull base surgery (ESBS).
Discussion
Despite the use of robotic systems in a variety of different surgical fields, no system was developed for ESBS. Transoral surgical modalities for head and neck tumors increased over the last years since the Food and Drug Administration (FDA) approved the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, California) in 2009. Even the flexible robotic systems (Medrobotics®) in transoral surgery were optimized and carried out as described before. -,,, - Transoral robotic surgery as well as the da Vinci Surgical System cannot be applied in skull base surgery due to the fact that these systems are too big for endonasal surgery. Moreover, there are no surgical instruments which can be used with these systems (eg, forceps, drill, piezo system). So far, no commercially available robotic system is suitable for ESBS. However, robotic surgery in ESBS would provide a great benefit in endoscopic guiding systems especially using a 4-hand technique, which has been implemented in the surgical routine for skull base surgery. , Bolzoni Villaret et al designed a prototype for hybrid robotic ESBS. However, reports on the clinical application are still missing. Over the last years, other systems have been tested, but their application is still prevented by excessive dimensions, unsafe systems, and surgical time prolongation : Nimsky et al used a prototype of a hexapod-based Evolution 1 robotic system in 2 patients with pituitary adenoma. In 2005, Wurm et al. described a telemanipulator named “A73 robot” for cadaver dissection. In the same year, they presented an advanced system interconnected with a redundant navigation system for increasing intraoperative safety but they concluded that this feature did not make the robot more accurate. They assumed that this advanced system added a potent safety feature to the system. Other groups described multiple cadaver studies about different endoscopic holding systems without satisfactory safety for transition into clinical application. - In 2013, Trevillot et al wrote about an improved robotic system consisting of several flexible, precurved tubes that were nested within each other. Up to now the system was tested for cadaver dissections only, but seems to be interesting for further studies. In 2004, Kraft et al described an automated endoscopic system for optical positioning (AESOP) during laparoscopic abdominal surgery. The benefit seems to be saving manpower, but the surgeon has to accept less comfort as well as a prolonged duration of surgery. Nevertheless, it is another interesting approach for endoscopic guiding systems. Nathan et al described the use of the FDA-approved robot AESOP in a cadaver study. The advanced part of this system was a video-assisted voice-controlled robot. This cadaver study postulated that the approach seems to be efficient, but further studies are missing. All together due to the narrow anatomy and working space, no robotic surgical tool was implemented in clinical routine for ESBS so far. Additionally, there were no surgical instruments that could be used for robotic systems in the nasal cavity.
Conclusions
The Medineering Robotic Endoscope Guiding System seems to be a safe and effective support in ESBS especially in the context of orbital decompression. It allows a 2- and 4-hand technique being really helpful to preserve important anatomical structures like the optic nerve. The setup time in these abovementioned 8 cases was prolonged while the operating time was not extended. One criticism seems to be that the robotic arm appears to be relatively heavy so that surgeons should familiarize with the system before use. To our knowledge, this is the first study describing the successful application of a robotic system in orbital surgery.
In future, the Medineering Robotic Endoscope Guiding System has to be optimized by implementing advanced control units, for example, joystick systems. In general, surgical robots will provide a programmable digital platform for the integration of preoperative 3D planning data from CT or MRI scans in order to determine surgical aims, to describe a surgical corridor, and to highlight surgical landmarks. This can help to reduce unwanted tissue damage and complications. Thus, the Medineering Robotic Endoscope Guiding System is an interesting platform for developing application-specific plug-and-play robotics in ESBS.
Acknowledgments
The authors wish to thank Susanne Zapf for her support in manuscript writing.
Authors’ Note Stefan Mattheis and Anke Schlüter contributed equally to this work.
Declaration of Conflicting Interests The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Stefan Mattheis received speaker fees.
Funding The author(s) received no financial support for the research, authorship, and/or publication of this article.
Anke Schlüter
https://orcid.org/0000-0003-4523-9798
References
- 1. Friedrich DT, Modes V, Hoffmann TK, Greve J, Schuler PJ, Burgner-Kahrs J. Teleoperated tubular continuum robots for transoral surgery—feasibility in a porcine larynx model. Int J Med Robot. 2018;14(5):e1928.
- 2. Friedrich DT, Scheithauer MO, Greve J, Hoffmann TK, Schuler PJ. Recent advances in robot-assisted head and neck surgery. Int J Med Robot. 2017;13(2):e1744.
- 3. Friedrich DT, Sommer F, Scheithauer MO, Greve J, Hoffmann TK, Schuler PJ. An innovate robotic endoscope guidance system for transnasal sinus and skull base surgery: proof of concept. J Neurol Surg B Skull Base. 2017;78(6):466–472.
- 4. Hasskamp P, Lang S, Holtmann L, Stuck BA, Mattheis S. First use of a new retractor in transoral robotic surgery (TORS). Eur Arch Otorhinolaryngol. 2016;273(7):1913–1917.
- 5. Lang S, Mattheis S, Hasskamp P, et al. A European multicenter study evaluating the flex robotic system in transoral robotic surgery. Laryngoscope. 2017;127(2):391–395.
- 6. Mattheis S, Hasskamp P, Holtmann L, et al. Flex Robotic System in transoral robotic surgery: the first 40 patients. Head Neck. 2017;39(3):471–475.
- 7. Schuler PJ, Hoffmann TK, Veit JA, et al. Hybrid procedure for total laryngectomy with a flexible robot-assisted surgical system. Int J Med Robot. 2017;13(2):e1749.
- 8. Bahn RS. Autoimmunity and Graves’ disease. Clin Pharmacol Ther. 2012;91(4):577–579.
- 9. Eckstein A, Dekowski D, Fuhrer-Sakel D, Berchner-Pfannschmidt U, Esser J. Graves’ ophthalmopathy [in German]. Ophthalmologe. 2016;113(4):349–364. quiz 465-346.
- 10. Eckstein A, Esser J, Mattheis S, Berchner-Pfannschmidt U. Graves’ orbitopathy. Klin Monbl Augenheilkd. 2016;12;233:1385–1407.
- 11. McKeag D, Lane C, Lazarus JH, et al. Clinical features of dysthyroid optic neuropathy: a European Group on Graves’ Orbitopathy (EUGOGO) survey. Br J Ophthalmol. 2007;91(4):455–458.
- 12. Oeverhaus M, Fischer M, Hirche H, Schluter A, Esser J, Eckstein AK. Tendon elongation with bovine pericardium in patients with severe esotropia after decompression in graves’ orbitopathy-efficacy and long-term stability. Strabismus. 2018;26(2):62–70.
- 13. Piantanida E, Bartalena L. Teprotumumab: a new avenue for the management of moderate-to-severe and active graves’ orbitopathy? J Endocrinol Invest. 2017;40(8):885–887.
- 14. Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748–1761.
- 15. Stahr K, Holtmann L, Schluter A, et al. The influence of orbital decompression on objective nasal function in patients with graves’ orbitopathy. Eur Arch Otorhinolaryngol. 2018;275(10):2507–2513.
- 16. European Group on Graves’ Orbitopathy, Mourits MP, Bijl H, et al. Outcome of orbital decompression for disfiguring proptosis in patients with graves’ orbitopathy using various surgical procedures. Br J Ophthalmol. 2009;93(11):1518–1523.
- 17. Leone CR Jr, Piest KL, Newman RJ. Medial and lateral wall decompression for thyroid ophthalmopathy. Am J Ophthalmol. 1989;108(2):160–166.
- 18. Stahr K, Eckstein A, Holtmann L, et al. A comparative analysis of piezosurgery and oscillating saw for balanced orbital decompression. Orbit. 2019;38(6):433–439. Epub 2018.
- 19. Schluter A, Ahmadipour Y, Vogelsang T, et al. Evaluation of the application of rhino-septal splints in endoscopic transsphenoidal skull base surgery. Eur Arch Otorhinolaryngol. 2016;273(12):4571–4578.
- 20. Hoffmann TK, Friedrich DT, Schuler PJ. Robot-assisted surgery in the head and neck region [in German]. HNO. 2016;64(9):658–666.
- 21. Mattheis S, Hoffmann TK, Schuler PJ, Dominas N, Bankfalvi A, Lang S. The use of a flexible CO2-laser fiber in transoral robotic surgery (TORS) [in German]. Laryngorhinootologie. 2014;93(2):95–99.
- 22. Mattheis S, Kansy B, Hasskamp P, Holtmann L, Lang S. Advances in transoral robotic surgery [in German]. HNO. 2015;63(11):752–757.
- 23. Mattheis S, Mandapathil M, Rothmeier N, Lang S, Dominas N, Hoffmann TK. Transoral robotic surgery for head and neck tumors: a series of 17 patients [in German]. Laryngorhinootologie. 2012;91(12):768–773.
- 24. Bolzoni Villaret A, Doglietto F, Carobbio A, et al. Robotic transnasal endoscopic skull base surgery: Systematic review of the literature and report of a novel prototype for a hybrid system (Brescia Endoscope Assistant Robotic Holder). World Neurosurg. 2017;105:875–883.
- 25. Castelnuovo P, Pistochini A, Locatelli D. Different surgical approaches to the sellar region: Focusing on the “two nostrils four hands technique. Rhinology. 2006;44(1):2–7.
- 26. Nimsky C, Rachinger J, Iro H, Fahlbusch R. Adaptation of a hexapod-based robotic system for extended endoscope-assisted transsphenoidal skull base surgery. Minim Invasive Neurosurg. 2004;47(1):41–46.
- 27. Wurm J, Bumm K, Steinhart H, et al. Development of an active robot system for multi-modal paranasal sinus surgery [in German]. HNO. 2005;53(5):446–454.
- 28. Wurm J, Dannenmann T, Bohr C, Iro H, Bumm K. Increased safety in robotic paranasal sinus and skull base surgery with redundant navigation and automated registration. Int J Med Robot. 2005;1(3):42–48.
- 29. Chan JY, Leung I, Navarro-Alarcon D, et al. Foot-controlled robotic-enabled endoscope holder for endoscopic sinus surgery: a cadaveric feasibility study. Laryngoscope. 2016;126(3):566–569.
- 30. Fischer M, Grobner C, Dietz A, Krinninger M, Luth TC, Strauss G. A technique with manipulator-assisted endoscope guidance for functional endoscopic sinus surgery: proof of concept. Otolaryngol Head Neck Surg. 2011;145(5):833–839.
- 31. Strauss G, Hofer M, Kehrt S, et al. Manipulator assisted endoscope guidance in functional endoscopic sinus surgery: proof of concept [in German]. HNO. 2007;55(3):177–184.
- 32. Trevillot V, Sobral R, Dombre E, Poignet P, Herman B, Crampette L. Innovative endoscopic sino-nasal and anterior skull base robotics. Int J Comput Assist Radiol Surg.2013;8(6):977–987.
- 33. Xia T, Baird C, Jallo G, et al. An integrated system for planning, navigation and robotic assistance for skull base surgery. Int J Med Robot. 2008;4(4):321–330.
- 34. Kraft BM, Jager C, Kraft K, Leibl BJ, Bittner R. The AESOP robot system in laparoscopic surgery: increased risk or advantage for surgeon and patient? Surg Endosc. 2004;18(8):1216–1223.
- 35. Nathan CO, Chakradeo V, Malhotra K, D’Agostino H, Patwardhan R. The voice-controlled robotic assist scope holder AESOP for the endoscopic approach to the sella. Skull Base. 2006;16(3):123–131.