Introduction
Fibrous dysplasia (FD) is an uncommon benign but progressive skeletal disorder that destroys and replaces the normal bone with fibrous bone tissue. Formation of pathological tissues can lead to deformities, fractures, pain, and functional impairment., The incidence of FD is approximately 1 to 2 per 30 000 people, and both sexes are equally affected. Fibrous dysplasia accounts for 2.5% of all osseous neoplasms and 7.0% of all benign bone tumors., Fibrous dysplasia may occur at any age and most commonly affects patients younger than 30 years. The etiology of FD is controversial. de Reynal B first described FD in 1938 and believed that it was caused by the defect in the differentiation of the mesenchymal bone precursor. In 1957, George found that fibroblast hyperplasia also played an important role in the pathogenesis of FD. In general, there are 3 variant types classified by the clinical presentation: monostotic, nonsyndromic polyostotic, and McCune-Albright syndrome (MAS).
Fibrous dysplasia shows a racial predilection in that Caucasians account for more than 80% of affected patients while Asians account for only 1%. At present, there are few reports on the characteristics of patients in Asia. Fibrous dysplasia can invade the bones of the whole body, but it mostly appears in the bones of the trunk, limbs, and craniofacial region. Of these, craniofacial lesions usually occur in the maxilla. The temporal bone is rarely affected.
Although FD of the temporal bone generally grows slowly, it tends to cause some complications with disease progression, such as stenosis of the external auditory canal (EAC), conductive hearing loss, secondary cholesteatoma, otorrhea, and so on. The optimal management of patients with temporal bone FD remains a topic of debate and controversy. This article reviewed and analyzed 11 patients with temporal bone FD who were treated in our center in recent 10 years, and we summarized the experience of diagnosis and treatment in the Chinese population in order to improve the life quality of those patients in the future.
Materials and Methods
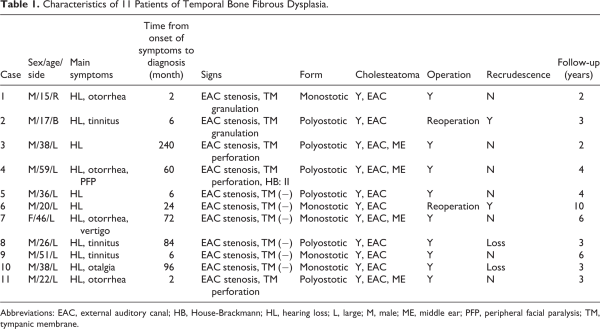
All patients with confirmed diagnosis of temporal bone FD by pathology results from August 2009 to August 2019 were included in this study. The patient’s medical records were reviewed for demographic data, clinical presentations, diagnostic findings, histopathology, serum indicators, tumor classification, treatment strategies, length of clinical follow-up, and prognosis. In addition, the involvement of individual anomalies was also analyzed. Ethical approval was obtained from the Medical Ethical Commission of the Eye and ENT Hospital affiliated to Fudan University. The characteristics of these 11 patients are presented in Table 1.
Participants with temporal bone FD underwent comprehensive otolaryngologic and audiological evaluation. Routine blood tests and general examinations were necessary before surgery. Pure tone average was determined by averaging hearing levels at 0.5, 1, 2, and 4 kHz. According to the involved scope, FD can be divided into 3 types: involvement of a single bone (monostotic form, MFD), multiple bones (polyostotic form, PFD), and multiple bones with pigmentation and endocrinology abnormalities (MAS). Facial nerve function was assessed using the House-Brackmann grading scale.
Surgical Technique
The aim of the surgery was to recover function and prevent complications. The choice of surgical approach was based on the extent of lesion involvement, physical examination, imaging studies, and pathology tests. In this study, the main operative technique consisted of canaloplasty of EAC and wide meatoplasty. A standardized approach was performed under assisted general anesthesia using an endaural incision as described in previous publications., After debridement of cholesteatoma keratin debris of the EAC, the membranous auditory canal was incised. The fibrous tissue was sharply detached from the bony ear canal down to the eardrum level. The bony ear canal was widened using diamond burrs of descending size. Subsequently, part of the concha, cartilaginous of EAC, and skin were removed, to create a widening of the cartilaginous portion of the EAC to adapt to the widened bony ear canal. The conchal flap is rotated toward the meatus to cover the part of the defect. Finally, an ear dressing with ribbon gauze was placed in the ear canal and soaked with antibiotic. If the middle ear lesions were severe, a retroauricular approach was performed. Four patients were underwent retroauricular approach in this series. Adjunctive procedures, including cholesteatoma resection, tympanoplasty, ossicular chain reconstruction, skin grafting, and facial nerve decompression, were performed when necessary. The long-term follow-up was performed in terms of postoperative hearing thresholds and EAC status with endoscope and high-resolution computed tomography (HRCT) of the temporal bone.
Statistical Analysis
Statistical analysis was performed using statistical software SPSS version 25 (SPSS Inc). Unless otherwise stated, results are presented as mean ± SD or as a percentage in case of categorical data. Normality of data distribution was analyzed using paired Student t test to compare results within the groups and the unpaired t test to compare differences between the groups. Otherwise the nonparametric Wilcoxon signed-rank test was used. The P value of <.05 was considered statistically significant.
Results
Demographics
Among the 11 (12 ears) patients with temporal bone FD managed at the authors’ centers, the mean age at time of diagnosis was 33.4 (15-59 years) years and 10 (90.9%) were men. Ages of onset in most patients were less than 30 years. One patient had bilateral disease. One patient reported a history of sudden sensorineural hearing loss (SSHL; Table 1).
Signs and Symptoms
The average time delay between first symptom onset and diagnosis was 54.3 months (range, 2-240 months) for the 11 patients with data available. The most common presenting symptoms were hearing loss (100%), tinnitus (36.4%), and otorrhea (36.4%), whereas otalgia (18.2%), vertigo (9.1%), and facial paresis (9.1%) were less common. Two patients were profound SSHL. One patient’s course was complicated by a subperiosteal abscess. All of the patients were found to have stenosis of the EAC by physical examination, and 8 of them were found to have intact tympanic membrane during operation (Table 1).
Supplementary Examination
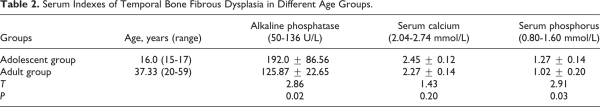
The level of alkaline phosphatase (ALP) and phosphorus in peripheral blood of the adolescent group (range from 15-17 years) was 192.0 ± 86.56 U/L and 1.27 ± 0.14 mmol/L, which was significantly higher than that of the adult group (range from 20-59 years; 125.87 ± 22.65 U/L and 1.02 ± 0.20 mmol/L, P < .05). The level of serum calcium of the adolescent group was slightly higher than the adult group (26.73 ± 12.49 dB vs 20.55 ± 9.58 dB), but the difference was not significant (P > .05; Table 2).
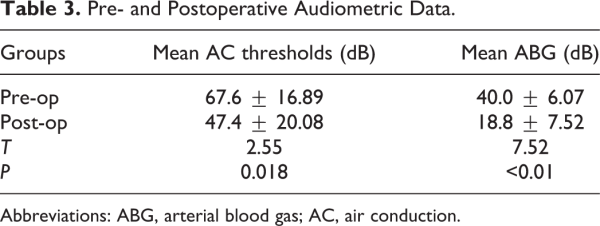
There was a significant difference between postoperative and preoperative mean AC thresholds (47.4 ± 20.08 dB vs 67.6 ± 16.89 dB, P < .05). The postoperative mean ABG was 18.8 ± 7.52 dB compared with 40.0 ± 6.07 dB in preoperative mean ABG and the difference was significant (P < .01; Table 3).
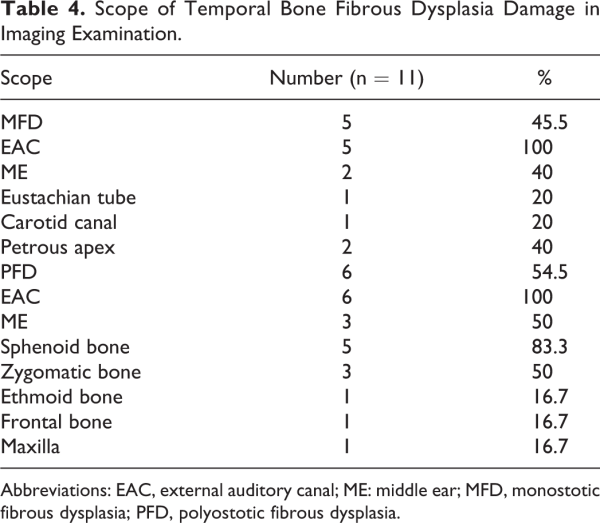
High-resolution computed tomography of temporal bone was used for initial evaluation and pretreatment planning (Figure 1). By virtue of inclusion criteria, all lesions originated from the temporal bone. Monostotic form of FD accounted for 45.5%, PFD 54.5%, and no MAS was found. The tumors mainly lead to stenosis of the external auditory meatus, especially at the osteochondral junction. In patients with MFD, 100% of tumors involved the EAC, 40% involved the middle ear or mastoid, 20% involved the Eustachian tube, 20% involved the carotid canal, and 20% involved the petrous bone. In patients with PFD, 100% of tumors involved the EAC, 50% involved the middle ear or mastoid, 83.3% involved the sphenoid bone, 50% involved zygomatic bone, and 16.7% for the ethmoid bone, frontal bone, maxilla, carotid canal, and petrous bone (Table 4). There were 11 cases of cholesteatoma in the EAC, 4 cases of cholesteatoma in the middle ear. One case of peripheral facial paralysis was caused by secondary cholesteatoma of middle ear.
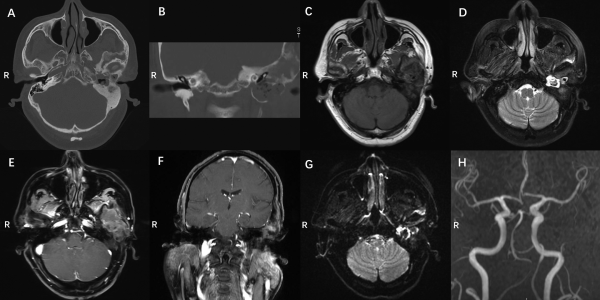
Figure 1
Preoperative imaging data: (A, B) preoperative CT images, (C) T1-weighted MRI, (D) T2 weighted, (E, F) enhanced scan imaging, (G) DWI imaging, and (H) MR angioplasty (MRA) of the carotid artery. Axial (A) and coronal (B) views of the noncontrast temporal HRCT demonstrating an expansile lesion with a “ground-glass” appearance in the left petrous bone, displacing the anterior wall of the EAC posteriorly. The lesions were located in temporal bone and sphenoid bone greater wing. The left EAC was compressed and narrowed, and a substantial soft-tissue density in the left EAC and middle ear, suspected recurrence of cholesteatoma. T1WI (C) showed moderate signal and T2WI (D) showed inhomogeneous high signal. DWI (G) indicates limited diffusion and no enhancement was found. T1WI (E) showed moderate signal and T2WI (F) showed slightly high or media and inhomogeneous enhancement on enhanced scans. The left carotid artery was surrounded by lesions and MRA showed slight stenosis (H). CT indicates computed tomography; DWI, diffusion-weighted imaging; EAC, external auditory canal; HRCT, high-resolution computed tomography; MRI, magnetic resonance imaging; T1WI, T1-weighted imaging; T2WI, T2-weighted imaging.
Management and Follow-Up Outcomes
All 11 patients underwent surgery. No patient was treated with radiotherapy. Canaloplasty of EAC and cholesteatoma resection were performed in all cases, including 10 with wide meatoplasty. Four patients underwent tympanoplasty and 5 patients underwent skin graft of EAC. One patient underwent decompression of facial nerve and 2 patients underwent reconstruction of ossicular chain.
There were no perioperative deaths in this series. Over a mean period of 4.2 years (range from 2-10 years), restenosis happened in 2 patients’ EAC in the third year after operation. So they underwent a secondary operation, and they were followed up for 2 years without recurrence. Two patients were lost to follow-up at 3 years (Table 5).
Discussion
Fibrous dysplasia is a rare benign lesion of bone disorder, characterized by a slow, progressive replacement of normal bone elements with proliferative fibrous tissue. Reports on cases with the temporal bone affected are uncommon, especially in Asians. Previous publications reported that there was no significant difference in gender, but 90% of our patients were male., Temporal bone FD occurs in 25% to 70% of patients with craniofacial skeleton involvement, most frequently in the setting of PFD. In this article, 6 cases of temporal bone FD were PFD, accounting for 54.5%. The most common clinical manifestations of the temporal bone FD were progressive stenosis of the EAC and conductive hearing loss, followed by tinnitus and otorrhea. There was no obvious clinical symptom in the early stage of the disease, and the median time delay between the first symptom onset and diagnosis was 54.2 months in this report. The lesion mainly leads to stenosis of the external auditory meatus, especially at the osteochondral junction. Cholesteatoma was the main complication of this disease, which was secondary to occlusion of the EAC with the growth of the lesion., All of the patients we reported have cholesteatoma, including 7 cases were limited to the EAC, and 4 cases invaded the middle ear. Two patients had SSHL, which may be due to cochlear destruction, inner auditory canal stenosis, or vestibular fistula. The prevalence of cranial neuropathy in temporal bone FD is rare. One of the patients in the current series had facial paresis caused by secondary cholesteatoma. For lesions beyond the temporal bone, the sphenoid and zygomatic bone were often invaded, accounting for 83.3% and 50%, respectively.
High-resolution computed tomography is especially important in the diagnosis of the temporal bone FD. The typical manifestations are homogenous ground-glass density, loss of the trabecular pattern, and asymmetrical thickening of the cortical wall. However, the imaging findings are not always typical and may be initially misdiagnosed by radiologists. Three types of CT findings are classified: pagetoid, sclerotic, and cystic types, and the pagetoid type is the end stage of sclerotic and cystic types. Besides, EAC and middle ear cholesteatomas can be well evaluated on HRCT. The magnetic resonance imaging appearance of FD is less characteristic and the typical characteristics are often very heterogenous pattern of enhancement in T1- and T2-weighted sequences. Magnetic resonance imaging might be useful in cases of cystic FD and in assessing the involvement of soft tissues. It is difficult to distinguish the temporal bone FD and some skull-base cases such as low-grade chondrosarcoma, atypical chordoma, or intraosseous meningioma from imaging and the final diagnosis depends on pathological examination. Fibrous dysplasia lesions may be quite vascular and bleeding can be brisk. If the lesion is quiescent or asymptomatic, and/or in the cranial base, a biopsy of FD may not be necessary. History, clinical examination, and the classic radiographic presentation are often adequate to establish the diagnosis of the temporal bone FD.
There is no unified guidance on treatment standards, and the treatments of temporal bone FD still remain diverse. Currently, there is as yet no effective drug treatment. Although radiotherapy can inhibit the progression of disease, it has been reported that radiation may increase the possibility of malignant transformation. Hence, radiotherapy is also not recommended as an optimal choice. If there is no complication, conservative treatment is preferred. However, surgical interventions are recommended in the following complications: secondary cholesteatoma, peripheral facial paralysis, recalcitrant infection, or biopsy for exclusion of malignancy. The aim of surgery is to restore function, prevent complications, and improve cosmetic demands. The selection of surgical indications in our case series was secondary cholesteatoma.
Our experience is that if the patient has no complications, he can receive surgery until adulthood. The narrowing of the EAC may result in significant cerumen and keratin debris build-up. Therefore, it is recommended that regular examinations are usually required by the otolaryngologist to maintain EAC patency. The timing of surgery is a key aspect of decision-making, and the abnormal skeletal tissue hyperplasia become active in childhood. It may be beneficial to wait until growth has slowed of the FD and the patient has progressed beyond adolescence. In this report, 2 patients underwent a secondary operation, and the first surgery was performed during adolescence. It is reported that FD was related to increased osteoclastic resorption and ALP, calcium, and phosphorus in serum had relation with FD progression., At the same time, we also found that serum phosphatase were significantly higher in adolescence than that in adulthood, suggesting that the FD was active bone growth before adulthood. Surgery may be performed before FD maturity if there are severe complications; however, patients must be aware of the risk of regrowth.
Canaloplasty of EAC and wide meatoplasty are first considered. The lesions of EAC, such as cholesteatoma, can be removed at the same time. Because the surgery itself is relatively not complicated, the complication will rarely occur and the results are satisfactory. A skin graft can be an optional procedure for canal coverage when the skin defect of EAC is large. Tympanoplasty should be performed if symptomatic middle or inner ear invasion. Temporal bone resection is only performed for extended bony lesions. Complete resection of all lesions is not necessary because function can be restored well and the residual lesions did not significantly progress postoperatively after adulthood. However, a relatively large sample investigation including treatment and long-term follow-up will be necessary to provide a more comprehensive overview of this disease.
Conclusion
Cholesteatoma is the main complication of temporal bone FD, which is secondary to occlusion of the EAC with the growth of the lesion. If the patient has no complications, they can be observed with serial cleanings/debridement in the clinic. Canaloplasty of EAC with wide meatoplasty is first considered, and if surgery itself is relatively not complicated, the complication will rarely occur and the results are satisfactory. Also, a long-term follow-up will be necessary for patients after surgery and that adolescent patients may be at higher risk of restenosis.
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article:The present study was supported in part by the National Natural Science Foundation of China (Number 81870726).
Kun Zhang
https://orcid.org/0000-0002-5280-4479
References
- 1. Riddle ND, Bui MM. Fibrous dysplasia. Arch Pathol Lab Med. 2013,137(1):134–138.
- 2. Roszko KL, Collins MT, Boyce AM. Mosaic effects of growth hormone on fibrous dysplasia of bone. N Engl J Med. 2018,379(20):1964–1965.
- 3. Frisch CD, Carlson ML, Kahue CN, et al. Fibrous dysplasia of the temporal bone: a review of 66 cases. Laryngoscope. 2015;125(6):1438–1443.
- 4. Rahman AM, Madge SN, Billing K, et al. Craniofacial fibrous dysplasia: clinical characteristics and long-term outcomes. Eye (Lond). 2009;23(12):2175–2181.
- 5. Sachdeva K. Extensive craniofacial fibrous dysplasia: an overview. Clin Rhinol. 2017;10(1):17–21.
- 6. de Reynal B. A case of fibrous dysplasia of bone of Jaffe-Lichtenstein. Rhumatologie. 1968;20(3):109–113.
- 7. George WC. Osteoblastic hyperplasia of bone: a histochemical appraisal of fibrous dysplasia of bone. Cancer. 1957:10(6):1157–1161.
- 8. Robinson C, Collins MT, Boyce AM. Fibrous dysplasia/McCune-Albright syndrome: clinical and translational perspectives. Curr Osteop Rep. 2016;14(5):178–186.
- 9. Megerian CA, Sofferman RA, McKenna MJ, et al. Fibrous dysplasia of the temporal bone: ten new cases demonstrating the spectrum of otologic sequelae. Am J Otol. 1995;6(1):408–419.
- 10. Marcela F, Bhimrao SK, Saxby AJ, et al. Fibrous dysplasia of the temporal bone systematic review of management and hearing outcomes. Otology Neurotol. 2014;35(10):1698–1706.
- 11. Dhooge I, D’hoop M, Loose D, et al. Acquired atresia of the external auditory canal: long-term clinical and audiometric results after surgery. Otol Neurotol. 2014;35(7):1196–1200.
- 12. Liu YH, Chang KP. Fibrous dysplasia of the temporal bone with external auditory canal stenosis and secondary cholesteatoma. J Int Adv Otol. 2016;12(1):125.
- 13. Couturier A, Aumaitre O, Gilain L, et al. Craniofacial fibrous dysplasia: a 10-case series. Eur Ann Otorhinolaryngol Head Neck Dis. 2017,134(4):229–235.
- 14. Frisch CD, Carlson ML, Kahue CN, et al. Fibrous dysplasia of the temporal bone: a review of 66 cases. Laryngoscope. 2015;125(6):1438–1443.
- 15. Lisle DA, Monsour PA, Maskiell CD. Imaging of craniofacial fibrous dysplasia. J Med Imaging Radiat Oncol. 2008,52(4):325–332.
- 16. Mehmet HA, Ismail S, Recep S, et al. CT and MR imaging in a large series of patients with craniofacial fibrous dysplasia. P P Radiol I Med Nuklear. 2015;80(1):232–240.
- 17. Bousson V, Rey-Jouvin C, Laredo JD, et al. Fibrous dysplasia and McCune-Albright syndrome: imaging for positive and differential diagnoses, prognosis, and follow-up guidelines. Eur J Radiol. 2014;83(10):1828–1842.
- 18. Lee JS, Fitz Gibbon EJ, Chen YR, et al. Clinical guidelines for the management of craniofacial fibrous dysplasia. Orphanet J Rare Dis. 2012;7(suppl 1):S2.
- 19. Li Z, Raynald, Wang Z, Haiyan Q. Malignant transformation of craniofacial fibrous dysplasia: a systematic review of overall survival. Neurosurg Rev. 2019;2:27.
- 20. Mierzwinski J, Kosowska J, Tyra J, et al. Different clinical presentation and management of temporal bone fibrous dysplasia in children. World J Surg Oncol. 2018,16(1):5.
- 21. Liens D, Delmas PD, Meunier PJ. Long-term effects of intravenous pamidronate in fibrous dysplasia of bone. Lancet. 1994;343(8903):953–954.
- 22. Hussein MA, Yun IS, Kim BO, et al. Craniofacial fibrous dysplasia: retrospective study on the relationship between the tumor volume changes and the circulating serum calcitonin and serum alkaline phosphatase. Ann Plast Surg. 2017;78(3):289–293.