Introduction
Facial synkinesis, a sequela of facial nerve injury, is the involuntary movement of the facial mimetic musculature accompanying volitional facial movement. These undesired muscle contractions result in aesthetically and functionally distressing symptoms, culminating in decreased quality of life. Uncoordinated facial musculature movement results in functional limitations, including oral incompetence limiting the patient’s ability to eat, drink, articulate, and display facial expressions (Figure 1).- The significant functional, social, and psychological morbidity associated with facial synkinesis accentuates the need for timely recognition and treatment of this disorder in patients suffering from clinical facial nerve disorders. Facial synkinesis may occur in a complex presentation with concomitant hyperkinesis of facial musculature leading to a non-flaccid facial paralysis. In these cases, patients may have abnormal movements in addition to a hypokinetic appearance with decreased mobility due to over-stimulation of antagonistic facial muscles.
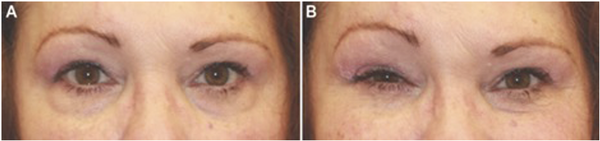
Figure 1
61-year-old patient with a remote history of Ramsay Hunt syndrome on the right with subsequent synkinesis development. Photos are of a patient without treatment. (A) Appearance of the eyes in repose. (B) Oral-ocular synkinesis with involuntary eye contracture and narrowing of the palpebral fissure during smiling.
Methods
A PubMed and Cochrane search was done with no date restrictions for English-language literature on facial synkinesis. The search terms used were “facial,” “synkinesis,” “palsy,” and various combinations of the terms.
Results
Facial synkinesis: pathophysiology
Although underlying pathophysiology has yet to be conclusively elucidated, facial synkinesis is postulated to result from insult to multiple levels along the neural pathway. Nonspecific axonal regeneration,- somatotopic reorganization,, anomalous ephaptic transmission,- hypersensitivity of the facial nucleus,, and aberrant central nervous system adaptation, have all been presented as possible mechanisms. Currently, the leading theory involves nonspecific aberrant regeneration of nerves due to ineffective myelination and reorganization of neural networks during regrowth resulting in “crosstalk” between distal nerve fibers coupled with hypersensitization of the facial nucleus. This miscommunication results in involuntary activation of facial musculature during attempted volitional movements. After the initial facial nerve injury, aberrant regeneration among nerve fibers can develop as early as three-to-four months and can continue for up to 2 years.
The current treatment paradigm for facial synkinesis focuses on suppressing undesirable facial movements while maximizing volitional movement. The main therapeutic strategies can be divided into rehabilitation or neuromuscular retraining,, and implementing botulinum toxin for chemodenervation., Severe synkinesis not amenable to conservative or minimally invasive measures may require surgical intervention to selectively ablate involved neural or muscular tissue. To supplement, functional transfer of muscles using free tissue transfer may be beneficial in specific patient subpopulations.- It is essential to note that these therapies are not meant to achieve premorbid facial function but rather to mitigate the results of synkinesis with improved facial symmetry and function.-
Evaluation of synkinesis
In addition to the above anatomically based clinical findings, several validated diagnostic tools have been developed to evaluate the severity of synkinesis. The Synkinesis Assessment Questionnaire (SAQ) is a 10-item validated patient-reported questionnaire used to guide botulinum toxin therapy (Figure 2). The Sunnybrook Facial Grading System (SBGS; Figure 3) and the electronic physician graded scale (eFACE) are just two of the multiple grading systems employed by clinicians. Sunnybrook Facial Grading System is a well-established grading system that incorporates 5 standard facial expressions to assess the movement and extent of synkinesis.- In a recent systematic review, the SBGS scale was the most frequently used outcome measure. The eFACE is an electronic scale incorporating both dynamic and smile scores with a synkinesis score, which is anatomically separated, and allows objective measurements to be obtained based on clinical photographs (Figure 4). This allows for both documentation of abnormal movements and evaluation of therapies employed (eg, botulinum toxin, neuromuscular retraining, or surgery). Regardless of the system implemented, clinicians are advised to utilize diagnostic tools to guide therapeutic interventions and document progress.
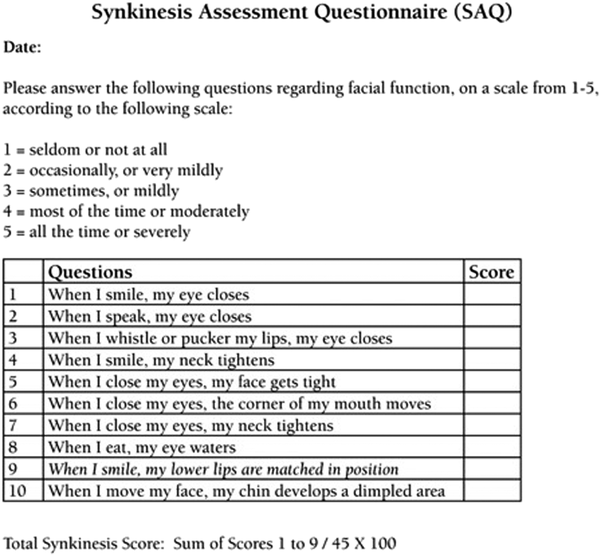
Figure 2
Synkinesis Assessment Questionnaire (adapted with permission from: Mehta RP, Wernick Robinson M, and Hadlock TA. Validation of the synkinesis assessment questionnaire. Laryngoscope 2007;117(5):924).
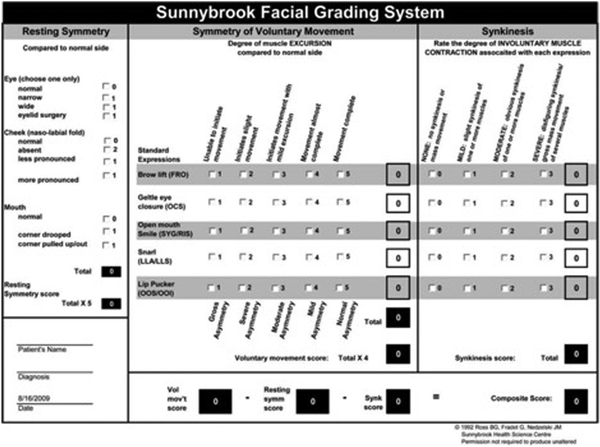
Figure 3
Sunnybrook Facial Grading System.
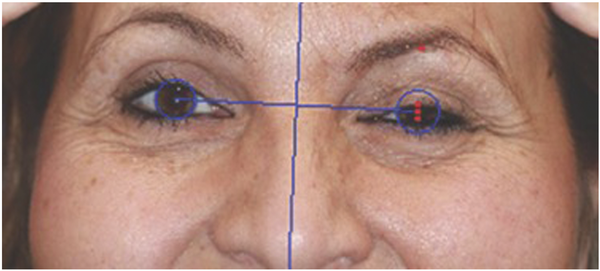
Figure 4
45-year-old patient with recurrent Bell’s palsy with persistent asymmetry and synkinesis. Photo shows use of the facial assessment by computer evaluation (FACE) program to obtain eye measurements in a patient with untreated oral-ocular synkinesis.
Treatment modalities
Hemifacial synkinesis following an injury to the facial nerve displays zonal permutations of hypokinesis and hyperkinesis, causing non-flaccid facial paralysis. Although many treatments for flaccid facial paralysis have been available, their use in non-flaccid facial paralysis is emerging. Miller and Hadlock proposed a treatment ladder to serve as a framework when approaching a patient with non-flaccid facial paralysis. Their algorithm begins with targeted facial rehabilitation, followed by chemodenervation therapy, selective myectomies, selective denervation, and gracilis muscle transfer, in a step-wise approach. Therefore, treatment techniques should aim to both restore normal movement and inhibit areas of aberrant activity. The following sections discuss treatment modalities, including physical therapy and rehabilitation, neuromodulation with botulinum toxin injection, and surgical interventions.
Physical rehabilitation
Physiotherapy is an important component in the treatment of synkinesis. The primary goal is to maximize volitional control of facial muscular contraction through the implementation of specific exercises. These techniques have been shown to improve zonal areas of hypokinesis while decreasing or further preventing the progression of synkinesis. Therapy consists of various techniques used concomitantly, including neuromuscular retraining or biofeedback, soft tissue massage, and mobilization, as well as meditation. Electromyographic feedback, electrical stimulation, and mime therapy have also shown promising results in this patient population.,,
A large retrospective study by Lindsay et al demonstrated that targeted facial rehabilitation therapy results in quantitative improvement in facial function scores using the facial grading scale in patients with non-flaccid facial paralysis. Their study consisted of 303 patients with facial paralysis that were evaluated by 1 physical therapist at a tertiary care facial nerve center during a 5-year period. Patients were evaluated by a Facial Grading Scale (FGS) scores. The patients underwent facial rehabilitation, including education, neuromuscular training, massage, meditation-relaxation, and individualized home programs. They found a statistically significant increase in FGS scores following treatment, indicating an improvement in facial function (P < .001).
Facial musculature lacks intrinsic sensory receptors. Therefore, neuromuscular retraining facilitates patient self-awareness of facial posture and movements. Patients perform controlled facial movements to increase their range of motion and promote symmetry. Electromyographic (EMG) biofeedback uses electrodes to record muscle contraction, providing patients with both auditory and visual feedback of muscle activity., A recent double-blinded randomized controlled trial investigating the effects of biofeedback therapy demonstrated a significant reduction in facial synkinesis in the treatment arm (P < .01).
Soft tissue mobilization, comprised of massaging areas of persistent aberrant muscle contraction, meditation therapy, and relaxation therapy, consisting of exercises that release tension in facial musculature, are also often implemented in facial rehabilitation and have shown efficacy when preceding neuromuscular retraining. Facial rehabilitation techniques are often used in combination to optimize softening of hyperkinetic strained musculature. A comprehensive rehabilitative plan should employ physical rehabilitation techniques combined with chemodenervation and/or surgical intervention in cases refractory to conservative therapy. Physical therapy’s therapeutic effects have shown to be additive when used with botulinum toxin chemodenervation therapy and neuromuscular therapy.,,
Chemodenervation
Chemodenervation with botulinum toxin aims to selectively weaken hyperkinetic or synkinetic musculature to facilitate volitional facial movement restoring symmetry and augmenting rehabilitative treatments. Botulinum toxin binds to presynaptic cholinergic nerve terminals preventing the release of acetylcholine with resultant neuromuscular blockade. Treatment is temporary, with botulinum toxin A effects persisting for approximately 3 months. Injection techniques are site-specific with the typical use of 10-40 units of botulinum toxin, distributed in aliquots throughout multiple locations.
Several studies have indicated that appropriately utilized chemodenervation therapy in patients suffering from facial synkinesis improves the quality of life, self-image, social interactions, mastication, and oral competence.-Figure 5 shows the facial musculature commonly targeted in the treatment of facial synkinesis. Injections should target hyperkinesis zonal areas while considering the anticipated vector of muscle contracture and facial movement. In a prospective cohort study of 99 patients treated with botulinum toxin for facial synkinesis, Shinn et al found significant improvement in post-treatment Synkinesis Assessment Questionnaire scores. Those with a greater degree of morbidity, younger patients, and females showed the most significant response. A brief overview of anatomic site-specific treatment is detailed below.
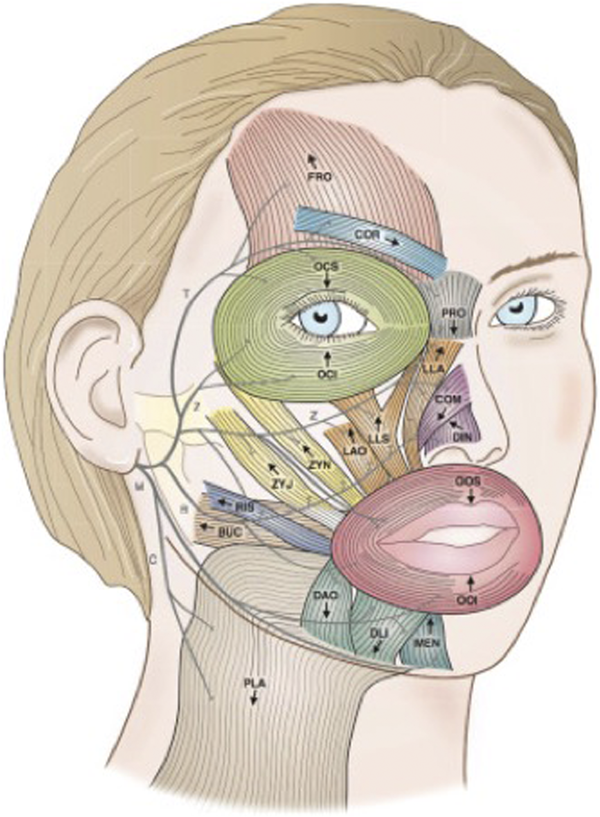
Figure 5
Muscles of facial expression; arrows indicate the directional vector of muscle contraction. Muscles: BUC, buccinators; COM, compressor naris; COR, corrugator; DAO, depressor anguli oris; DIN, dilator naris; DLI, depressor labii inferioris; FRO, frontalis; LAO, levator anguli oris; LLA, levator labii alaeque nasi; LLS, levator labii superioris; MEN, mentalis; OCI, orbicularis oculi inferioris; OCS, orbicularis oculi superioris; OOI, orbicularis oris inferioris; OOS, orbicularis oris superioris; PLA, platysma; PRO, procerus; RIS, risorius; ZYJ, zygomaticus major; ZYN, zygomaticus minor. Facial nerve branches: B, buccal; C, cervical; M, mandibular; T, temporal; Z, zygomaticus. (Adapted with permission from: Mehdizadeh et al. OB, Diels J, White WM. Botulinum toxin in the treatment of facial paralysis. Facial Plastic Surgery Clinics. 2016 Feb 1; 24 (1):11-20.)
Periocular musculature
Periocular synkinesis often manifests as blepharospasm with resultant narrowing of the palpebral fissure and visual obstruction. Botulinum toxin treatment of the orbicularis oculi weakens the muscle, thereby preventing blepharospasm and allowing for maintained eye-opening during volitional facial movements. Previously documented doses used to treat orbicularis oculi synkinesis vary. The muscle is targeted along with the orbit’s lateral extent or within the upper and lower eyelids within the subcutaneous plane. Familiarity with the anatomy and precise technique is required to prevent inadvertent injection of the levator palpebrae superioris muscle, resulting in ptosis. Overtreatment is not advised as excessive dosages may result in incomplete eye closure, dry eye, and damage to the ocular surface.,
Perioral musculature
Synkinesis of the lip depressors results in inadequate smile excursion with a limited oral commissure elevation, due primarily to aberrant concomitant activation of antagonistic lip elevator and depressor musculature. Therefore, selective botulinum toxin application to depressor muscles enables the unopposed action of lip elevators with improved oral commissure excursion and smile symmetry. Conservative dosing is recommended as over-weakening perioral musculature may result in oral incompetence with functional implications in speech and mastication. The depressor anguli oris (DAO) muscle, which originates at the inferior aspect of the mandible and inserts at the modiolus, functions in the oral commissure’s inferolateral movement. Therefore, tailored injection of this muscle results in improved excursion of the corner of the mouth. Injections to target this muscle begin at the modiolus, along the oral commissure, progressing from a subcutaneous plane more deeply along the mandible’s inferior border. Approximate dosages of 5-10 units of the toxin are often used. It is essential to note the relationship between the platysma, which interdigitates with DAO fibers, as treatment of platysmal banding inferior to the DAO along the medial neck may further augment oral commissure excursion. Additional muscles influencing lower lip positioning include the depressor labii inferioris (DLI) and mentalis. Patients with synkinesis will often display hyperkinesis of the contralateral unaffected DLI with hypokinesis of the ipsilateral paretic DLI. The ensuing imbalance results in the hyperkinetic side effectively pulling the weakened lip toward the mouth, with evidence of excessive unilateral dental show on the contralateral side. Therefore, selective injection of the contralateral DLI with 5 units of toxin results in an equalization of lower lip position and mandibular dental show. Conversely, excessive weakening of the DLI can completely paralyze the lower lip with exacerbation of drooling and oral incompetence.
Hyperfunction of the mentalis muscle in synkinetic patients may also be problematic, resulting in chin dimpling and superior displacement of the lower lip with resultant limitations to labial speech. Chemodenervation of the mentalis may be performed with dosing of approximately ten units of the toxin with improved muscle tone of the mentum.
Midface
The hyperkinetic activity of midface musculature may be demonstrated in patients as deepening of the nasolabial fold during rest as well as unintended contracture during eye closure. Overly aggressive treatment of this area may result in loss of smile excursion and apparent facial asymmetry. Therefore, midface synkinesis should first be addressed with soft tissue massage and rehabilitation. In patients with intractable midface synkinetic contraction, low dosages of 1.5 to 4 units of toxin injection are recommended, with the targeting of the zygomaticus major muscle.
Aberrant nasal muscle hyperfunction may also result in persistent nasal obstruction. Anecdotally, it has been our experience that these patients often experience concomitant midface synkinesis. Therefore, addressing midfacial synkinetic muscle activity with chemodenervation while performing soft tissue nasal massage may yield the best outcome. In addition, low-dose botulinum toxin to the alaeque nasi muscle may improve symmetry.
Buccinator
Physicians should suspect buccinator synkinesis when patients display retraction and decreased mobility of the oral commissure or difficulty with mastication or manipulation of the food bolus in the oral phase. Chemodenervation of the buccinator muscle has been shown to improve mastication, facial expressivity, and the pronunciation of fricative consonants while decreasing trauma to the buccal mucosa. Injection of the buccinator is typically performed intraorally, directly into the area with which exhibits the greatest contraction. Conservative doses, starting with 2 units, are recommended, as excessive weakening may lead to oral incompetence, air escape during an attempted speech, or worsening of cheek biting or midface spasms.,
Platysmal synkinesis
Platysmal hyperfunction is often identified as banding in the craniocaudal dimension. The weakening of this muscle along its length significantly relieves banding and subjective tightness resulting in overall neck comfort. Total dosing of botulinum will vary, depending on muscle bulk and degree of banding, but ranges from 10 to 65 units distributed as aliquots along with the muscle., Injections should be given in the subcutaneous plane given the superficial location of the muscle. Deeper injections risk paresis of the deeper neck musculature and resultant dysphagia.
Treatment of the contralateral face
Patients with synkinesis often experience changes to the contralateral, unaffected facial musculature, which may develop static or dynamic hyperkinesis. Several mechanisms have been proposed to account for these changes. The most widely accepted states that a compensatory mechanism resulting from the affected side occurs in the unaffected side, leading to increased muscle contraction and cortical reorganization., In conjunction with chemodenervation therapy, physical rehabilitation has been shown to decrease this hyperfunction while improving symmetry. Chemodenervation of the frontalis muscle and corrugator supercilii, on the unaffected side, with 10-15 units of botulinum toxin relieves asymmetry and discomfort due to increased tension. In addition, chemodenervation of the contralateral levator labii superioris alaeque nasi muscle may reduce inadvertent visibility of upper teeth, while treatment of the contralateral DLI will improve the mandibular dental show. Choi et al recently described a series of 42 patients with unilateral facial synkinesis treated with chemodenervation to both sides of the face and reported improved quality of life, facial symmetry, socialization, and personal image, and oral intake with a bilateral facial treatment. Despite these findings, there continues to be some controversy surrounding chemodenervation to the contralateral side of the face.
Surgical treatment modalities
Multiple novel surgical techniques have been developed to improve synkinesis symptoms by inhibiting hyperkinetic areas while restoring or augmenting normal movement. A step-wise approach often implementing physical rehabilitation and chemodenervation is utilized. However, certain patients develop neutralizing antibodies following repeated botulinum toxin injections rendering treatment ineffective. Clinicians should familiarize themselves with surgical options to allow for appropriate patient evaluations, patient counseling, and referrals to surgeons.
Selective neurolysis
Selective neurolysis refers to a form of intervention in which particular facial nerve branches, identified to be contributory to synkinetic activity, are resected, resulting in decreased aberrant facial nerve activity and involuntary muscle contraction. Several studies advocate for removing small nerve segments to prevent recurrent synkinesis attributable to nerve regrowth rather than transection. Neurolysis has been utilized in patients with periocular synkinesis refractory to chemodenervation secondary to the formation of neutralizing antibodies.,
A recent study by van Veen et al investigated the long-term outcomes of selective neurectomy for refractory periocular synkinesis. The majority of patients were found to require recommencement of chemodenervation treatment within a median time of 1.2 years. Although their study demonstrated that patients required reinstitution of pharmacological therapy following surgery, patients were shown to experience a symptom-free interval following surgery and demonstrated a sustained increase in palpebral fissure width following the procedure. Furthermore, these previously refractory patients also demonstrated renewed response to botulinum toxin treatment. More recently, selective neurectomy has been used in larger cohorts of patients by Azizzadeh, with excellent results reported in the midface, lower face, and cervical regions. Although additional studies and longer-term follow-up are needed, selective neurectomy offers a promising surgical treatment for this complex patient population.
Selective myectomy
Selective myectomy removes a segment of muscle contributing to the synkinetic movement. Generally, myectomy should not be first-line therapy as this treatment is irreversible. This technique is most commonly utilized to address hyperkinesis or synkinesis of the platysma musculature, although resection of DAO, DLI, and orbicularis oculi muscles has been reported.,-
Modified selective neurectomy combined with a platysmal myotomy was described by Azizzadeh et al, in which preservation of zygomatic and marginal mandibular branches that innervate key smile muscles and ablation of buccal and cervical branches that cause lateral and/or inferior excursion of the oral commissure was performed. Benefits include an outpatient procedure where patients are discharged the same day, and authors note objective improvements in electronic clinician-graded facial function scale score, House–Brackmann score, and decreased botulinum toxin A requirements with results lasting 12 months after the procedure.
Nerve transfers and free muscle transfer
Nerve transfer is commonly used in facial paralysis with proximal nerve injury while distal nerve branches and innervated musculature remain viable. Donor nerves such as the contralateral unaffected facial nerve, masseteric nerve, or the hypoglossal nerve are connected to distal facial nerve branches to establish tone and movement. In patients who exhibit partial recovery, with subsequent development of synkinesis, nerve transfers have shown utility in reestablishing tone and overall control of the face., One case series described successful masseter-to-facial nerve transfers with selective neurectomies for smile rehabilitation and demonstrated improvement in overall smile symmetry and reduction in facial synkinesis, as measured by the eFACE tool. Another recently published case series described success with a single-stage masseter-to-zygomatic nerve transfer with selective neurectomy to manage eye closure-smile excursion synkinesis. Overall, five-to-seven nerve transfers can serve as an adjunct to primary repair in augmenting a functional smile in patients with flaccid or non-flaccid facial nerve palsy; however, the role of nerve transfers is not as consistent in patients with non-flaccid facial paralysis and synkinesis. Further studies are needed to evaluate their efficacy as a sole surgical intervention to “supercharge” facial movement or in combination with selective neurectomy.
The use of free gracilis muscle transfer (FGMT), in which the lower extremity muscle is transferred to the face for reanimation of the smile, has shown particular efficacy in patients with flaccid facial paralysis. Its use in synkinetic patients with non-flaccid facial paralysis is less clear, although promising. Lindsay et al reported a case series of 20 patients that underwent FGMT with innervation via a cross-facial nerve grafting (CFNG) and/or masseteric nerve graft and reported significant improvements in their smiles. Their criteria for consideration for candidacy for FGMT included less than 2 mm of oral commissure excursion despite exhaustive utilization of conservative therapies, including rehabilitation and chemodenervation. Additionally, Vincent et al reported a case series of 7 patients who underwent masseteric-to-facial nerve transfer with selective neurectomy to rehabilitate the synkinetic smile and found significant improvement in postoperative eFACE scores. Unfortunately, studies investigating surgical interventions display insufficient objective, quantifiable data, and variability concerning outcomes despite promising preliminary data. Therefore, they should be considered a last resort in intractable synkinesis cases due to the risk of a potential loss of residual facial function.
Conclusion
Facial synkinesis is a distressing disorder resulting in undesired facial movement accompanying volitional movement and expression, particularly when it presents as a non-flaccid facial paralysis. It may develop weeks to months following facial nerve injury and commonly involves perioral, periocular, midfacial, and neck musculature. The asymmetric facial appearance and uncoordinated facial movements hamper an individual’s emotional expressivity and prevent normal social interaction resulting in decreased quality of life, poor self-image, and depression. Facial rehabilitation, including neuromuscular retraining, soft tissue massage, and relaxation therapy in addition to chemodenervation with botulinum toxin, remains the cornerstone of treatment. Ultimately, therapy should be tailored to the severity and pattern of synkinesis, and each patient approached on a case-by-case basis. In severe, intractable synkinesis cases, surgical interventions, including selective neurectomy, selective myectomy, nerve transfer, or free muscle transfer, may play a more significant role in alleviating symptoms. A multidisciplinary approach involving therapists, clinicians, and surgeons is necessary to develop a comprehensive treatment regimen that will result in optimal outcomes.
Author Contributions All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.Study concept and design: Shokri and Lighthall.Acquisition, analysis, or interpretation of data: All authors.Drafting of the manuscript: All authors.Critical revision of the manuscript for important intellectual content: Shokri and Lighthall.Administrative, technical, or material support: Shokri and Lighthall.Study supervision: Lighthall.
Declaration of conflicting interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval The authors confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1. Salles AG, da Costa EF, Ferreira MC, do Nascimento Remigio AF, Moraes LB, Gemperli R. Epidemiologic overview of synkinesis in 353 patients with longstanding facial paralysis under treatment with botulinum toxin for 11 years. Plast Reconstr Surg. 2015;136(6):1289–1298.
- 2. VanSwearingen JM, Cohn JF, Bajaj-Luthra A. Specific impairment of smiling increases the severity of depressive symptoms in patients with facial neuromuscular disorders. Aesthetic Plast Surg. 1999;23(6):416–423.
- 3. Filipo R, Spahiu I, Covelli E, Nicastri M, Bertoli GA. Botulinum toxin in the treatment of facial synkinesis and hyperkinesis. Laryngoscope. 2012;122(2):266–270.
- 4. Husseman J, Mehta R. Management of synkinesis. Facial Plast Surg. 2008;24(2):242–249.
- 5. Crumley RL. Mechanisms of synkinesis. Laryngoscope. 1979;89(11):1847–1854.
- 6. Chaco J. Misdirection of facial nerve fibers in Bell's palsy. ORL (Basel). 1974;36(4):205–208.
- 7. Bratzlavsky M, Eecken HV. Altered synaptic organization in facial nucleus following facial nerve regeneration: an electrophysiological study in man. Ann Neurol. 1977;2(1):71–73.
- 8. Baker RS, Stava MW, Nelson KR, May PJ, Huffman MD, Porter JD. Aberrant reinnervation of facial musculature in a subhuman primate. Neurology. 1994;44(11):2165.
- 9. Choi D, Raisman G. Disorganization of the facial nucleus after nerve lesioning and regeneration in the rat: effects of transplanting candidate reparative cells to the site of injury. Neurosurgery. 2005;56(5):1093–1100. discussion 1093-1100.
- 10. Sadjadpour K. Postfacial palsy phenomena: faulty nerve regeneration or ephaptic transmission? Brain Research. 1975;95(2-3):403–406.
- 11. Maeyama H, Aoyagi M, Tojima H, Inamura H, Kohsyu H, Koike Y. Electrophysiological study on the pathology of synkinesis after facial nerve paralysis. Acta Otolaryngol. 1994;114:161–164.
- 12. Blumberg H, Jänig W. Activation of fibers via experimentally produced stump neuromas of skin nerves: ephaptic transmission or retrograde sprouting? Exp Neurol. 1982;76(3):468–482.
- 13. Thomander L. Reorganization of the facial motor nucleus after peripheral nerve regeneration. An HRP study in the rat. Acta Oto-Laryngologica. 1984;97(5-6):619–626.
- 14. VanSwearingen JM, Brach JS. Changes in facial movement and synkinesis with facial neuromuscular reeducation. Plast Reconstr Surg. 2003;111(7):2370–2375.
- 15. Placheta E, Tzou C-HJ, Hold A, Pona I, Frey M. Facial synkinesia before and after surgical reanimation of the paralyzed face. Plast Reconstr Surg. 2014;133(6):842e–851e.
- 16. Çelik M, Forta H, Vural Ç. The development of synkinesis after facial nerve paralysis. Eur Neurol. 2000;43(3):147–151.
- 17. Pourmomeny AA, Asadi S, Cheatsaz A. Management of facial synkinesis with a combination of BTX-A and biofeedback: a randomized trial. Iranian journal of otorhinolaryngology. 2015;27(83):409–415.
- 18. Nakamura K, Toda N, Sakamaki K, Kashima K, Takeda N. Biofeedback rehabilitation for prevention of synkinesis after facial palsy. Otolaryngol Head Neck Surg. 2003;128(4):539–543.
- 19. Hadlock TA, Greenfield LJ, Wernick-Robinson M, Cheney ML. Multimodality approach to management of the paralyzed face. Laryngoscope. 2006;116(8):1385–1389.
- 20. Lindsay RW, Bhama P, Hadlock TA. Quality-of-life improvement after free gracilis muscle transfer for smile restoration in patients with facial paralysis. JAMA Facial Plastic Surgery. 2014;16(6):419–424.
- 21. Bhama PK, Weinberg JS, Lindsay RW, Hohman MH, Cheney ML, Hadlock TA. Objective outcomes analysis following microvascular gracilis transfer for facial reanimation. JAMA Facial Plastic Surgery. 2014;16(2):85–92.
- 22. Lindsay RW, Bhama P, Weinberg J, Hadlock TA. The success of free gracilis muscle transfer to restore smile in patients with nonflaccid facial paralysis. Ann Plast Surg. 2014;73(2):177–182.
- 23. Mehta RP, WernickRobinson M, Hadlock TA. Validation of the synkinesis assessment questionnaire. Laryngoscope. 2007;117(5):923–926.
- 24. Kang TS, Vrabec JT, Giddings N, Terris DJ. Facial nerve grading systems (1985-2002): beyond the House-Brackmann scale. Otol Neurotol : Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 1985-2002;23(5):767–771.
- 25. Neely JG, Cherian NG, Dickerson CB, Nedzelski JM. Sunnybrook facial grading system: reliability and criteria for grading. Laryngoscope. 2010;120(5):1038–1045.
- 26. Hu WL, Ross B, Nedzelski J. Reliability of the sunnybrook facial grading system by novice users. J Otolaryngol. 2001;30(4):208–211.
- 27. Lapidus JB, Lu JC-Y, Santosa KB, et al. Too much or too little? A systematic review of postparetic synkinesis treatment. J Plast Reconstr Aesthetic Surg. 2020;73(3):443–452.
- 28. Banks CA, Bhama PK, Park J, Hadlock CR, Hadlock TA. Clinician-graded electronic facial paralysis assessment. Plast Reconstr Surg. 2015;136(2):223e–230e.
- 29. Banks CA, Jowett N, Azizzadeh B, et al. Worldwide testing of the eFACE facial nerve clinician-graded scale. Plast Reconstr Surg. 2017;139(2):491e–498e.
- 30. Choi CJ, Lefebvre DR, Yoon MK. Validation of the facial assessment by computer evaluation (FACE) program for software-aided eyelid measurements. Orbit. 2016;35(3):117–120.
- 31. Miller MQ, Hadlock TA. Beyond botox: contemporary management of nonflaccid facial palsy. Facial Plastic Surgery & Aesthetic Medicine. 2020;22(2):65–70.
- 32. Teixeira LJ, Valbuza JS, Prado GF. Physical therapy for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2011(12):Cd006283.
- 33. Lindsay RW, Robinson M, Hadlock TA. Comprehensive facial rehabilitation improves function in people with facial paralysis: a 5-year experience at the Massachusetts Eye and Ear Infirmary. Phys Ther. 2010;90(3):391–397.
- 34. Brach JS, VanSwearingen JM, Lenert J, Johnson PC. Facial neuromuscular retraining for oral synkinesis. Plast Reconstr Surg. 1997;99(7):1922–1931. discussion 1932-1923.
- 35. Ross B, Nedzelski JM, McLean JA. Efficacy of feedback training in long-standing facial nerve paresis. Laryngoscope. 1991;101(7 Pt 1):744–750.
- 36. Cattaneo L, Pavesi G. The facial motor system. Neurosci Biobehav Rev. 2014;38:135–159.
- 37. Brudny J. Biofeedback in facial paralysis: electromyographic rehabilitation. In: The Paralyzed Face. St Louis, MO: Mosby Yearbook; 1991:247–264.
- 38. Baumel JJ. Trigeminal-facial nerve communications: their function in facial muscle innervation and reinnervation. Arch Otolaryngol Head Neck Surg. 1974;99(1):34–44.
- 39. Pereira L, Obara K, Dias J, Menacho M, Lavado E, Cardoso J. Facial exercise therapy for facial palsy: systematic review and meta-analysis. Clin Rehabil. 2011;25(7):649–658.
- 40. Wernick Robinson M, Baiungo J, Hohman M, Hadlock T Facial rehabilitation. Operat Tech Otolaryngol Head Neck Surg, 23(4);2012:288–296.
- 41. Bikhazi NB, Maas CS. Refinement in the rehabilitation of the paralyzed face using botulinum toxin. Otolaryngology-Head Neck Surg (Tokyo). 1997;117(4):303–307.
- 42. Toffola ED, Furini F, Redaelli C, Prestifilippo E, Bejor M. Evaluation and treatment of synkinesis with botulinum toxin following facial nerve palsy. Disabil Rehabil. 2010;32(17):1414–1418.
- 43. Choi KH, Rho SH, Lee JM, Jeon JH, Park SY, Kim J. Botulinum toxin injection of both sides of the face to treat post-paralytic facial synkinesis. J Plast Reconstr Aesthetic Surg. 2013;66(8):1058–1063.
- 44. Rzany B, Dill-Müller D, Grablowitz D, Heckmann M, Caird D. Repeated botulinum toxin A injections for the treatment of lines in the upper face: a retrospective study of 4,103 treatments in 945 patients. Dermatol Surg : official publication for American Society for Dermatologic Surgery [et al.]. 2007;33(1 Spec No):S18–S25.
- 45. Mehta RP, Hadlock TA. Botulinum toxin and quality of life in patients with facial paralysis. Arch Facial Plast Surg. 2008;10(2):84–87.
- 46. Shinn JR, Nwabueze NN, Du L, et al. Treatment patterns and outcomes in botulinum therapy for patients with facial synkinesis. JAMA Facial Plastic Surgery. 2019;21(3):244–251.
- 47. Laskawi R. The use of botulinum toxin in head and face medicine: an interdisciplinary field. Head Face Med. 2008;4:5.
- 48. Laskawi R, Damenz W, Roggenkämper P, Baetz A. Botulinum toxin treatment in patients with facial synkinesis. Eur Arch Oto-Rhino-Laryngol : Official Journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : Affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 1994:S195–S199.
- 49. Labbé D, Bénichou L, Iodice A, Giot J-P. Signe du depressor anguli oris (DAO) dans les parésies faciales. Comment le rechercher et libérer le sourire (note technique). Ann Chir Plast Esthet. 2012;57(3):281–285.
- 50. Lindsay RW, Edwards C, Smitson C, Cheney ML, Hadlock TA. A systematic algorithm for the management of lower lip asymmetry. Am J Otolaryngol. 2011;32(1):1–7.
- 51. Wiener A, Touloei K, Glick BP. A novel long-term therapy of facial synkinesis with botulinum Neurotoxins Type A and Fillers. The Journal of clinical and aesthetic dermatology. 2011;4(3):45–49.
- 52. Markey JD, Loyo M. Latest advances in the management of facial synkinesis. Curr Opin Otolaryngol Head Neck Surg. 2017;25(4):265–272.
- 53. de Sanctis Pecora C, Shitara D. Botulinum toxin type a to improve facial symmetry in facial palsy: a practical guideline and clinical experience. Toxins. 2021;13(2):159.
- 54. Wei LA, Diels J, Lucarelli MJ. Treating buccinator with botulinum toxin in patients with facial synkinesis. Ophthalmic Plast Reconstr Surg. 2016;32(2):138–141.
- 55. Patel PN, Owen SR, Norton CP, et al. Outcomes of buccinator treatment with botulinum toxin in facial synkinesis. JAMA Facial Plastic Surgery. 2018;20(3):196–201.
- 56. Boahene K. Reanimating the paralyzed face. F1000prime reports. 2013;5:49.
- 57. Laskawi R, Rohrbach S, Rödel R. Surgical and nonsurgical treatment options in patients with movement disorders of the platysma. J Oral Maxillofac Surg. 2002;60(2):157–162.
- 58. Cabin JA, Massry GG, Azizzadeh B. Botulinum toxin in the management of facial paralysis. Curr Opin Otolaryngol Head Neck Surg. 2015;23(4):272–280.
- 59. Öge AE, Yayla V, Demir GA, Eraksoy M. Excitability of facial nucleus and related brain-stem reflexes in hemifacial spasm, post-facial palsy synkinesis and facial myokymia. Clin Neurophysiol. 2005;116(7):1542–1554.
- 60. Salles AG, Toledo PN, Ferreira MC. Botulinum toxin injection in long-standing facial paralysis patients: improvement of facial symmetry observed up to 6 months. Aesthetic Plast Surg. 2009;33(4):582–590.
- 61. Bakheit AMO, Liptrot A, Newton R, Pickett AM. The effect of total cumulative dose, number of treatment cycles, interval between injections, and length of treatment on the frequency of occurrence of antibodies to botulinum toxin type A in the treatment of muscle spasticity. Int J Rehabil Res. 2012;35(1):36–39.
- 62. Hohman MH, Lee LN, Hadlock TA. Two-step highly selective neurectomy for refractory periocular synkinesis. Laryngoscope. 2013;123(6):1385–1388.
- 63. Bran GM, Lohuis PJFM. Selective neurolysis in post-paralytic facial nerve syndrome (PFS). Aesthetic Plast Surg. 2014;38(4):742–744.
- 64. van Veen MM, Dusseldorp JR, Hadlock TA. Long-term outcome of selective neurectomy for refractory periocular synkinesis. Laryngoscope. 2018;128(10):2291–2295.
- 65. Azizzadeh B, Frisenda JL. Surgical management of postparalysis facial palsy and synkinesis. Otolaryngol Clin. 2018;51(6):1169–1178.
- 66. Chuang DC-C, Chang TN-J, Lu JC-Y. Postparalysis facial synkinesis. Plastic and Reconstructive Surgery - Global Open. 2015;3(3):e320.
- 67. Terzis JK, Karypidis D. Therapeutic strategies in post-facial paralysis synkinesis in adult patients. Plast Reconstr Surg. 2012;129(6):925e–939e.
- 68. Guerrissi JO. Selective myectomy for postparetic facial synkinesis. Plast Reconstr Surg. 1991;87(3):459–466.
- 69. Lai C-S, Lu S-R, Yang S-F, Teh L-S, Lee S-S. Surgical treatment of the synkinetic eyelid closure in Marin-Amat syndrome. Ann Plast Surg. 2011;67(5):498–501.
- 70. Vincent AG, Bevans SE, Robitschek JM, Wind GG, Hohman MH. Masseteric-to-facial nerve transfer and selective neurectomy for rehabilitation of the synkinetic smile. JAMA Facial Plastic Surgery. 2019;21(6):504–510.
- 71. Gray ML, Hu S, Gorbea E, Mashkevich G. Masseteric-zygomatic nerve transfer for the management of eye closure-smile excursion synkinesis. Am J Otolaryngol. 2020;41(4):102479.
- 72. Banks CA, Jowett N, Iacolucci C, Heiser A, Hadlock TA. Five-year experience with fifth-to-seventh nerve transfer for smile. Plast Reconstr Surg. 2019;143(5):1060e–1071e.
