Introduction
The nonconventional yeast Yarrowia lipolytica has a potent ability to utilize hydrophobic substrates, including n-alkane and fatty acid, as sole carbon and energy sources, making it as a model organism for studying the metabolism of these hydrophobic substrates in fungi (Barth and Gaillardin , , Fickers et al. , Nicaud ). Additionally, Y. lipolytica can synthesize and accumulate substantial amounts of lipids within its cells. These characteristics have generated significant attention, making Y. lipolytica as a host for converting hydrophobic substrates into useful lipids and for bioremediation of environments contaminated with petroleum or oil. To optimize Y. lipolytica for these applications, it is critical to understand the metabolism of hydrophobic substrates and its regulation in this yeast.
In Y. lipolytica, n-alkane is first hydroxylated to fatty alcohol by cytochrome P450 (P450ALK) of the CYP52 family in the endoplasmic reticulum (ER) (Fukuda , , Fukuda and Ohta , ). Yarrowia lipolytica has 12 genes, ALK1–ALK12, encoding CYP52-family P450s, with ALK1 being primarily responsible for n-alkane assimilation (Iida et al. , , Takai et al. , Iwama et al. ). Fatty alcohol is then oxidized to fatty aldehyde by fatty alcohol dehydrogenase in the ER or fatty alcohol oxidase in the peroxisome (Iwama et al. ). Subsequently, fatty aldehyde is oxidized to fatty acid by fatty aldehyde dehydrogenase in either the ER or the peroxisome (Iwama et al. ). Fatty acid is activated to acyl-CoA by acyl-CoA synthetase and then either metabolized through β-oxidation or used for lipid synthesis (Tenagy et al. , ). The transcription of genes involved in n-alkane metabolism, including ALK1, is upregulated in the presence of n-alkane and repressed in the presence of glycerol (Iida et al. , , Mori et al. ). The transcription of ALK1 is regulated by the basic helix-loop-helix transcription activators Yas1 and Yas2, and the Opi1-family transcription repressor Yas3 (Yamagami et al. , Endoh-Yamagami et al. , Hirakawa et al. , Fukuda and Ohta ). Yas1 and Yas2 form a heterocomplex that is constitutively present in the nucleus. In the absence of n-alkane, Yas3 binds to Yas2 in the nucleus and represses ALK1 transcription. When n-alkane is present, Yas3 is retained at the ER membrane, possibly through interaction with phosphatidic acid or phosphoinositide, allowing the Yas1–Yas2 complex to activate ALK1 transcription (Kobayashi et al. , , , Fukuda and Ohta ).
Oxysterol-binding protein (OSBP)-related proteins (ORPs) are a family of lipid transfer proteins (LTPs) widely conserved in eukaryotes (Arora et al. ). Initially identified as proteins binding to oxysterol, further research has revealed that some ORPs can also bind cholesterol or ergosterol, the latter being a major sterol in fungi. The yeast Saccharomyces cerevisiae has seven ORP genes, OSH1–OSH7. While none of these OSH genes are essential for growth individually, a strain with all seven OSH genes deleted is inviable, indicating that Osh proteins share a common, essential function, which remains undetermined (Beh et al. ). While all Osh proteins have been shown to transport sterol between membranes in vitro (Raychaudhuri and Prinz , Tian et al. , ), Osh6 and Osh7 specifically are reported to be involved in transporting phosphatidylserine (PS) from the ER to the plasma membrane (Maeda et al. ). Additionally, ORPs have been proposed to function as regulators or lipid sensors in processes, such as endocytosis and exocytosis (Kentala et al. ). Yarrowia lipolytica has four ORP genes, OSH1, OSH3, OSH4, and OSH6, which are orthologs of OSH1, OSH3, OSH4, and OSH6, respectively, in S. cerevisiae. We have previously shown that deletion mutants of OSH3 and OSH6 exhibit defects in growth on n-alkanes in Y. lipolytica (Iwama et al. ). In the OSH6 deletion mutant, although the transcription of ALK1 was upregulated in response to n-alkane, the production of functional P450 induced by n-alkane was not observed. These results, along with the proposed role of Osh6 in S. cerevisiae, suggest that PS or sterol may accumulate in the ER membrane, where P450s function, in the OSH6 deletion mutant. This accumulation, due to a deficiency in PS or sterol transport in the OSH6 deletion mutant of Y. lipolytica, likely leads to defects in the activity or folding of P450s, including Alk1.
The phospholipid synthesis pathway in fungi has been extensively studied and elucidated in S. cerevisiae (Fig. S1) (Henry et al. ). In the cytidine diphosphate-diacylglycerol (CDP-DAG) pathway, PS and phosphatidylinositol (PI) are synthesized from CDP-DAG and serine or myo-inositol in the ER by PS synthase Pss1/Cho1 or PI synthase Pis1, respectively. PS is decarboxylated to phosphatidylethanolamine (PE) by PS decarboxylases Psd1 in the mitochondria or the ER, or by Psd2 in endosome, Golgi, or vacuole. PE is subsequently trimethylated to phosphatidylcholine (PC) by PE methyltransferases Pem1/Cho2 and Pem2/Opi3 in the ER. Additionally, PE and PC are also synthesized from ethanolamine (Etn) and choline (Cho), respectively, through the Kennedy pathway. In the Kennedy pathway, Etn and Cho are phosphorylated by Eki1 and Cki1 to form phosphoethanolamine (P-Etn) and phosphocholine (P-Cho). P-Etn and P-Cho are then converted to CDP-ethanolamine (CDP-Etn) and CDP-choline (CDP-Cho) by Ect1 and Pct1, respectively. Finally, PE and PC are synthesized from DAG and CDP-Etn and CDP-Cho by Ept1 and Cpt1. PE and PC are essential for the growth of S. cerevisiae. However, PS is not essential for growth, and a PSS1 deletion mutant can grow if Etn is supplemented. Moreover, the PSS1 deletion mutant can grow in the presence of Cho because PC is synthesized from exogenous Cho through the Kennedy pathway, and PE is synthesized from P-Etn produced in the degradation of phosphorylated sphingoid base by Dpl1.
Although S. cerevisiae can grow without PS, PSS1 is required for or involved in various cellular processes. These processes include the polarized localization of Cdc42 and the establishment of cell polarity (Fairn et al. ), shmoo formation and endocytosis of mating pheromone (Kashikuma et al. ), vacuolar morphology (Hamamatsu et al. ), tryptophane uptake (Nakamura et al. ), plasma membrane protein localization (Guo et al. , Kashikuma et al. ), plasma membrane structure (Mioka et al. ), resistance to higher temperatures (Jarolim et al. , Nomura et al. ), and TORC2 signaling (Nomura et al. ). In contrast, the roles of PS in Y. lipolytica are not well understood. In this study, we investigated the role of the ortholog of S. cerevisiae PSS1/CHO1 in Y. lipolytica. Our results indicate that the PS synthase is involved in the utilization of n-alkane and the production of the functional P450 required for the hydroxylation of n-alkane in Y. lipolytica.
Materials and methods
Strains and growth conditions
Yeast strains used in this study are shown in Table 1. Yarrowia lipolytica strain CXAU1 (MATA ura3 ade1) and CXAU/A1 (MATA ura3 ade1::ADE1) were used as wild-type strains (Iida et al. , Yamagami et al. ). To delete PSS1 (YALI0D08514g, NCBI accession number XP_502577.1), ADE1-carrying fragment that was obtained by digestion of pBPSS1PT-ADE1 (Table 2) with ApaI and XbaI was used as a deletion cassette. Correct integration was confirmed by Polymerase Chain Reaction (PCR).
An appropriate carbon source was added to YNB [0.17% yeast nitrogen base without amino acids and ammonium sulfate (Becton, Dickinson and Company, Sparks, MD) and 0.5% ammonium sulfate (Fujifilm Wako Pure Chemical, Osaka, Japan)] as follows: 2% (w/v) glucose (Fujifilm Wako Pure Chemical) (SD); 2% (w/v) glycerol (Kanto Chemicals, Tokyo, Japan) (SG); 2% (v/v) n-decane (TCI, Tokyo, Japan) (C10); 2% (v/v) n-dodecane (TCI) (C12); 2% (v/v) n-tetradecane (TCI) (C14); or 2% (v/v) n-hexadecane (TCI) (C16). Uracil (24 mg/l) and/or adenine (24 mg/l) were/was added, if necessary. For solid media, 2% agar (Kanto Chemicals) was added. n-Alkanes were supplied in the vapor phase to YNB solid media as described previously (Endoh-Yamagami et al. ). YPD medium [1% yeast extract (Becton, Dickinson and Company), 2% Hipolypepton (Nihon Pharmaceutical, Tokyo, Japan), and 2% glucose] was also used. Yeast cells were grown at 30°C.
Plasmids
Plasmids used in this study are shown in Table 2, and sequences of used primers are listed in Table S1.
The deletion cassette for PSS1 was constructed as follows: The 5′- and 3′-adjacent regions of PSS1 were amplified from CXAU1 total DNA by PCR using primers PSS1P-F, PSS1P-R, PSS1T-F, and PSS1T-R. Amplified fragments were cloned into pBluescript II SK (+) to obtain pBPSS1PT. pBPSS1PT was digested with BamHI and ligated with the ADE1-carrying BamHI fragment of pSAT4 to obtain pBPSS1PT-ADE1.
The plasmid to express mNeonGreen-Pss1 was constructed as follows: the DNA fragments of the 5′-adjacent region of PSS1 and the ORF with the 3′-adjacent region of PSS1 were amplified from CXAU1 total DNA by PCR using primers pSUT5-EcoRIsite-PSS1ncr-F and PSS1p-mNG-R, and PSS1ORF-F and PSS1t-pSUT5-EcoRIsite-R, respectively. The mNeonGreen gene was amplified from pFA6a-mNeonGreen-KanMX6 by PCR using primers mNeoGreen-F and mNG-PSS1ORF-R. The codon usage of mNeonGreen in the plasmid pFA6a-mNeonGreen-KanMX6 was modified for expression in the yeast S. cerevisiae. These fragments were cloned into the EcoRI site or pSUT5 using SLiCE (Motohashi ) to obtain pmNG-PSS1.
The plasmid to overexpress PSS1 using TEF1 promoter was constructed as follows: the ORF of PSS1 was amplified from CXAU1 total DNA by PCR using primers pSUTEF1-PSS1-F and pSUTEF1-PSS1-R, and cloned into EcoRI site of pSUTEF1 (Iwama et al. ) using SLiCE to obtain pSUTEF1-PSS1.
The plasmid to express the fusion protein of Alk1 with mNeonGreen at its C-terminus under the control of ALK1 promoter was constructed as follows: the DNA fragment of the ORF with the 5′-adjacent region of ALK1 was amplified from CXAU1 total DNA by PCR using primers pSUT5-EcoRI-ALK1p-F and ALK1-nonSC-R. The mNeonGreen gene was amplified from pFA6a-mNeonGreen-KanMX6 by PCR using primers ALK1-mNeonGreen-F and mNeoGreen-pSUT5-EcoRIsite-R. These fragments were cloned into the EcoRI site of pSUT5 using SLiCE (Motohashi ) to obtain pSALK1-mNG.
Transformation of Y. lipolytica
Yarrowia lipolytica was transformed by electroporation as described previously (Iida et al. ).
Quantitative real time PCR
Harvested cells were broken in the buffer provided with the kit with 0.5 mm glass beads using Cell Disruptor Micro Smash MS-100R (TOMY) and total RNAs were extracted using Plant Total RNA Mini Kit (Favorgen). Reverse transcription of RNA was performed using ReverTra Ace® qPCR RT Master Mix with gDNA Remover (TOYOBO). Quantitative PCR was performed using Applied Biosystems 7300 Real Time PCR System with THUNDERBIRD SYBR qPCR Mix (TOYOBO) with gene primers: ALK1_U3 and ALK1_L3 for ALK1, PSS1_U and PSS1_L for PSS1, and HHT1_U and HHT1_L for YALI0F25905g (HHT1) encoding histone H3 (Table S1). HHT1 was used as a normalizer.
Measurement of reduced CO-difference spectra
Cells were cultured in the SD medium for 24 h, after which n-dodecane was added and the cells were further cultured for 6 h. Reduced CO-difference spectra were measured as described previously (Takai et al. ).
Lipid analysis
Cells were precultured in the SD medium for 48 h. The wild-type strain and the wild-type strain harboring pSUTEF1-PSS1 or pSUT5 were seeded at an initial OD600 = 0.05 and the pss1Δ strain was seeded at an initial OD600 = 0.2 to the SD medium because the growth of the pss1Δ strain was slower than the wild-type strain. The cells were incubated for 24 h and harvested. Lipids were extracted from harvested cells using the BUME method (Löfgren et al. ). As internal standards, 15:0–18:1-d7-PC, 15:0–18:1-d7-PE, 15:0–18:1-d7-PS, and 15:0–18:1-d7-PI (Avanti Polar Lipids) were added. The extracted lipids were analysed by the method described by Nakao et al. (). The liquid chromatography–mass spectrometry (LC–MS) system for the lipidome analysis consisted of a Prominence UFLC system (Shimadzu, Kyoto, Japan) coupled to a 3200 QTRAP System (SCIEX, Redwood City, CA). The optimal conditions for the ionization and fragmentation of each lipid were determined. MS analysis was run in the positive ion mode with the following instrument parameters: curtain gas of 10 psi, collision gas of 6 psi, gas temperature of 200°C, and ion spray voltage of 5500 V. The levels of declustering potential, entrance potential, collision energy, cell exit potential, temperature, nebulizer gas, and auxiliary gas were optimized for each target. The individual lipid contents were quantified by comparing the peak areas of analyte to their corresponding internal standards. Data acquisition and analysis were performed using Analyst Software (SCIEX).
Western blot analysis
Cells were collected, washed with PBS, and disrupted in the breaking buffer [25 mM Hepes-KOH (pH 7.4), 100 mM KCl, 10% glycerol, 1 mM dithiothreitol, and protease inhibitor cocktail (cOmplete™ Mini Protease Inhibitor Cocktail, Merck)] with glass beads using Multibeads shocker (Yasui Kikai). Proteins were separated by 10% SDS-polyacrylamide gel and transferred to a PVDF membrane. Alk1-mNeonGreen and mNeonGreen-Pss1 were detected with mNeonGreen Monoclonal antibody (32F6) (ChromoTek) diluted 1:1000. HRP-linked antimouse IgG (Cell Signaling Technology; 1:5000) was used as a secondary antibody. TBS-T buffer [50 mM Tris–HCl (pH 7.6), 150 mM KCl, 0.05% Tween 20] was used for dilution of the antibodies.
Microscopy
Microscopic images were acquired using IX73 microscope equipped with a microscope digital camera DP80 (Olympus, Tokyo, Japan).
Results
Growth and P450ALK production of the deletion mutant of PSS1 of Y. lipolytica
We searched the genome database of Y. lipolytica for the ortholog of PSS1/CHO from S. cerevisiae and identified a gene YALI0D08514g. This gene is predicted to be 711 base pairs in length and encodes a 236-amino-acid protein (Fig. S2). The product of YALI0D08514g showed ~60% identity with the Pss1 of S. cerevisiae. This gene was named PSS1.
To investigate the role of PSS1 of Y. lipolytica, we constructed a deletion mutant of PSS1 (pss1Δ) and characterized it. The pss1Δ strain did not grow on minimal medium containing glucose (SD), but did grow when supplemented with 1 mM Etn, 1 mM Cho, or both (Fig. 1A). The colonies of the pss1Δ strain were smaller than those of the wild-type strain, suggesting the importance of PS for growth on glucose (Fig. 1B and Fig. S3). The pss1Δ strain was able to grow in Cho-supplemented media, probably because PE is synthesized from P-Etn, produced in the degradation of phosphorylated sphingoid base by the Dpl1 homolog. However, the colonies of the pss1Δ strain on SD medium containing Cho were smaller than those on SD medium containing Etn. It is possible that PE synthesized in the Kennedy pathway from P-Etn produced in the degradation of phosphorylated sphingoid base was insufficient in Y. lipolytica in the absence of exogenous Etn.
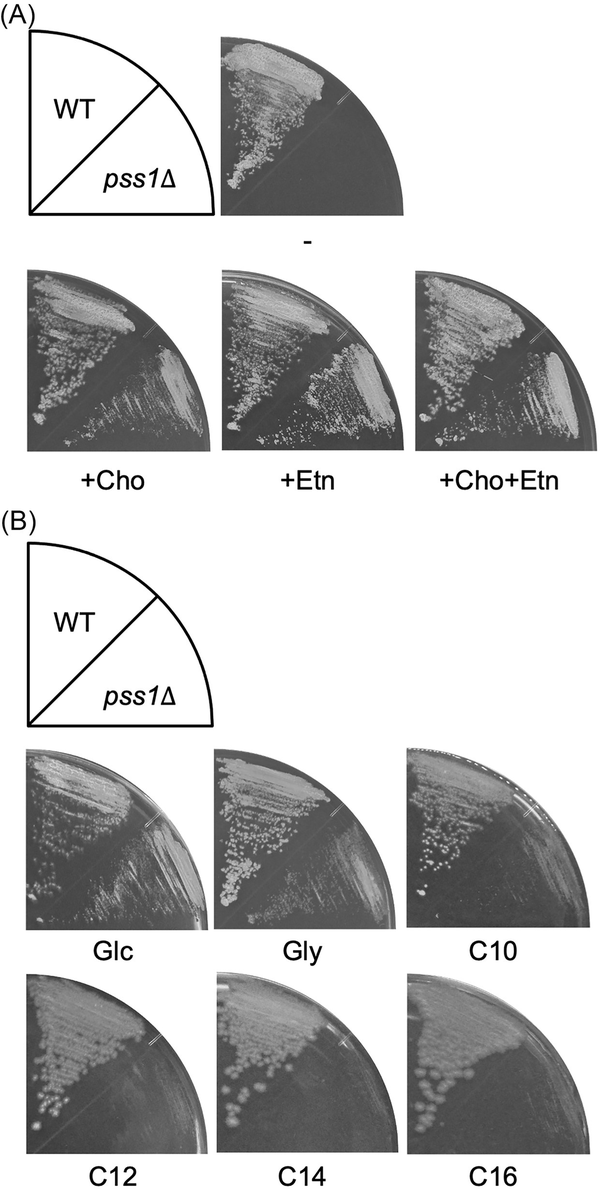
Figure 1
Growth of the deletion mutant of PSS1. (A) The wild-type strain CXAU/A1 and the pss1Δ strain were cultured on the solid SD medium in the presence or absence of Etn and/or Cho for 2 days. (B) The wild-type strain CXAU/A1 and the pss1Δ strain were cultured on the solid SD medium (Glc) and SG medium (Gly) for 2 days, and on the YNB medium containing n-decane (C10), n-dodecane (C12), n-tetradecane (C14), or n-hexadecane (C16) for 5 days in the presence of Etn and Cho.
We next analysed the growth of the pss1Δ strain on medium containing glycerol or n-alkane, with the presence of Etn and Cho (Fig. 1B and Fig. S3). The pss1Δ strain could grow on the medium containing glycerol, but its growth was slower than that of the wild-type strain. In contrast, the pss1Δ strain exhibited severe growth defects on medium containing n-decane, n-dodecane, n-tetradecane, and n-hexadecane. These results suggest that PSS1 plays critical roles in the utilization of n-alkanes in Y. lipolytica.
As mentioned in the section “Introduction,” the transcription of genes involved in n-alkane metabolism, including ALK1, is upregulated in the presence of n-alkane. The transcription of ALK1 is regulated by the Yas1–Yas2–Yas3 system in response to n-alkane (Fukuda and Ohta , Fukuda ). Because Yas3 is suggested to localize to the ER membrane through interaction with phosphatidic acid or phosphoinositide in the presence of n-alkane, it is considered that phospholipids might be involved in the transcriptional regulation of genes required for n-alkane metabolism. Therefore, we analysed the transcript level of ALK1 by quantitative real time PCR (qRT-PCR) (Fig. 2A). The wild-type and pss1Δ strains, cultured for 24 h in a glucose-containing medium with Etn and Cho, were incubated in medium containing glucose, glycerol, n-dodecane, or n-hexadecane with Etn and Cho for 1 h. Significant differences were not observed in the transcript levels of ALK1 between the wild-type and pss1Δ strains when cultured in the media containing glucose, glycerol, or n-dodecane, while the ALK1 transcript level was slightly lower in the pss1Δ strain compared with the wild-type strain when cultured in the medium containing n-hexadecane. These results imply that the transcription of ALK1 is also activated in the pss1Δ strain.
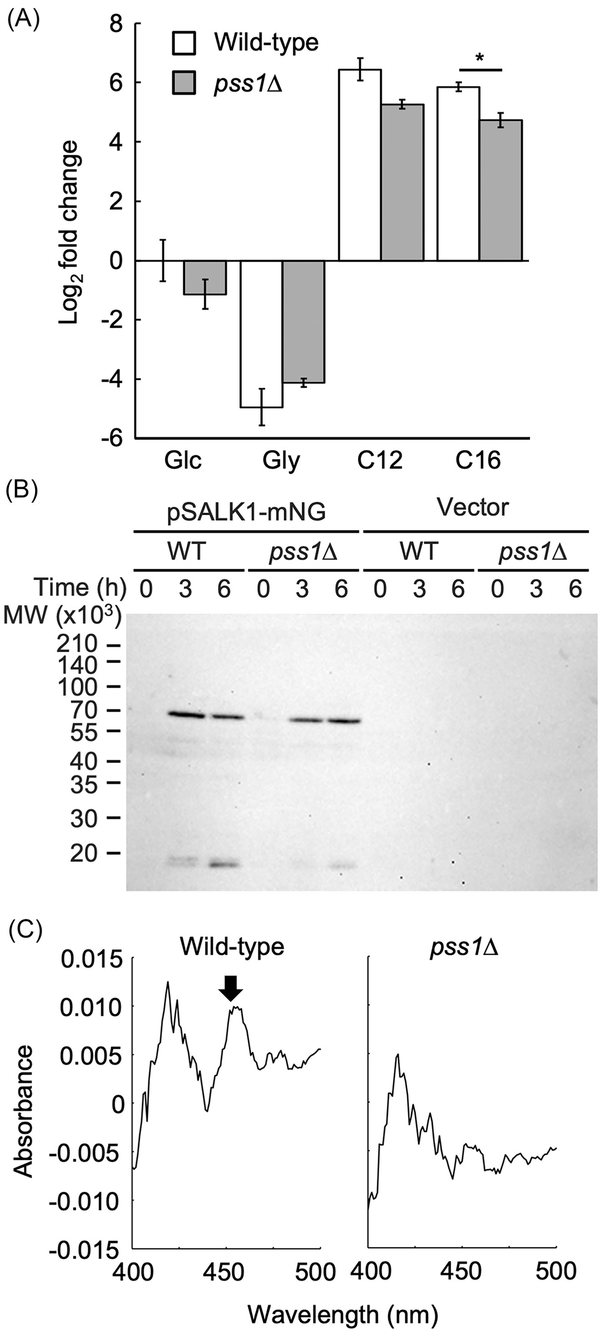
Figure 2
Expression of ALK1 and production of P450 in the deletion mutant of PSS1. (A) Expression of ALK1 in the pss1Δ strain. The wild-type strain CXAU/A1 (white bars) and the pss1Δ strain (gray bars) were cultured to logarithmic phase in the SD medium containing Etn and Cho, and shifted to the SD (Glc), SG (Gly), or the YNB medium containing n-dodecane (C12) or n-hexadecane (C16) in the presence of Etn and Cho, after which they were further incubated for 1 h. Cells were collected and the amount of ALK1 mRNA was quantified by qRT-PCR according to the section “Materials and methods.” Fold changes compared to the wild-type cells incubated in the SD medium were calculated. Bars indicate the mean of three biological replicates. Error bars represent SE. *, statistically significant difference relative to the wild-type strain (*, P < .05, Student's t-test). (B) Production of Alk1-mNeonGreen in the pss1Δ strain. The wild-type strain CXAU/A1 and the pss1Δ strain harboring the empty vector or the plasmid to express Alk1-mNeonGreen (pSALK1-mNG) were cultured to logarithmic phase in the SD medium containing Etn and Cho (0 h), and shifted to the SD medium containing n-dodecane with Etn and Cho, after which cells were further incubated for 3 or 6 h. The cell extracts were prepared and subjected to western blot analysis using anti-mNeonGreen antibody. (C) Production of functional P450 in the pss1Δ strain. The wild-type strain CXAU/A1 (WT) and the pss1Δ strain were cultured to logarithmic phase in the SD medium containing Etn and Cho, after which n-dodecane was added and the cells were further incubated for 6 h. Reduced CO-difference spectra of whole yeast cells were measured. The peak corresponding to P450 is indicated by an arrow. Similar results were obtained in two independent experiments.
We also assessed whether the translation products of ALK1 increased in the pss1Δ strain in response to n-alkane. A low-copy plasmid, pSALK1-mNeonGreen, was constructed to express Alk1 tagged with the fluorescent protein mNeonGreen at its C-terminus (Alk1-mNeonGreen) from the ALK1 promoter. The deletion mutant of 12 ALK genes cannot grow on the medium containing n-alkanes (Takai et al. ), but its growth was restored by introducing this plasmid, indicating that Alk1-mNeonGreen is functional in Y. lipolytica (Fig. S4). Next, the wild-type and pss1Δ strains containing pSALK1-mNeonGreen, precultured in medium containing glucose with Etn and Cho were shifted to medium containing n-dodecane with Etn and Cho and incubated for 3 and 6 h. The amounts of Alk1-mNeonGreen in these extracts were analysed by western blot (Fig. 2B). The amounts of Alk1-mNeonGreen increased in the pss1Δ strain cultured in medium containing n-dodecane compared with those in the medium containing glucose, similar to the wild-type strain. This suggests that ALK1 mRNA is translated normally in the pss1Δ strain.
We next analysed the production of functional P450 in response to n-alkane. The wild-type and pss1Δ strains that were cultured in medium containing glucose with Etn and Cho, were incubated in medium containing n-dodecane and glucose with Etn and Cho for 6 h. Reduced CO-difference spectra were measured in these cells (Fig. 2C). A distinct peak was observed at around 450 nm in the wild-type strain, but not in the pss1Δ strain, indicating that the pss1Δ strain has a defect in producing functional P450 in response to n-alkane.
Cellular phospholipid composition in the deletion mutants of PSS1
The cellular phospholipid composition in the pss1Δ strain cultured in the glucose medium with Etn and Cho was analysed. As shown in Fig. 3, PS species were not detected in the lipid extracts of the pss1Δ strain, confirming that PSS1 encodes a PS synthase in Y. lipolytica. Instead, the levels of various PI species, including 32:2, 34:0, 34:1, 36:1, and 36:2, were elevated in the pss1Δ strain compared with the wild-type strain, likely because excess CDP-DAG was used for PI synthesis in the pss1Δ strain due to the defect in PS synthesis.
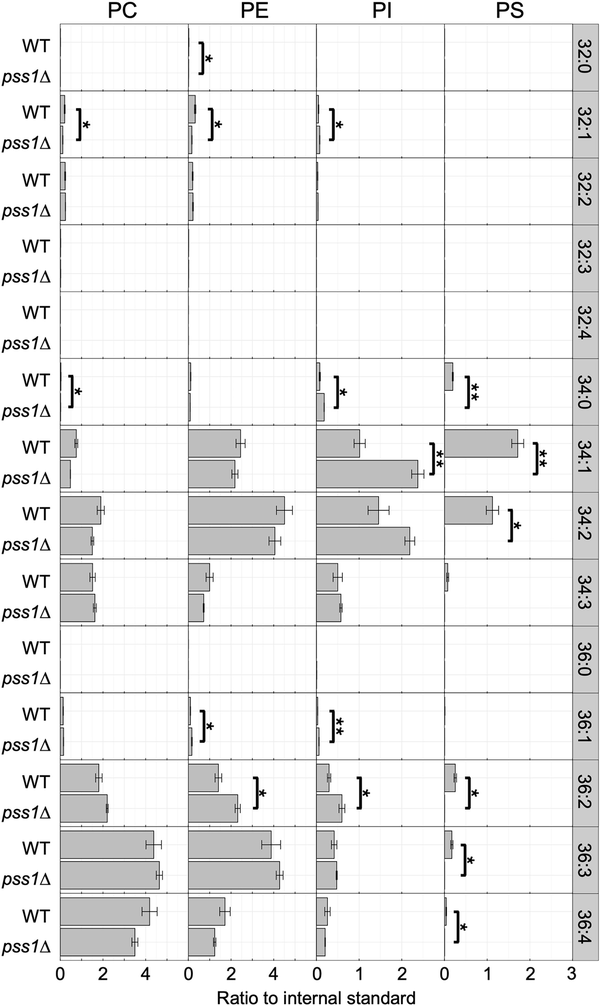
Figure 3
Phospholipid composition of the deletion mutants of PSS1. The wild-type strain CXAU/A1 (WT) and the pss1Δ strain were cultured to logarithmic phase in the SD medium containing Etn and Cho. Lipids were extracted and analysed as described in the section “Materials and methods.” Acyl chain profiles of each phospholipid species are shown. Data are the means of three independent assays. Error bars represent SE. * and **, statistically significant difference relative to the wild-type strain (*, P < .05; **, P < .01, Student’s t-test).
Subcellular localization of Pss1
The subcellular localization of Pss1 in Y. lipolytica were determined. Pss1 fused with mNeonGreen at its C terminus (Pss1-mNeonGreen) or N terminus (mNeonGreen-Pss1) was expressed from the PSS1 promoter using a low-copy plasmid in the pss1Δ strain. The expression of mNeonGreen-Pss1 restored the growth defect of the pss1Δ strain in the absence of Ent or Cho (Fig. 4A), indicating that mNeonGreen-Pss1 functions as Pss1 in Y. lipolytica. In contrast, Pss1-mNeonGreen did not restore the growth defect of the pss1Δ strain in the absence of Ent or Cho (data not shown), implying that Pss1-mNeonGreen does not function as Pss1. In the western blot analysis of the extract of the cells expressing mNeonGreen-Pss1, a band corresponding to the molecular weight of the full-length mNeonGreen-Pss1 was detected using the anti-mNeonGreen antibody (Fig. 4B), indicating that mNeonGreen-Pss1 is expressed in the cells. Next, the localization of mNeonGreen-Pss1 in cells expressing Sec63-mCherry as an ER marker was observed. The fluorescent signals of mNeonGreen-Pss1 were observed in almost the same compartments as Sec63-mCherry (Fig. 4C). These results suggest that Pss1 of Y. lipolytica is localized to the ER, similarly to Pss1 of S. cerevisiae.
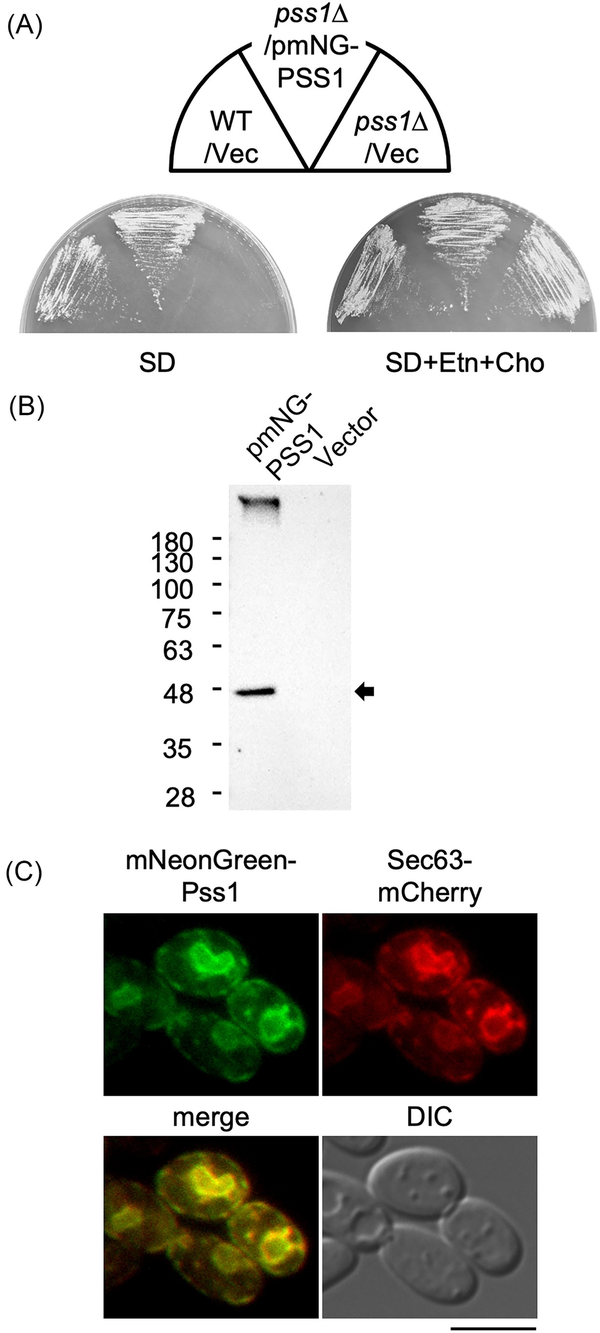
Figure 4
Subcellular localization of Pss1. (A) Growth of the strain expressing mNeonGreen-Pss1. The wild-type strain CXAU/A1 (WT) and the pss1Δ strain harboring the empty vector (Vec) or the plasmid to express mNeonGreen-Pss1 (pmNG-PSS1) were cultured on the solid SD medium in the presence or absence of Etn and Cho for 2 days. (B) Production of mNeonGreen-Pss1. The wild-type strain CXAU/A1 harboring the empty vector or the plasmid to express mNeonGreen-Pss1 (pmNG-PSS1) was cultured to logarithmic phase in the SD medium. The cell extracts were prepared and subjected to western blot analysis using anti-mNeonGreen antibody as described in the section “Materials and methods.” The band corresponding to the molecular weight of the full-length mNeonGreen-Pss1 is indicated by an arrow. (C) The strain expressing Sec63-mCherry and mNeonGreen-Pss1 was cultured to logarithmic phase in the SD medium. The localization of Sec63-mCherry and mNeonGreen-Pss1 was observed as described in in the section “Materials and methods.” Bar, 5 µm.
Expression level of PSS1
The expression level of PSS1 in wild-type cells cultured in media with various carbon sources was analysed using qRT-PCR. No significant difference in PSS1 transcript levels was observed between cells cultured in medium containing glucose or glycerol (Fig. 5). However, PSS1 transcript level was slightly decreased in cells cultured in medium containing n-decane and increased in those cultured in the medium containing n-hexadecane.
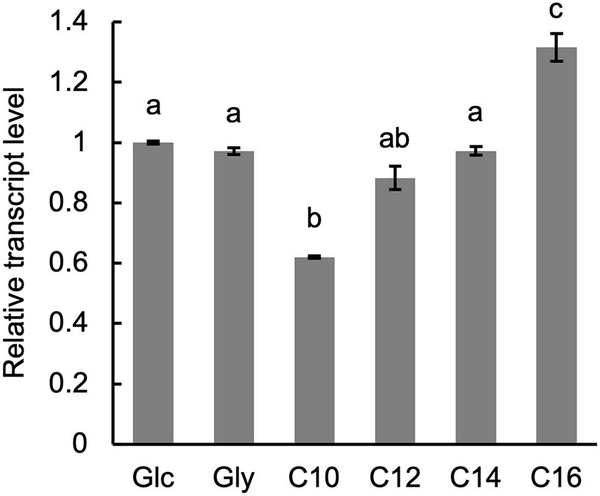
Figure 5
Expression of PSS1. The wild-type strain CXAU/A1 was cultured to logarithmic phase in the SD medium, and shifted to the SD (Glc), SG (Gly), or the YNB medium containing n-decane (C10), n-dodecane (C12), n-tetradecane (C14), or n-hexadecane (C16), after which they were further incubated for 1 h. Cells were collected and the amounts of PSS1 mRNA were quantified by qRT-PCR according to the section “Materials and methods.” Bars indicate the mean of three biological replicates. Error bars represent SE. a, b, and c, statistically significant differences among PSS1 transcript levels are indicated by different letters above bars (P < .01; Tukey HSD test).
Overexpression of PSS1
The deletion mutant of OSH6 of Y. lipolytica showed defects in growth on n-alkanes and in the production of functional P450 in response to n-alkane (Iwama et al. ). These results raised the possibility that the deletion of OSH6 caused the accumulation of PS in the ER membrane, negatively affecting the folding or activity of Alk proteins in the OSH6 deletion mutant. To examine this possibility, PSS1 was overexpressed in Y. lipolytica. A plasmid to express PSS1 from the constitutive promoter of TEF1 (Iwama et al. ) was constructed and introduced into the wild-type strain. qRT-PCR analysis showed that the transcript level of PSS1 was highly increased in the strain containing the PSS1-overexpression plasmid (Fig. 6A). Next, we examined the growth of this strain on media containing various carbon sources. The strain overexpressing PSS1 exhibited similar growth levels on the medium containing glucose, glycerol, or n-alkanes (Fig. 6B). Additionally, the strain overexpressing PSS1 showed no significant alteration in the composition of phospholipids (Fig. 6C), suggesting that a regulatory system maintaining phospholipid composition functions in Y. lipolytica even when PSS1 is overexpressed.
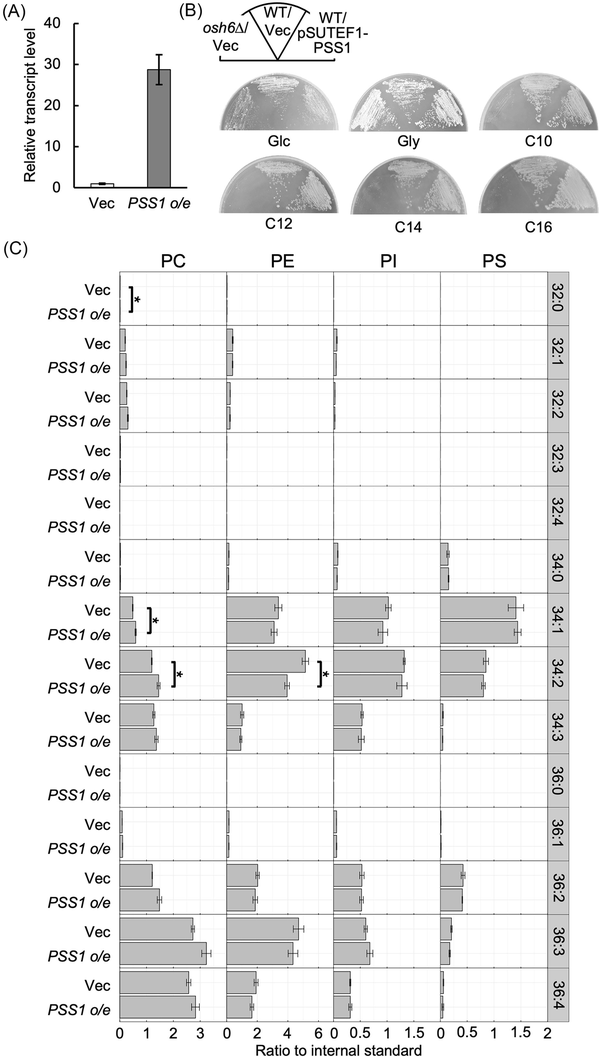
Figure 6
Overexpression of PSS1. (A) Expression of PSS1. The wild-type strain CXAU/A1 strain containing the empty vector (Vec) or the plasmid to overexpress PSS1 (PSS1 o/e) was cultured to logarithmic phase in the SD medium. Cells were collected and the amount of PSS1 mRNA was quantified by qRT-PCR according to the section “Materials and methods.” The relative transcript level of PSS1 in the strain containing the plasmid to overexpress PSS1 to that in vector-containing strain is shown. Bars indicate the mean of three biological replicates. Error bars represent SE. (B) Growth of the strain containing the plasmid to overexpress PSS1. The wild-type strain CXAU/A1 (WT) containing the empty vector (Vec) or the plasmid to overexpress PSS1 (pSUTEF1-PSS1) and the osh6Δ containing the empty vector were cultured on the solid SD medium (Glc) for 2 days, SG medium (Gly) for 2 days, and on the YNB medium containing n-decane (C10), n-dodecane (C12), n-tetradecane (C14), or n-hexadecane (C16) for 5 days. (C) Phospholipid composition of the strain containing the plasmid to overexpress PSS1. The wild-type strain CXAU/A1 containing the empty vector (Vec) or the plasmid to overexpress PSS1 (PSS1 o/e) were cultured to logarithmic phase in the SD medium. Lipids were extracted and analysed as described in the section “Materials and methods.” Acyl chain profiles of each phospholipid species are shown. Data are the means of three independent assays. Error bars represent SE. *, statistically significant difference relative to the wild-type strain (*, P < .05, Student’s t-test).
Discussion
In this study, we characterized the PS synthase gene PSS1 in Y. lipolytica. The deletion mutant of PSS1 exhibited defects in growth on media containing n-alkanes as carbon sources and in the production of functional P450 in response to n-alkane, although the transcription of ALK1 was upregulated in response to n-alkane and the ALK1 transcript was translated in the pss1Δ strain. These results suggest that PSS1 plays a critical role in the n-alkane-induced production of P450 and the utilization of n-alkane.
The roles of PS in the activity and membrane binding of various P450s have been analysed in vitro. Studies have reported that PS stimulates the catalytic activities of P450s, CYP17A1, CYP1A1, and CYP3A4 of human, and CYP2B1 of rat (Kisselev et al. , Kim et al. , , Peng and Auchus ). Additionally, PS has been shown to enhance the membrane binding of rabbit CYP1A2 (Ahn et al. ). In contrast, PS does not affect the catalytic activity or conformation of human CYP1B1 (Jang et al. ). It has also been reported that PS inhibits the 5-cholestane-3,7,12-triol hydrolase activity of human CYP27A1, while having no effect on its cholesterol hydroxylase activity (Murtazina et al. ). Thus, PS plays important roles in the activity and membrane binding of a subset of P450s in vitro. However, the roles of PS in P450 activity and membrane binding in vivo remain poorly understood. In this study, it was suggested that PS is required for the production of functional Alk proteins of the CYP52 family in Y. lipolytica in vivo. In S. cerevisiae, ERG11, which encodes the CYP51 family P450 responsible for C-14 demethylation of lanosterol in the ergosterol synthesis pathway, is essential for growth under standard conditions (Kalb et al. ). Erg11 is conserved in eukaryotes and Y. lipolytica contains a gene, YALI0B05126g, that encodes a protein homologous to Erg11 of S. cerevisiae. It is possible that functional Erg11 is produced in the pss1Δ strain of Y. lipolytica since this strain can grow in glucose-containing medium as long as Eth or Cho is added. Therefore, the effects of PS on P450 functions or membrane binding may vary among different P450 species within Y. lipolytica.
The pss1Δ strain was able to grow on SD medium supplemented with Etn or Cho. Yarrowia lipolytica possesses genes that encode proteins homologous to the enzymes of the Kennedy pathway of S. cerevisiae, including YALI0B09515g and YALI0E16907g (EKI1 or CKI1), YALI0C06303g (ECT1), YALI0D18271g (PCT1), and YALI0C10989g and YALI0E26565g (EPT1 or CPT1). Consequently, PE and PC can be produced from Etn and Cho, respectively, via the Kennedy pathway in Y. lipolytica, similarly to S. cerevisiae (Henry et al. ). Additionally, Y. lipolytica contains genes, YALI0E06061g (PEM1) and YALI0E12441g (PEM2), encoding proteins homologous to the PE methyltransferases Pem1 and Pem2, respectively, of S. cerevisiae. Therefore, when Etn is supplemented in the medium, PE produced through the Kennedy pathway may be trimethylated to PC by the orthologs of Pem1 and Pem2. Furthermore, Y. lipolytica has a gene, YALI0E28237g, encoding a protein homologous to S. cerevisiae Dpl1, which degrades phosphorylated sphingoid base into fatty aldehyde and P-Etn. When Cho is supplemented in the absence of Etn, it is likely that PC is synthesized from Cho and PE is synthesized from P-Etn produced in the degradation of phosphorylated sphingoid base by the Dpl1 homolog through the Kennedy pathway. The colonies of the pss1Δ strain on SD medium containing Etn, Cho, or both were smaller than those of the wild-type strain. These results suggest that PS plays an important role in growth on glucose-containing medium. Growth retardation due to the deletion of the PS synthase gene in the presence of Etn or Cho has also been observed in S. cerevisiae (Hikiji et al. ).
In Y. lipolytica, the deletion mutant of SFH21, which encodes a Sec14-family protein, exhibited defects in the utilization of n-alkane and filamentous growth in response to n-alkane (Watanabe et al. ). Sec14-family proteins are a group of LTPs that are widely conserved in eukaryotes. It has been reported that these proteins promote phosphoinositide production by extracting PI from membranes and presenting it to PI kinases (Schaaf et al. ). They are also hypothesized to facilitate phospholipid transport between cellular membranes (Mizuike et al. ). The defects observed in the SFH21 deletion mutant regarding n-alkane utilization and filamentous growth raise the possibility that phospholipid distribution is crucial for these processes in Y. lipolytica. However, the deletion mutant still produced functional P450 in response to n-alkane. Additionally, the pss1Δ strain exhibited filamentous growth on solid medium containing either glucose or n-alkanes, although its growth was poorer compared with the wild-type strain (Fig. S3). Therefore, the causes of the defects in n-alkane utilization in the pss1Δ strain are likely different from those in the deletion mutant of SFH21.
The deletion mutant of OSH6 exhibited defects in producing functional P450 in response to n-alkane and in growing on media containing n-alkanes (Iwama et al. ). Since Osh6 is known to transport PS (Maeda et al. ) and sterol (Tian et al. ) between membranes in vitro in S. cerevisiae, it is presumed that an increased level of PS or sterol in the ER membrane, where P450s localize and function, interferes with the folding, activity, or membrane binding of Alk proteins in the OSH6 deletion mutant. Because both the PSS1 deletion mutant and the OSH6 deletion mutant exhibited a similar phenotype regarding the defect in the production of functional P450, it is possible that the optimal amount of PS in the ER is crucial for the functionality of P450. To test this hypothesis, we attempted to increase PS levels in Y. lipolytica by overexpressing PSS1, but no increase in PS was observed in the cells (Fig. 6). It would be interesting to test whether overexpression of PSD1 or PSD2 could compensate for the growth deficiency of the OSH6 deletion mutant in n-alkanes, if their overexpression leads to a reduction in the PS content of the ER membrane. To better understand the defects in functional P450 production in the OSH6 deletion mutant, an analysis of the lipid composition of the purified ER membrane from this deletion mutant of OSH6 is needed.
Despite the elevated transcript level of PSS1 due to expression from the TEF1 promoter, no significant change in PS levels was detected. Previous studies have reported that overexpression of PSS1 leads to growth retardation in S. cerevisiae (Makanae et al. ). Therefore, it is possible that PS levels are more tightly regulated in Y. lipolytica compared with S. cerevisiae.
The enzymatic reactions involved in n-alkane metabolism occur in the ER and peroxisome. It is highly plausible that the phospholipids composing the membranes of these organelles play critical roles in their function. Investigating the roles of phospholipids other than PS in n-alkane metabolism in Y. lipolytica is of significant interest.
Acknowledgements
This work was performed using the facilities of the Agro-Biotechnology Research Center of The University of Tokyo.
References
- Ahn T, Guengerich FP, Yun CH. Membrane insertion of cytochrome P450 1A2 promoted by anionic phospholipids. Biochemistry. 1998;37:12860–6.
- Arora A, Taskinen JH, Olkkonen VM. Coordination of inter-organelle communication and lipid fluxes by OSBP-related proteins. Prog Lipid Res. 2022;86:101146.
- Barth G, Gaillardin C. Yarrowia Lipolytica. In: Wolf K (ed.), Non-Conventional Yeast in Biotechnology a Handbook. Berlin, Heidelberg, New York: Springer, 1996, 313–88.
- Barth G, Gaillardin C. Physiology and genetics of the dimorphic fungus Yarrowia lipolytica. FEMS Microbiol Rev. 1997;19:219–37.
- Beh CT, Cool L, Phillips J, et al Overlapping functions of the yeast oxysterol-binding protein homologues. Genetics. 2001;157:1117–40.
- Endoh-Yamagami S, Hirakawa K, Morioka D, et al Basic helix-loop-helix transcription factor heterocomplex of Yas1p and Yas2p regulates cytochrome P450 expression in response to alkanes in the yeast Yarrowia lipolytica. Eukaryot Cell. 2007;6:734–43.
- Fairn GD, Hermansson M, Somerharju P, et al Phosphatidylserine is polarized and required for proper Cdc42 localization and for development of cell polarity. Nat Cell Biol. 2011;13:1424–30.
- Fickers P, Benetti PH, Waché Y, et al Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res. 2005;5:527–43.
- Fukuda R. Metabolism of hydrophobic carbon sources and regulation of it in n-alkane-assimilating yeast Yarrowia lipolytica. Biosci Biotechnol Biochem. 2013;77:1149–54.
- Fukuda R. Utilization of n-alkane and roles of lipid transfer proteins in Yarrowia lipolytica. World J Microbiol Biotechnol. 2023;39:97.
- Fukuda R, Ohta A. Utilization of hydrophobic substrate by Yarrowia lipolytica. In: Barth G (ed.), Yarrowia Lipolytica Genetics, Genomics, and Physiology. Heidelberg, New York, Dordrecht, London: Springer, 2013, 111–9.
- Fukuda R, Ohta A. Genetic features and regulation of n-alkane metabolism in yeasts. In: Rojo F (ed.), Aerobic Utilization of Hydrocarbons, Oils and Lipids, Handbook of Hydrocarbon and Lipid Microbiology. Heidelberg, New York, Dordrecht, London: Springer, 2017a.
- Fukuda R, Ohta A. Enzymes for aerobic degradation of alkanes in yeasts. In: Rojo F (ed.), Aerobic Utilization of Hydrocarbons, Oils and Lipids, Handbook of Hydrocarbon and Lipid Microbiology. Heidelberg, New York, Dordrecht, London: Springer, 2017b.
- Guo Y, Au WC, Shakoury-Elizeh M, et al Phosphatidylserine is involved in the ferrichrome-induced plasma membrane trafficking of Arn1 in Saccharomyces cerevisiae. J Biol Chem. 2010;285:39564–73.
- Hamamatsu S, Shibuya I, Takagi M, et al Loss of phosphatidylserine synthesis results in aberrant solute sequestration and vacuolar morphology in Saccharomyces cerevisiae. FEBS Lett. 1994;348:33–36.
- Henry SA, Kohlwein SD, Carman GM. Metabolism and regulation of glycerolipids in the yeast Saccharomyces cerevisiae. Genetics. 2012;190:317–49.
- Hikiji T, Miura K, Kiyono K, et al Disruption of the CHO1 gene encoding phosphatidylserine synthase in Saccharomyces cerevisiae. J Biochem. 1988;104:894–900.
- Hirakawa K, Kobayashi S, Inoue T, et al Yas3p, an Opi1 family transcription factor, regulates cytochrome P450 expression in response to n-alkanes in Yarrowia lipolytica. J Biol Chem. 2009;284:7126–37.
- Iida T, Ohta A, Takagi M. Cloning and characterization of an n-alkane-inducible cytochrome P450 gene essential for n-decane assimilation by Yarrowia lipolytica. Yeast. 1998;14:1387–97.
- Iida T, Sumita T, Ohta A, et al The cytochrome P450ALK multigene family of an n-alkane-assimilating yeast, Yarrowia lipolytica: cloning and characterization of genes coding for new CYP52 family members. Yeast. 2000;16:1077–87.
- Iwama R, Hara M, Mizuike A, et al Osh6p, a homologue of the oxysterol-binding protein, is involved in production of functional cytochrome P450 belonging to CYP52 family in n-alkane-assimilating yeast Yarrowia lipolytica. Biochem Biophys Res Commun. 2018;499:836–42.
- Iwama R, Kobayashi S, Ishimaru C, et al Functional roles and substrate specificities of twelve cytochromes P450 belonging to CYP52 family in n-alkane assimilating yeast Yarrowia lipolytica. Fungal Genet Biol. 2016;91:43–54.
- Iwama R, Kobayashi S, Ohta A, et al Fatty aldehyde dehydrogenase multigene family involved in the assimilation of n-alkanes in Yarrowia lipolytica. J Biol Chem. 2014;289:33275–86.
- Iwama R, Kobayashi S, Ohta A, et al Alcohol dehydrogenases and an alcohol oxidase involved in the assimilation of exogenous fatty alcohols in Yarrowia lipolytica. FEMS Yeast Res. 2015;15:fov014.
- Jang HH, Kim DH, Ahn T, et al Functional and conformational modulation of human cytochrome P450 1B1 by anionic phospholipids. Arch Biochem Biophys. 2010;493:143–50.
- Jarolim S, Ayer A, Pillay B, et al Saccharomyces cerevisiae genes involved in survival of heat shock. G3. 2013;3:2321–33.
- Kalb VF, Woods CW, Turi TG, et al Primary structure of the P450 lanosterol demethylase gene from Saccharomyces cerevisiae. DNA. 1987;6:529–37.
- Kashikuma R, Nagano M, Shimamura H, et al Role of phosphatidylserine in the localization of cell surface membrane proteins in yeast. Cell Struct Funct. 2023;48:19–30.
- Kentala H, Weber-Boyvat M, Olkkonen VM. OSBP-related protein family: mediators of lipid transport and signaling at membrane contact sites. Int Rev Cell Mol Biol. 2016;321:299–340.
- Kim KH, Ahn T, Yun CH. Membrane properties induced by anionic phospholipids and phosphatidylethanolamine are critical for the membrane binding and catalytic activity of human cytochrome P450 3A4. Biochemistry. 2003;42:15377–87.
- Kim KH, Kim DH, Jang HH, et al Lateral segregation of anionic phospholipids in model membranes induced by cytochrome P450 2B1: bi-directional coupling between CYP2B1 and anionic phospholipid. Arch Biochem Biophys. 2007;468:226–33.
- Kisselev P, Schwarz D, Platt KL, et al Epoxidation of benzo[a]pyrene-7,8-dihydrodiol by human CYP1A1 in reconstituted membranes. Effects of charge and nonbilayer phase propensity of the membrane. Eur J Biochem. 2002;269:1799–805.
- Kobayashi S, Hirakawa K, Fukuda R, et al Disruption of the SCS2 ortholog in the alkane-assimilating yeast Yarrowia lipolytica impairs its growth on n-decane, but does not impair inositol prototrophy. Biosci Biotechnol Biochem. 2008;72:2219–23.
- Kobayashi S, Hirakawa K, Horiuchi H, et al Phosphatidic acid and phosphoinositides facilitate liposome association of Yas3p and potentiate derepression of ARE1 (alkane-responsive element one)-mediated transcription control. Fungal Genet Biol. 2013;61:100–10.
- Kobayashi S, Tezaki S, Horiuchi H, et al Acidic phospholipid-independent interaction of Yas3p, an Opi1-family transcriptional repressor of Yarrowia lipolytica, with the endoplasmic reticulum. Yeast. 2015;32:691–701.
- Löfgren L, Ståhlman M, Forsberg GB, et al The BUME method: a novel automated chloroform-free 96-well total lipid extraction method for blood plasma. J Lipid Res. 2012;53:1690–700.
- Maeda K, Anand K, Chiapparino A, et al Interactome map uncovers phosphatidylserine transport by oxysterol-binding proteins. Nature. 2013;501:257–61.
- Makanae K, Kintaka R, Makino T, et al Identification of dosage-sensitive genes in Saccharomyces cerevisiae using the genetic tug-of-war method. Genome Res. 2013;23:300–11.
- Mioka T, Guo T, Wang S, et al Characterization of micron-scale protein-depleted plasma membrane domains in phosphatidylserine-deficient yeast cells. J Cell Sci. 2022;135:jcs256529.
- Mizuike A, Kobayashi S, Rikukawa T, et al Suppression of respiratory growth defect of mitochondrial phosphatidylserine decarboxylase deficient mutant by overproduction of Sfh1, a Sec14 homolog, in yeast. PLoS One. 2019;14:e0215009.
- Mori K, Iwama R, Kobayashi S, et al Transcriptional repression by glycerol of genes involved in the assimilation of n-alkanes and fatty acids in yeast Yarrowia lipolytica. FEMS Yeast Res. 2013;13:233–40.
- Motohashi K. A simple and efficient seamless DNA cloning method using SLiCE from Escherichia coli laboratory strains and its application to SLiP site-directed mutagenesis. BMC Biotechnol. 2015;15:47.
- Murtazina DA, Andersson U, Hahn IS, et al Phospholipids modify substrate binding and enzyme activity of human cytochrome P450 27A1. J Lipid Res. 2004;45:2345–53.
- Nakamura H, Miura K, Fukuda Y, et al Phosphatidylserine synthesis required for the maximal tryptophan transport activity in Saccharomyces cerevisiae. Biosci Biotechnol Biochem. 2000;64:167–72.
- Nakao N, Ueno M, Sakai S, et al Natural ligand-nonmimetic inhibitors of the lipid-transfer protein CERT. Commun Chem. 2019;2:2098.
- Nicaud JM. Yarrowia lipolytica. Yeast. 2012;29:409–18.
- Nomura W, Ito Y, Inoue Y. Role of phosphatidylserine in the activation of Rho1-related Pkc1 signaling in Saccharomyces cerevisiae. Cell Signal. 2017;31:146–53.
- Nomura W, Ng SP, Takahara T, et al Roles of phosphatidylserine and phospholipase C in the activation of TOR complex 2 signaling in Saccharomyces cerevisiae. J Cell Sci. 2022;135:jcs259988.
- Peng HM, Auchus RJ. The action of cytochrome b5 on CYP2E1 and CYP2C19 activities requires anionic residues D58 and D65. Biochemistry. 2013;52:210–20.
- Raychaudhuri S, Prinz WA. Uptake and trafficking of exogenous sterols in Saccharomyces cerevisiae. Biochem Soc Trans. 2006;34:359–62.
- Schaaf G, Ortlund EA, Tyeryar KR, et al Functional anatomy of phospholipid binding and regulation of phosphoinositide homeostasis by proteins of the sec14 superfamily. Mol Cell. 2008;29:191–206.
- Takai H, Iwama R, Kobayashi S, et al Construction and characterization of a Yarrowia lipolytica mutant lacking genes encoding cytochromes P450 subfamily 52. Fungal Genet Biol. 2012;49:58–64.
- Tenagy IR, Kobayashi S, Shiwa Y, et al Acyl-CoA synthetases, Aal4 and Aal7, are involved in the utilization of exogenous fatty acids in Yarrowia lipolytica. J Gen Appl Microbiol. 2021;67:9–14.
- Tenagy PJS, Iwama R, Kobayashi S, et al Involvement of acyl-CoA synthetase genes in n-alkane assimilation and fatty acid utilization in yeast Yarrowia lipolytica. FEMS Yeast Res. 2015;15:fov031.
- Tian S, Ohta A, Horiuchi H, et al Evaluation of sterol transport from the endoplasmic reticulum to mitochondria using mitochondrially targeted bacterial sterol acyltransferase in Saccharomyces cerevisiae. Biosci Biotechnol Biochem. 2015;79:1608–14.
- Tian S, Ohta A, Horiuchi H, et al Oxysterol-binding protein homologs mediate sterol transport from the endoplasmic reticulum to mitochondria in yeast. J Biol Chem. 2018;293:5636–48.
- Watanabe N, Iwama R, Murayama R, et al Orthologs of Saccharomyces cerevisiae SFH2, genes encoding Sec14 family proteins, implicated in utilization of n-alkanes and filamentous growth in response to n-alkanes in Yarrowia lipolytica. FEMS Yeast Res. 2022;22:foac006.
- Yamagami S, Iida T, Nagata Y, et al Isolation and characterization of acetoacetyl-CoA thiolase gene essential for n-decane assimilation in yeast Yarrowia lipolytica. Biochem Biophys Res Commun. 2001;282:832–8.
- Yamagami S, Morioka D, Fukuda R, et al A basic helix-loop-helix transcription factor essential for cytochrome P450 induction in response to alkanes in yeast Yarrowia lipolytica. J Biol Chem. 2004;279:22183–9.


