Introduction
Oxidative permanent and semi-permanent hair dyes usually contain p-phenylenediamine (PPD), toluene-2,5-diamine (p-toluylenediamine; PTD), substituted para-diamines such as 2-methoxymethyl-p-phenylenediamine (ME-PPD), or ortho- or para-aminophenols. These colourless precursors react with a developer such as hydrogen peroxide in a series of oxidative reactions, eventually yielding large coloured molecules which in the course of polymerisation become trapped in the hair cortex, thus changing the hair colour. Many of these chemicals are classified with regard to their toxicological properties and labelled according to the Regulation (EC) No 1272/2008 on classification, labelling and packaging of substances and mixtures (CLP). For example, the most common precursors, PPD, PTD and ME-PPD, are classified as acutely toxic in contact with skin, and skin sensitizers (i.e. can also induce allergic reaction (elicitation) in already sensitized individuals)., However, under the CLP Regulation, none of these precursors are classified as carcinogenic or mutagenic.
In the Scientific Committee on Consumer Safety (SCCS) opinion on PPD published in 2012, PPD is considered non genotoxic, but with a note that information submitted was insufficient to allow a final risk assessment to be carried out. As the clastogenic effects found in vitro were not confirmed in in vivo tests, the SCCS considered PTD to have no in vivo genotoxic potential, and a similar conclusion was reached for ME-PPD in spite of equivocal results in some of the in vivo assays. Animal studies on carcinogenicity of oxidative hair dye ingredients, which were included in the SCCS opinions and in the International Agency for Cancer Research (IARC) opinion on risks of personal hair dye use and occupational exposure, mostly dated from 1970s and 1980s, with serious deviations from the current Organization for Economic Co-operation and Development Test Guidelines (OECD TGs) study design. More recent in vitro experiments indicated that PPD causes DNA damage, increases intracellular levels of reactive oxygen species leading to activation of apoptotic signalling pathways and induces cytotoxic effects through alteration of microRNA expression levels.,, In the new analysis of mutagenic components of oxidative hair dyes, however, a high rate of positive in vitro results was considered poorly predictive for in vivo assays but also for the outcomes of animal carcinogenicity assays.
There is a concern that prolonged personal use of oxidative hair dyes might lead to bladder, hematopoietic and breast cancer.,, In addition, hairdressers could also be at increased risk due to higher exposure to hairdressing chemicals at work compared to consumers., Increased risk of bladder cancer among hairdressers in comparison to the general population was noted in the study from New Zealand. Carcinogenicity could be the result of a genotoxic mode of action. A recent meta-analysis showed increased micronucleus frequencies in buccal swabs from hairdressers compared to non-exposed persons.
There is a clear need to assess the newer experimental findings, which would facilitate the interpretation of epidemiological studies. Therefore, the aim of the present systematic review was to evaluate recent in vitro and in vivo studies published in the last two decades investigating genotoxicity of oxidative hair dye precursors.
Methods
Search strategy
This study is part of a project reviewing toxicity of important hair and nail cosmetic ingredients in hairdressers. In this systematic review, the focus was on genotoxic effects of oxidative hair dye ingredients identified in in vitro or in vivo studies. A detailed protocol for systematic reviews performed within this project has previously been published and registered under the PROSPERO registration number CRD42021238118. It is based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) guidelines. We systematically searched the electronic databases MEDLINE, Web of Science - core collection, and Cochrane Library, in addition to Scientific Committee on Consumer Safety (SCCS) opinions, and toxicological dossiers of the German Committee for the determination of occupational exposure limits (‘MAK-Commission’), for publications in English, published from 2000 onwards, reporting results of any type of experimental studies investigating genotoxic effects of oxidative hair dyes or their components. In view of the complex compositions of these products, from November to December 2020 a DELPHI survey was conducted among different stakeholders, including members of the StanDerm network (EU Horizon 2020 COST Action TD 1206), the project-sponsoring social partners and the project steering group. Based on the results of the survey, substances included into the systematic review as the most relevant ingredients of oxidative hair dyes were: p-Phenylenediamine (PPD; CAS no. 106-50-3) and its salts (CAS no. 624-18-0, 16245-77-5), toluene-2,5-diamine (p-toluylenediamine; PTD; CAS no. 95-70-5) and its sulfate (CAS no. 615-50-9), and 2-methoxymethyl-PPD (mePPD; CAS no. 337906-36-2). The full search string used is shown in Supplemental Appendix 1. Initial search was performed in April 2021. Search results were imported into Zotero citation management software, where bibliographical duplicates were identified and removed. The remaining publications were imported into a Rayyan tool (Rayyan QCRI, https://rayyan.qcri.org/welcome) for shared screening by two reviewers for eligibility based on title, key words and abstract. Reference lists of already identified eligible publications were checked, a forward-snowballing citation analysis and additional manual search was conducted through Google Scholar for potentially relevant publications that may have been missed in the first systematic search.
Publication selection
The process of publication selection was performed in accordance with the PRISMA-P guidelines (Figure 1). Two researchers (Ž.B. and R.T.) independently screened publications (2000-2021) listed in search results for any relevant product identifiers and toxicological endpoints. Full text articles were retrieved and assessed for eligibility in the same manner as described above.
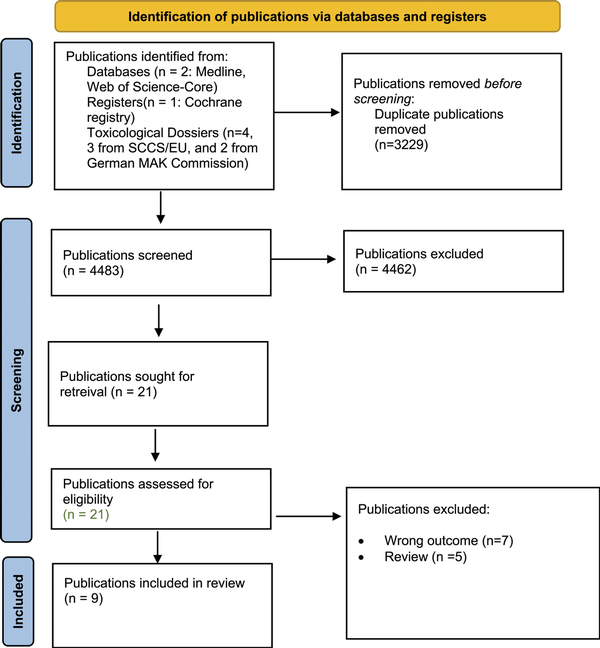
Figure 1
Prisma Flow diagram for the screening and selection of publications. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. doi: 10.1136/bmj.n71.
Data extraction
Data were extracted by two researchers (Ž.B. and R.T.) using standardized publication record forms (data extraction sheets). The extracted data included the publication ID, year of study conduct if reported, funding source, test method and guidelines followed, test article, study design (test system/animals, number of observational units and dose or concentration tested) and main outcomes.
Quality assessment
Criteria for the evaluation of quality and risk of bias for this systematic review were made by authors using four sources: i) mixed methods research appraisal; ii) Cochrane collaboration; iii) working group of the US EPA; and iv) animal studies guidance developed by the US National Toxicology Program’s Office of Health Assessment and Translation (OHAT) outlined in OHAT’s risk of bias documentation, and adopted to fit the genotoxicity studies design. The criteria described in detail in Supplemental Appendix 2 consisted of three parts regarding appropriate design and sample, justification of methodology, and presentation of results. A maximal score of 15 was possible and a score yielding a proportion ≥70% (i.e. ≥10.5 points) was considered good quality and a score <70% to be of lower quality.
Data synthesis
Characteristics and main findings of included studies were summarized tabularly, presenting the reported adverse effects in relation to administered dose or concentration of oxidative hair dye ingredient. Because of diversity of relevant outcomes and limitation to include only recent studies, results cannot be directly pooled for quantitative analyses, but the results, including quantifications from single studies considered sufficiently valid, were qualitatively synthetized by textual explanation, addressing the sources of heterogeneity and giving an overall conclusion.
Results
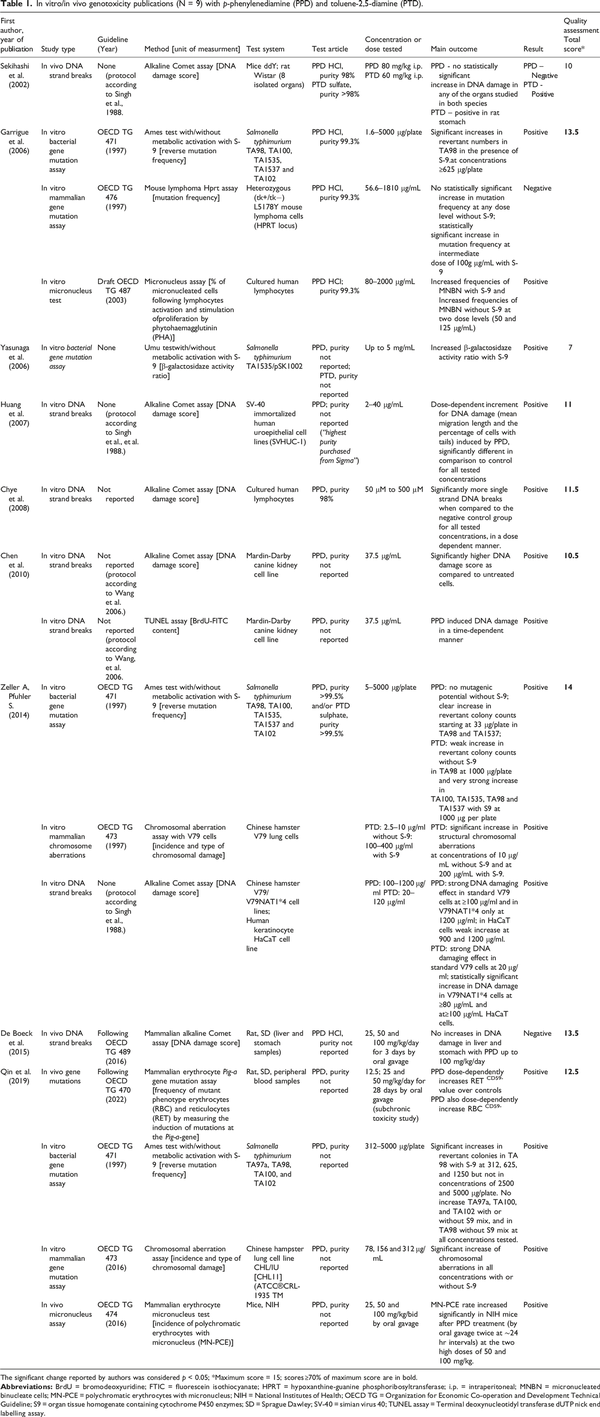
A total of 9 publications were analysed with the results of 17 studies published from 2000 onwards that investigated genotoxic effects of hair dye precursors (Table 1). All tested the effects of PPD and only three also included PTD. Only two studies were considered to be of lower quality,, mainly due to the methodological problems and incomplete result presentation.
In vitro bacterial gene mutation assay was performed in three studies,,, all of good quality, adhering to relevant OECD TGs. All studies had comparable protocols for in vitro bacterial assay following the current OECD guideline which was adopted in 1997, used similar PPD dose ranges, and provided comparable results. PPD tested positive only in one Salmonella typhimurium strain (TA 98) in a dose-related manner, but only in the presence of metabolic activation with rat liver S-9 mix. In other strains tested no increase in the number of revertant colonies was seen with or without metabolic activation. PPD metabolites MAPPD (N-monoacetyl-p-phenylenediamine (N-acetyl-p-phenylenediamine, 4-aminoacetanilide) and DAPPD (N,N’-diacetyl-p-phenylenediamine (N,N’-p-phenylenebisacetamide), formed by N-acetylation in human and mammalian skin and in human hepatocytes, were negative in the Ames test. PTD exhibited a weak increase in revertant colony counts without activation in TA98 and very strong increase in TA98, TA100, TA1535, and TA1537 following metabolic activation with S9. N-acetylated PTD metabolites 2-mono-Ac-PTD (4-amino-2-methylacetanilide), 5-mono-Ac-PTD (4-amino-3-methyl-acetanilide) and di-Ac-PTD (2,5-diacetamino-toluene), were also negative in the Ames test. Both PPD and PTD tested positive in an additional bacterial test in Salmonella typhimurium TA1535/pSK1002 strain with metabolic activation. The method, however, was not fully validated and the study did not follow OECD guidelines.
Three studies also included an in vitro mammalian gene mutation assay with PPD, and PTD. For PPD, the results were clearly positive in the chromosomal aberration assay with or without S-9, in Chinese hamster lung cell line and in compliance with a more recent OECD guideline. However, in the mouse lymphoma Hprt assay statistically significant increase in mutation frequency was observed following treatment with PPD at 100 μg/mL only with metabolic activation. The authors argued that there was no evidence of a dose-response and that the increase in mutation frequency was small in magnitude and within the historical control range. The chance event of no biological relevance should therefore be considered. PTD, when tested in Chromosomal aberration assay with V79 cells, showed clastogenic potential but no evidence of aneugenicity.
A newly-developed rodent in vivoPig-a gene mutation assay in mutant red blood cells or reticulocytes (defined as RBCCD59- or RETCD59-, respectively) conducted in line with recently adopted OECD TGs 470 also showed the mutagenic effect of PPD in accordance with classical genotoxicity test battery (gene mutations, structural and numerical chromosomal aberrations) recommended by the OECD.
PPD tested positive in mammalian cell micronucleus test in vitro in cultured human lymphocytes, performed in line with the 2003 draft OECD TGs 487, which was confirmed by in vivo murine erythrocyte micronucleus test conducted in line with the most recent guideline. N-acetylated derivatives of PPD and PTD, did not show a genotoxic potential in the human lymphocyte micronucleus assay in vitro,
Four in vitro studies investigated DNA breaks caused by PPD,,,, and PTD, all by alkaline Comet assay, and one of them additionally also by a terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay. The test systems were a human uroepithelial cell line, cultured human lymphocytes, a canine kidney cell line, Chinese hamster V79/V79NAT1*4 cell lines and human keratinocyte HaCaT cells. The protocols were designed by the authors with reference to their previous publications, and although mostly described in detail, a reference to standardized procedures was lacking. For example, the test substance incubation periods before the comet assay analysis varied: 24 h in Huang et al., 2007, 2 hours in Chye et al., 2008, 3 hours in Chen et al., 2010 and three and 48 h in Zeller et al., 2014. Nevertheless, in all four studies, DNA damage scores were significantly increased in a dose-dependent manner in cells treated with PPD and PTD in comparison to the controls. None of the N-acetylated PPD or PTD metabolites induced a biologically relevant increase in any of the Comet parameters. The results of the TUNEL assay after the incubation of canine kidney cell line with 37.5 μg/mL PPD for 3–12 h also showed increasing DNA damage compared to control, in a time-dependent manner.
Two studies used the in vivo rodent alkaline comet assay to detect DNA damage in mice and rat isolated tissues, following administration of PPD and PTD at doses of 80 and 60 mg/kg b.w., respectively, as a single i.p. dose or 3 consecutive daily doses of 25–100 mg/kg/day of PPD by oral gavage. The study design broadly followed the later OECD TG 486 which was not formally adopted until 2016. PPD did not induce DNA damage in any of the organs studied in both species, while PTD tested positive in rat stomach only.
Discussion
Findings from in vitro and in vivo experimental studies included in this review point to genotoxic potential of PPD and PTD, which are common precursors of oxidative hair dyes. Although the number of reviewed studies is limited, this finding indicates that a risk of genotoxicity after exposure to PPD and PTD can hitherto not be ruled out.
Nine publications were identified describing 17 assays, covering main genotoxicity endpoints with 10 different testing protocols (Table 1). Except for the bacterial reverse mutation assays for which the OECD TGs were not revised since 1997, only one mammalian cell chromosomal aberration test in vitro and one erythrocyte MN test in vivo were performed according to the updated OECD TGs, while two more in vivo assays closely followed the current TGs on the alkaline comet assay and Pig-a-gene mutations,, respectively.
An adequate chemical characterization of samples tested, which is lacking in several studies, is also critical for reliable results, as impurities may significantly affect the genotoxic response both in vitro and in vivo.
The studies of good quality designed in compliance with the OECD TGs,, showed positive results of in vitro bacterial mutation assays for both PPD and PTD, depending on the bacterial strain and mainly with previous metabolic activation. Compared to mammalian cells, high levels of reductive enzymes of bacteria can efficiently activate nitro and azo compounds to electrophilic metabolites. A lack of Phase II enzymes in liver S-9 preparations for metabolic activation can furthermore lead to misleading positive test results in standard in vitro genotoxicity assays. According to the conclusions of the EURL ECVAM Workshop an Ames positive result may be due to the conditions that favour high levels of oxidative metabolism. Similar experiments in mammalian cells and/or in vivo assays may show lower genotoxic potential, unless the doses of chemical used exceed the capacity of detoxication pathways, leading to metabolic activation of toxic pathways and resulting in genotoxicity but with a possible threshold dose-response. However, when tested in a newly developed Rodent Pig-a assay in vivo PPD also induced gene mutations in rat peripheral blood after 28-day oral exposure. Additionally, positive results indicating clastogenic potential of PPD and PTD were obtained by in vitro chromosomal aberration assay, although PPD was negative in the mouse lymphoma assay. PTD tested positive in all in vitro assays identified in this search,, however in the thymidine kinase gene mutation study (TK6 assay) using human lymphoblastoid TK6 cells, which express human metabolic enzymes, no increase in mutation frequency was observed after 4-h treatment in the absence or presence of S9 metabolic activation nor after 24-h treatment without S9. PPD was positive in one in vitro micronucleus assay in cultured human lymphocytes, and showed increased micronucleus frequencies in mice erythrocytes following high dose acute oral exposure in vivo Another indicator of genotoxicity, namely DNA breaks, were investigated in four in vitro studies,,, by an alkaline comet assay, and significantly higher DNA damage scores after PPD and PTD exposure was noted in all of them. The in vitro comet assay is considered to provide complementary information on genotoxicity and mechanism of action of chemicals, but it is not a standard battery test for mutagenic, clastogenic and aneugenic potential, and consequently has not been implemented into official regulatory testing guidelines. High concentrations of test agents may cause cytotoxicity or cell death, and give rise to false positive results, but further research is needed to establish a threshold value to distinguish between true and potentially false positive genotoxic effects detected by the comet assay. The effect of cytotoxicity may explain why in vitro comet assay results for PPD and PTD were not fully confirmed by the in vivo comet assay where PPD was clearly negative while PTD exhibited positive result in rat stomach only, following i.p. application., Nevertheless, it should be noted that in vivo comet assay also has important limitations: measurement of transient DNA lesion instead of irreversible gene and chromosomal mutations; sensitivity to indirect genotoxicity mechanisms linked to toxicity or cellular stress but low sensitivity to detect aneugenicity and DNA crosslinking.
Considering previous Scientific Committee on Consumer Safety (SCCS) reviews, the results of the present systematic review may help in the interpretation of epidemiological studies on cancer risk among hairdressers. Overall, SCCS did not consider PTD or PPD alone as genotoxic, but pointed out positive findings from in vivo and in vitro genotoxicity studies of PPD in combination with couplers or hydrogen peroxide., Recent studies reported increased micronucleus frequencies in hairdressers,, a population heavily exposed to hairdressing products including oxidative hair dyes. In addition, significantly higher DNA damage scores were found among hairdressers compared to controls, based on the comet assay performed on blood samples. Nevertheless, linking experimental results with epidemiological studies is difficult owing to, among other things, the kinetic properties of oxidative hair dye precursors. Dermal absorption of PPD and PTD is poor., No reliable data exist on inhalation exposure uptake, which may be relevant in occupationally exposed population. There are indications that PPD, PTD and related compounds undergo N-acetylation in the skin which is a detoxification mechanism resulting in non-genotoxic N- acetylated metabolites,, although at higher exposure levels, the detoxification capabilities of the skin may become a limiting factor, exposing the organism to the parent molecules. Lastly, there is no evidence of oxidative metabolism of absorbed PPD or PTD by human hepatic cytochrome P450 enzymes, a reaction that is presumably an activation step of carcinogenic aromatic amines.,
On the other hand, as demonstrated in several experimental studies, DNA damage induced by PPD and PTD is obviously dose-dependent which may be important in occupational conditions, as significantly higher exposure frequency in hairdressers than in clients and personal hair dye users were already confirmed. Little is known about the level of occupational external or systemic exposure of hairdressers to hair dye ingredients. Occupational exposure of hairdressers can be quantified by the use of biological monitoring of urinary PPD and PTD. However, the extent of exposure in hairdressers was found to be 2–3 orders of magnitude lower than after personal application of hair dyes, and the use of protective gloves did not influence the results. Another biomonitoring study showed that proper use of gloves in hairdressers may significantly reduce systemic exposure to PTD but not to PPD. To our knowledge, only one complete investigation of the occupational exposure of hairdressers to oxidative hair dyes was published, but it was performed under controlled conditions with a small number of subjects and only focused on PPD exposure. The authors concluded that occupational use of oxidative hair dyes produces negligible local or systemic exposure to PPD thus posing no risk to human health and that current protective measures were sufficient to limit exposure to acceptable levels. However, such definite conclusions evidently need further confirmation, as the problem of genotoxic potential of PPD and its derivatives is still not fully resolved. It was suggested that well designed epidemiological studies with high quality measurements of personal exposure levels in hair salons and possible biological exposure indices in hairdressers, as well as using for example the Pig-a assay for the detection of genetic effects, may further elucidate the suspected genotoxic potential of PPD and its derivatives. As animal testing of cosmetic ingredients is not allowed under the current EU Cosmetics Regulation, the use of micronucleus and comet assays in three-dimensional reconstituted human skin models is proposed as an alternative.
An increased incidence of tumour formation was already reported in some animal studies with exposure to hair dye precursors. Several meta-analyses found significant 20%–30% higher risk for bladder cancer in hairdressers compared to the general population or non-exposed occupations, and significantly increased frequency of other types of cancers in hairdressers, specifically laryngeal cancer and multiple myeloma.,,, Hairdressers and barbers were therefore classified under occupational exposure group 2A as probable human carcinogens by the International Agency for Research on Cancer, but personal use of hair dyes was categorized as not classifiable as to its carcinogenicity to humans (Group 3). The SCCS also considered PPD, PTD and ME-PPD in hair dyes safe for consumers and concluded that the weight of evidence supports PPD and derivatives not being mutagenic and carcinogenic in humans.,,
The results of this review add further knowledge about hairdresser’s exposure to potentially genotoxic agents which may initiate a sequence of events that leads to cancer formation. Because of the high relevance of genotoxicity information for the mode of action of any potential carcinogen, it is important to critically review recent publications to find more evidence whether PPD and its derivatives are mutagenic or clastogenic. Also, the OECD recently made significant revisions to the genotoxicity test guidelines, regarding the conduct of assays and the interpretation of test results, thus rendering older studies less reliable than the more recent ones conducted in accordance with modern standards.,
The main limitation of our review is a small number of identified eligible publications, often reporting studies not conducted in line with recent OECD guidelines or with significant deviations from the new OECD recommendations.
To conclude, although an interest for this research area is limited in the scientific community, a number of classical genotoxicity assays recommended by OECD TGs indicate that PPD and its derivatives have genotoxic potential and thus may pose an important public health concern. A significant segment of general population is exposed to hair dyes during adulthood, also including occupational exposure. More in-depth experimental studies conducted in line with recent OECD guidelines, as well as high quality epidemiological studies are imperative to further elucidate the risks associated with exposure to hair dye precursors, particularly in occupationally exposed populations such as hairdressers but also workers in the plastic and chemical industries where these compounds are also used.
Authors contributions All authors contributed to the study conception and design. Literature search and data analysis were performed by Željka Babić and Rajka Turk. The first draft of the manuscript was written by Željka Babić and Rajka Turk and all authors commented on previous versions of the manuscript and critically revised the work. All authors read and approved the final manuscript.
Declaration of conflicting interests The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: W. Uter has received a honorarium for a lecture on contact allergy from mixed dermatopharmaceutical sponsors (GEIDAC, Toledo, Sept. 2018) and travel reimbursement for participation in study meetings of the IDEA project (IFRA). W. Uter is external expert for the SCCS. Other authors: None conflict of interest to declare.
Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: With the support of the European Commission, DG Employment, Social Affairs and Inclusion; VS/2019/0440
Supplemental material Supplemental material for this article is available online.
References
- 1. Saitta P, Cook CE, Messina JL, et al. Is there a true concern regarding the use of hair dye and malignancy development?: a review of the epidemiological evidence relating personal hair dye use to the risk of malignancy. J Clin Aesthet Dermatol 2013; 6: 39–46.
- 2. Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006 https://echa.europa.eu/hr/regulations/clp/legislation (2022, accessed 16 November 2022).
- 3. Registration dossier 2-(methoxymethyl)benzene-1,4-diamine. https://echa.europa.eu/hr/registration-dossier/-/registered-dossier/5680/1/2 (2021, accessed 16 November 2022).
- 4. Scientific Committee on Consumer Safety (SCCS). OPINION ON p-Phenylenediamine. SCCS/1443/11. Revision of 18 September 2012 https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_094.pdf (2012, accessed 16 November 2022).
- 5. Scientific Committee on Consumer Safety (SCCS). OPINION ON Toluene-2,5-diamine and its sulfate. SCCS/1479/12. Revision of 18 September 2012, https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_093.pdf (2012, accessed 16 November 2022).
- 6. Scientific Committee on Consumer Safety (SCCS). OPINION ON 2-Methoxy-methyl-p-phenylenediamine and its sulfate salt. SCCS/1491/12. 26 February 2013. https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_123.pdf (2013, accessed 16 November 2022).
- 7. World Health Organization International Agency for Research on Cancer (IARC). Occupational Exposures of Hairdressers and Barbers and Personal Use of Hair Colourants. In: World Health Organization IARC. Some Aromatic Amines, Organic Dyes, and Related Exposures. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono93.pdf (2010, accessed 16 November 2022).
- 8. Organisation for Economic Co-operation and Development (OECD). Overview of the set of OECD genetic Toxicology test guidelines and updates performed in 2014-2015. Series on testing & assessment, report 238. Paris: Organisation for Economic Cooperation and Development. http://www.oecd.org/officialdocuments/displaydocument/?cote5env/jm/mono(2016)33&doclanguage5en (2017, accessed 16 November 2022).
- 9. Chye SM, Tiong YL, Yip WK, et al. Apoptosis induced by para-phenylenediamine involves formation of ROS and activation of p38 and JNK in chang liver cells. Environ Toxicol 2014; 29: 981–990.
- 10. Lee OK, Cha HJ, Lee MJ, et al. Implication of microRNA regulation in para-phenylenediamine-induced cell death and senescence in normal human hair dermal papilla cells. Mol Med Rep 2015; 12: 921–936.
- 11. Reena K, Ng KY, Koh RY, et al. para-Phenylenediamine induces apoptosis through activation of reactive oxygen species-mediated mitochondrial pathway, and inhibition of the NF-κB, mTOR, and Wnt pathways in human urothelial cells. Environ Toxicol 2017; 32: 265–277.
- 12. Zhou S, Li R, Zhang Z, et al. Analysis of mutagenic components of oxidative hair dyes with the Ames test. Hum Exp Toxicol 2021; 40: 1921–1937.
- 13. Andrew AS, Schned AR, Heaney JA, et al. Bladder cancer risk and personal hair dye use. Int J Cancer 2004; 109: 581–586.
- 14. Rauscher GH, Shore D, Sandler DP. Hair dye use and risk of adult acute leukemia. Am J Epidemiol 2004; 160: 19–25.
- 15. Heikkinen S, Pitkäniemi J, Sarkeala T, et al. Does hair dye use increase the risk of breast cancer? a population-based case-control study of finnish women. PLoS One 2015; 10(8): e0135190. DOI: .
- 16. Kezic S, Nunez R, Babić Ž, et al. Occupational exposure of hairdressers to airborne hazardous chemicals: a scoping review. Int J Environ Res Public Health 2022; 19(7): 4176. DOI: .
- 17. Symanzik C, Johansen JD, Weinert P, et al. Differences between hairdressers and consumers in skin exposure to hair cosmetic products: A review. Contact Dermatitis 2022; 86: 333–343.
- 18. Dryson E, 't Mannetje A, Walls C, et al. Case-control study of high risk occupations for bladder cancer in New Zealand. Int J Cancer 2008; 122: 1340–1346.
- 19. Hopf NB, Bolognesi C, Danuser B, et al. Biological monitoring of workers exposed to carcinogens using the buccal micronucleus approach: A systematic review and meta-analysis. Mutat Res Rev Mutat Res 2019; 781: 11–29.
- 20. Uter W, Johansen JD, Havmose MS, et al. Protocol for a systematic review on systemic and skin toxicity of important hazardous hair and nail cosmetic ingredients in hairdressers. BMJ Open 2021; 11(12): e050612. https://bmjopen.bmj.com/content/11/12/e050612 (2021, accessed 16 November 2022).
- 21. Uter W, Johansen JD, Havmose MS, et al. Protocol for a systematic review on skin and systemic toxicity of important hazardous substances in hair cosmetics and hand eczema in hairdressers. PROSPERO 2021 CRD42021238118 https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021238118 (2021, accessed 16 November 2022).
- 22. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation, BMJ 2015; 350: g7647. https://www.bmj.com/content/349/bmj.g7647 (2015, accessed 16 November 2022).
- 23. Pluye P, Gagnon M-P, Griffiths F, et al. A scoring system for appraising mixed methods research, and concomitantly appraising qualitative, quantitative and mixed methods primary studies in Mixed Studies Reviews. Int J Nurs Stud 2009; 46: 529–546.
- 24. Sterne JAC, Hernán MA, McAlleenan A, et al. Assessing risk of bias in a non-randomized study. In: Higgins JPT, Thomas J, Chandler J, et al. (eds) Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester UK: John Wiley & Sons, 2019, pp. 621–641.
- 25. United States Environmental Protection Agency. A summary of general assessment factors for evaluating the quality of scientific and technical information - prepared for the U.S. Environmental protection agency by members of the assessment factors Workgroup, a group of the EPA’s science policy Council. https://www.epa.gov/sites/default/files/2015-01/documents/assess2.pdf (2003, accessed 16 November 2022).
- 26. National Toxicology Program. Handbook for conducting a literature-based health assessment using office of health assessment and translation (OHAT) approach for systematic review and evidence integration. https://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookmarch2019_508.pdf (2019, accessed 16 November 2022).
- 27. Sekihashi K, Yamamoto A, Matsumura Y, et al. Comparative investigation of multiple organs of mice and rats in the comet assay. Mutat Res 2002; 517: 53–75.
- 28. Yasunaga K, Kiyonari A, Nakagawa M, et al. Different results of the salmonella umu test between three isomers of phenylenediamine (pda) derivatives. Drug Chem Toxicol 2006; 29: 203–213.
- 29. Garrigue JL, Ballantyne M, Kumaravel T, et al. In vitro genotoxicity of para-phenylenediamine and its N-monoacetyl or N,N’-diacetyl metabolites. Mutat Res 2006; 608: 58–71.
- 30. Zeller A, Pfuhler S. N-acetylation of three aromatic amine hair dye precursor molecules eliminates their genotoxic potential. Mutagenesis 2014; 29: 37–48.
- 31. Qin M, Chen R, Huang Z, et al. Application of the in vivo Pig-a gene mutation assay to test the potential genotoxicity of p-phenylenediamine. Food Chem Toxicol 2019; 123: 424–430.
- 32. Organisation for Economic Co-operation and Development (OECD). Overview of the set of OECD genetic Toxicology test guidelines and updates performed in 2014-2015. https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JM-MONO(2016)33/rev1&doclanguage=en (2017, accessed 16 November 2022).
- 33. Organisation for Economic Co-operation and Development (OECD). Test No. 471: bacterial reverse mutation test. OECD Guidelines for the Testing of Chemicals (2020, accessed 16 November 2022).
- 34. Organisation for Economic Co-operation and Development (OECD). Test No. 473. In Vitro mammalian chromosomal aberration test, oecd guidelines for the testing of chemicals (2016, accessed 16 November 2022).
- 35. Organisation for Economic Co-operation and Development (OECD). Test No. 487: In Vitro mammalian cell micronucleus test, OECD guidelines for the testing of chemicals. https://www.oecd.org/env/ehs/testing/E487_2010.pdf (2016, accessed 16 November 2022).
- 36. Organisation for Economic Co-operation and Development (OECD). Test No. 474: mammalian erythrocyte micronucleus test, OECD guidelines for the testing of chemicals (2016, accessed 16 November 2022).
- 37. Huang YC, Hung WC, Kang WY, et al. p-Phenylenediamine induced DNA damage in SV-40 immortalized human uroepithelial cells and expression of mutant p53 and COX-2 proteins. Toxicol Lett 2007; 170: 116–123.
- 38. Chye SM, Hseu YC, Liang SH, et al. Single strand DNA breaks in human lymphocytes exposed to para-phenylenediamine and its derivatives. Bull Environ Contam Toxicol 2008; 80: 58–62.
- 39. Chen SC, Chen CH, Tioh YL, et al. Para-phenylenediamine induced DNA damage and apoptosis through oxidative stress and enhanced caspase-8 and -9 activities in Mardin-Darby canine kidney cells. Toxicol In Vitro 2010; 24: 1197–1202.
- 40. De Boeck M, van der Leede Bj, De Vlieger K, et al. Evaluation of p-phenylenediamine, o-phenylphenol sodium salt, and 2,4-diaminotoluene in the rat comet assay as part of the Japanese Center for the Validation of Alternative Methods (JaCVAM)-initiated international validation study of in vivo rat alkaline comet assay. Mutat Res 2015; 786–788: 151–157.
- 41. Organisation for Economic Co-operation and Development (OECD). Test No. 489: in vivo mammalian alkaline comet assay, OECD guidelines for the testing of chemicals, section 4. Paris: OECD Publishing (2016, accessed 16 November 2022).
- 42. Kirkland D, Zeiger E, Madia F, et al. Can in vitro mammalian cell genotoxicity test results be used to complement positive results in the Ames test and help predict carcinogenic or in vivo genotoxic activity? I. Reports of individual databases presented at an EURL ECVAM Workshop. Mutat Res 2014; 775–776: 55–68.
- 43. Kirkland DJ, Müller L. Interpretation of the biological relevance of genotoxicity test results: the importance of thresholds. Mutat Res 2000; 464: 137–147.
- 44. Yasui M, Fukuda T, Ukai A, et al. Weight of evidence approach using a TK gene mutation assay with human TK6 cells for follow-up of positive results in Ames tests: a collaborative study by MMS/JEMS. Genes Environ 2021; 43: 7. https://genesenvironment.biomedcentral.com/articles/10.1186/s41021-021-00179-1 (2021, accessed 27 December 2022).
- 45. Frötschl R. Experiences with the in vivo and in vitro comet assay in regulatory testing. Mutagenesis 2015; 30: 51–57.
- 46. Azqueta A, Stopper H, Zegura B, et al. Do cytotoxicity and cell death cause false positive results in the in vitro comet assay? Mutat Res Genet Toxicol Environ Mutagen 2022; 881; 503520. DOI: .
- 47. Cordelli E, Bignami M, Pacchierotti F. Comet assay: a versatile but complex tool in genotoxicity testing. Toxicol Res 2021; 10: 68–78.
- 48. Farhadi S, Jolehar M, Safapour F. Micronucleus assay of buccal mucosal cells in hairdressers: the importance of occupational exposure. Asian Pac J Cancer Prev 2018; 19: 2131–2134.
- 49. Symanzik C, Weinert P, Babić Ž, et al. Skin toxicity of selected hair cosmetic ingredients: a review focusing on hairdressers. Int J Environ Res Public Health 2022; 19(13): 7588. DOI: .
- 50. Galiotte MP, Kohler P, Mussi G, et al. Assessment of occupational genotoxic risk among Brazilian hairdressers. Ann Occup Hyg 2008; 52: 645–651.
- 51. Pot LM, Scheitza SM, Coenraads PJ, et al. Penetration and haptenation of p-phenylenediamine. Contact Dermatitis 2013; 68: 193–207.
- 52. Nohynek GJ, Skare JA, Meuling WJA, et al. Human systemic exposure to [14C]-paraphenylenediamine-containing oxidative hair dyes: Absorption, kinetics, metabolism, excretion and safety assessment. Food Chem Toxicol 2015; 81: 71–80.
- 53. Stanley LA, Skare JA, Doyle E, et al. Lack of evidence for metabolism of p-phenylenediamine by human hepatic cytochrome P450 enzymes. Toxicology 2005; 210: 147–157.
- 54. Skare JA, Hewitt NJ, Doyle E, et al. Metabolite screening of aromatic amine hair dyes using in vitro hepatic models. Xenobiotica 2009; 39: 811–825.
- 55. Gube M, Heinrich K, Dewes P, et al. Internal exposure of hairdressers to permanent hair dyes: a biomonitoring study using urinary aromatic diamines as biomarkers of exposure. Int Arch Occup Environ Health 2011; 84: 287–292.
- 56. Geens T, Aerts E, Borguet M, et al. Exposure of hairdressers to aromatic diamines: an interventional study confirming the protective effect of adequate glove use. Occup Environ Med 2016; 73: 221–228.
- 57. Hueber-Becker F, Nohynek GJ, Dufour EK, et al. Occupational exposure of hairdressers to [14C]-para-phenylenediamine-containing oxidative hair dyes: a mass balance study. Food Chem Toxicol 2007; 45: 160–169.
- 58. Pfuhler S, Kirst A., Aardema M., et al. A tiered approach to the use of alternatives to animal testing for the safety assessment of cosmetics: genotoxicity. A COLIPA analysis. Regul Toxicol Pharmacol 2010; 57: 315–324.
- 59. Reulen RC, Kellen E, Buntinx F, et al. A meta-analysis on the association between bladder cancer and occupation. Scand J Urol Nephrol 2008; 42: 64–78.
- 60. Takkouche B, Regueira-Méndez C, Montes-Martínez A. Risk of cancer among hairdressers and related workers: a meta-analysis. Int J Epidemiol 2009; 38: 1512–1531.
- 61. Harling M, Schablon A, Schedlbauer G, et al. Bladder cancer among hairdressers: a meta-analysis. Occup Environ Med 2010; 67: 351–358.
- 62. Bayer O, Cámara R, Zeissig SR, et al. Occupation and cancer of the larynx: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 2016; 273: 9–20.
- 63. Thybaud V, Lorge E, Levy DD, et al. Main issues addressed in the 2014–2015 revisions to the OECD genetic toxicology test guidelines. Environ Mol Mutagen 2017; 58: 284–295.