Introduction
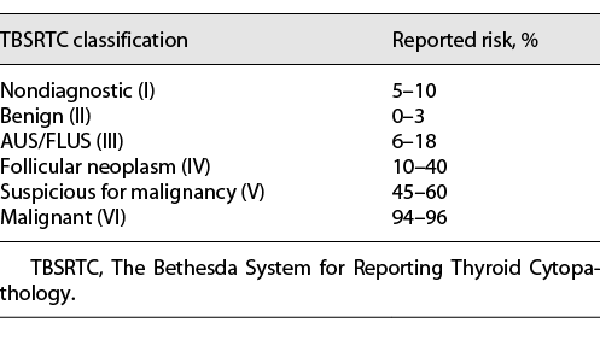
Fine-needle aspiration (FNA) biopsy is a valuable tool for the assessment of thyroid lesions in the pediatric population due to its accuracy, cost-efficiency, and minimally invasive nature [-]. FNA biopsies are evaluated using known cytomorphologic criteria and classified according to The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC); each category in TBSRTC is associated with a range of malignancy risk (shown in Table 1) [-]. While the majority of biopsied thyroid nodules are accurately classified as benign (TBSRTC II) or malignant (TBSRTC VI), 15–30% of thyroid FNAs are diagnosed as indeterminate or suspicious for neoplasm or malignancy []. The three indeterminate categories of TBSRTC are as follows: (1) atypia/follicular lesion of undetermined significance (AUS/FLUS; TBSRTC III), (2) follicular neoplasm/suspicious for follicular neoplasm (FN/SFN; TBSRTC IV), and (3) suspicious for malignancy (SM; TBSRTC V) [, ].
Cytologically indeterminate nodules remain a challenge for pediatric clinicians as the risks of thyroid malignancy vary remarkably and are higher than encountered in adults. Reported malignancy rates from available pediatric FNA studies range from 0 to 50% for AUS/FLUS, 20–100% for FN/SFN, and 40–100% for SM [-]. Variation in providers’ interpretations of cytopathology results may lead to conflicting clinical recommendations, unnecessary surgical interventions, and additional costs incurred to deliver care. Accordingly, adjunct ancillary techniques, including molecular testing, have become an area of interest in the pediatric population [].
Advancements in comprehensive next-generation sequencing (NGS) panels have informed the management of indeterminate thyroid nodules []. In 2015, the American Thyroid Association (ATA) published revised guidelines and recommended molecular testing as an adjunct to indeterminate FNA in adults [, ]. Correspondingly, diagnostic molecular testing is being increasingly integrated into the diagnosis and management of thyroid nodules []. Enhanced understanding of the histologic diagnoses and genetic alterations of pediatric indeterminate thyroid FNAs may provide an opportunity to refine surgical management.
In this study, we report our institutional experience of indeterminate thyroid FNA with a convenience cohort of pediatric patients followed at the Children’s Hospital of Philadelphia (CHOP). We focused on the histopathologic features and molecular markers of thyroid lesions in children and adolescents presenting with AUS/FLUS, FN/SFN, or SM cytology to (1) identify the predominant histologic subtypes of differentiated thyroid cancer (DTC), (2) determine association(s) between cytologic diagnosis, thyroid malignancy, and genetic alterations, and (3) gain insight into the diagnostic utility of molecular testing on surgical decision-making and clinical outcomes.
Materials and Methods
A retrospective chart review was conducted of patients who underwent thyroid FNA(s) at CHOP between January 2010 and December 2021. Records were reviewed to collect information pertaining to patient demographics, FNA cytology, surgical approach, surgical pathology, and mutational analysis. Histopathologic characteristics, including histologic subtype, primary tumor focality and laterality, encapsulation, capsular invasion, vascular invasion, lymphatic invasion, extrathyroidal extension, and extranodal extension were reviewed. Primary tumor (T), regional lymph nodes (N), and distant metastasis (M) were assigned using the 7th or 8th edition of the American Joint Committee on Cancer (AJCC) classification for DTC [, ]. Thyroid cancer risk stratification was adopted from the 2015 ATA Management Guidelines for Children with Thyroid Nodules and DTC []. Molecular driver alterations and copy number variations (CNVs) were identified using either of the following targeted NGS assays: (1) ThyroSeq version 3 (v.3) performed on cytology specimens or (2) CHOP’s Comprehensive Solid Tumor Panel (CSTP) performed on histopathologic specimens. Thyroseq v.3 analyzes 112 thyroid-related genes for single nucleotide variants, indels, gene fusions, CNVs, and gene expression alterations and applies a Genomic Classifier to discriminate benign and malignant lesions []. CSTP encompasses 238 genes and more than 600 fusions to detect single nucleotide variants, indels, gene fusions, and CNVs [-]. CNVs were categorized using three distinct classes: (1) tumors lacking significant gains or losses (CNV-quiet), (2) tumors characterized by few CNV events (CNV-low), and (3) tumors characterized by a high frequency of gains or losses (CNV-high).
Statistical analyses were performed using R 4.1.0 and RStudio 1.4.1717 with packages tidyverse and rstatix [-]. Data with parametric distributions were reported as mean ± standard deviation. Histopathologic features between pediatric patients with AUS/FLUS, FN/SFN, and SM cytology were compared using two-tailed Fisher’s exact test. Age at the time of thyroid surgery and cumulative radioactive iodine dosage for patients with AUS/FLUS, FN/SFN, and SM cytology were compared using one-way ANOVA tests without subsequent post-hoc comparison. p values ≤0.05 were considered statistically significant. Risk of malignancy for patients presenting AUS/FLUS cytology was calculated under the assumption that the patients who did not undergo surgery were followed for benign nodule(s).
Results
Cytopathology
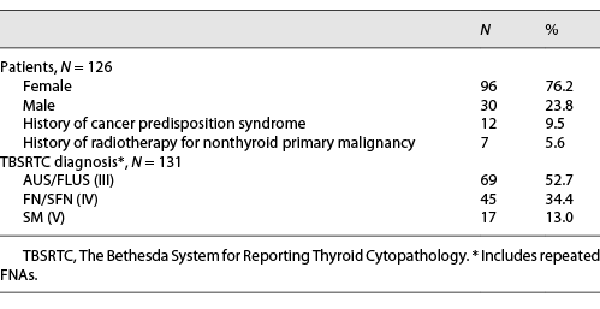
Over the course of the study, 597 FNAs were performed at CHOP, including 146 FNAs ultimately rendering indeterminate cytology (shown in Fig. 1). Twenty FNAs were excluded due to (1) FNA performed after 19 years of age (n = 17), (2) limited information in the hospital electronic medical record (n = 2), or (3) subsequent repeat FNA classified as malignant (n = 1). A final cohort of 131 indeterminate FNAs of 126 patients (96 females and 30 males; 14.9 ± 2.4 years as the mean age at the time of FNA) was confirmed. In this cohort, 12 (10%) patients were previously diagnosed with a cancer predisposition syndrome (PTEN hamartoma tumor syndrome: n = 10; DICER1 syndrome: n = 2), and 7 (6%) patients had previous treatment history of radiotherapy for their nonthyroid primary malignancy. Based on TBSRTC, 69 (53%) FNA specimens were reported as AUS/FLUS, 45 (34%) as FN/SFN, and 17 (13%) as SM; 5 (4%) patients with initial AUS/FLUS cytology underwent repeat FNA (shown in Table 2).
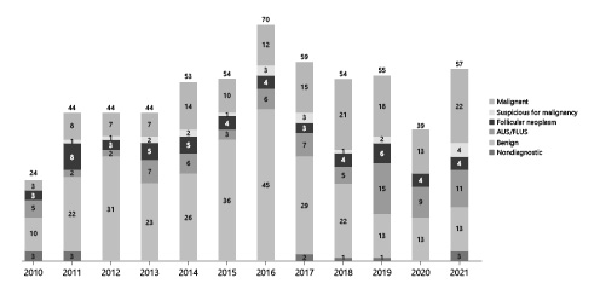
Fig. 1
Annual number of FNAs performed at the Children’s Hospital of Philadelphia’s Thyroid Center.
Surgical Outcomes and Final Diagnoses
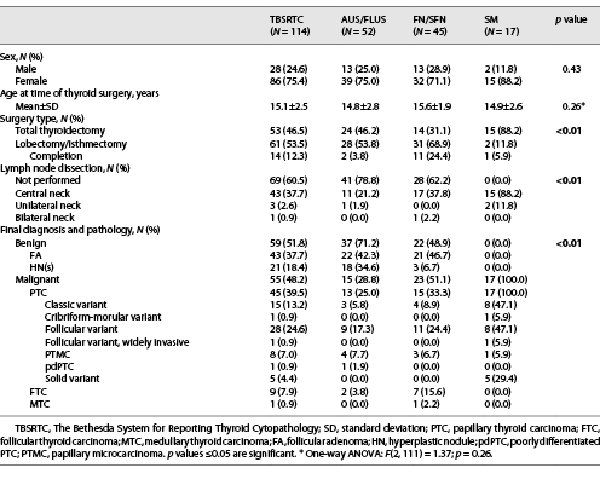
Surgical outcomes and final diagnoses for this cohort are presented in Table 3. Surgical resection was performed in 114 (90%) patients, and 55 (48%) were diagnosed with a thyroid malignancy on histology. Approximately 94% (17/18) of patients with a previously diagnosed cancer predisposition syndrome and/or treatment history of radiotherapy underwent surgery; 76% (13/17) of these patients were diagnosed with benign thyroid disease; and 24% (4/17) were diagnosed with thyroid cancer classified as ATA low risk. Of the 12 patients who pursued surveillance, 100% (12/12) had AUS/FLUS cytology, and 58% (7/12) had their FNA performed in the last 3 years (2019–2021). Risk of malignancy increased with TBSRTC classification: 23% (15/64) for AUS/FLUS, 51% (23/45) for FN/SFN, and 100% (17/17) for SM nodules. Significant differences were observed in surgical approach (p < 0.01), performance of lymph node dissection (p < 0.01), and histologic diagnosis (p < 0.01) between TBSRTC categories. Follicular variant of papillary thyroid carcinoma (fvPTC) was the most common malignancy across all indeterminate TBSRTC diagnoses: 60% (9/15) for AUS/FLUS, 48% (11/23) for FN/SFN, and 47% (8/17) for SM. One patient with SM cytology and diagnosed with fvPTC prior to 2017 was reclassified as benign noninvasive follicular thyroid neoplasm with papillary-like nuclear features using the 4th edition of the World Health Organization classification guidelines [].
Clinicopathologic Characteristics of Thyroid Malignancies
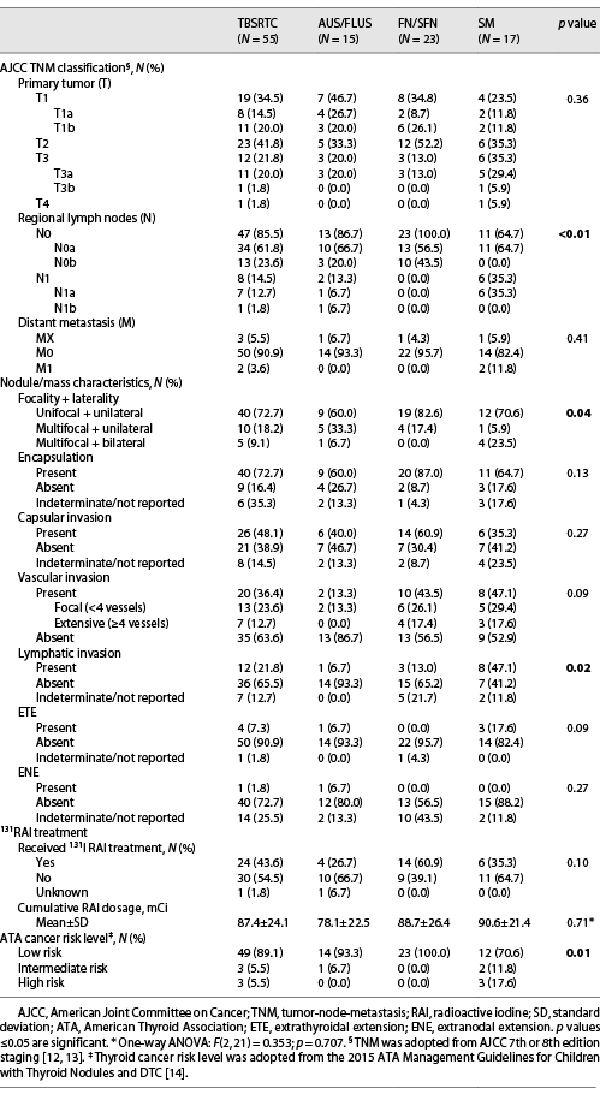
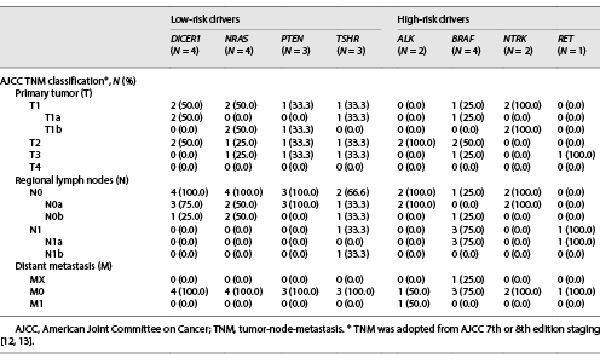
Clinicopathologic features of the 55 patients with thyroid cancer are presented in Table 4. AJCC lymph node (N) status correlated with TBSRTC cytology (p < 0.01), while tumor (T) and metastasis (M) status revealed no significant differences between TBSRTC categories (p = 0.36; p = 0.41). In addition, significant differences were observed between primary tumor focality/laterality (p = 0.04) and the presence of lymphatic invasion on pathology (p = 0.02). Unifocal, unilateral DTC was noted in the majority of patients across all indeterminate TBSRTC categories. An increasing trend was observed for the presence of lymphatic invasion with indeterminate categories; however, 1 patient with AUS/FLUS cytology demonstrated PTC with regional lymph node metastasis (LNM) as well as extranodal extension on pathology. As per the ATA cancer risk stratification, 89% (49/55) of patients with indeterminate cytology were found to have ATA low-risk disease, and 5 of 6 patients with ATA intermediate- or high-risk disease had SM cytology. Of the 3 patients with ATA high-risk disease, all were diagnosed with SM cytology and LNM (n = 1), metastasis to the lungs (n = 1), or metastasis to the frontal skull bone (n = 1).
Mutational Analysis
Somatic molecular testing was performed in 40% (51/126) of patients using CHOPs CSTP (34/51) or ThyroSeq v.3 (17/51), with 64% (33/51) of patients found to have a driver alteration potentially associated with thyroid carcinogenesis. DICER1 (n = 5), NRAS (n = 5), and TSHR (n = 5) mutations were the most common driver alterations in this cohort. Of the 31 driver-positive patients who underwent surgery, 27 (87%) had malignant histology, and 4 (13%) had benign histology. Of the two driver-positive patients who did not pursue surgery, both presented with AUS/FLUS cytology, with one tumor harboring a DICERE1813K mutation and the other a TSHRM453T activating mutation. Regarding the 18 patients with no identifiable driver alteration, 14 patients pursued surgical resection; 8 patients had malignant histology; and 6 patients had benign histology.
Of the 59 benign surgical specimens, 10 (17%) had molecular testing performed. Clinically significant variants were detected in 40% (4/10) of benign cases with AUS/FLUS (n = 3) or FN/SFN (n = 1) cytology, including PTEN (n = 1), NKX2.1 (n = 1), NRAS (n = 1), and TSHR (n = 1). Regarding the 55 malignant surgical specimens, 35 (64%) had molecular testing performed. Twenty-seven (27/35; 77%) malignant cases were positive on molecular analysis for driver alteration(s), including 14 (52%) drivers associated with low-invasive risk (DICER1, PTEN, RAS, and TSHR mutations), 9 (33%) drivers associated with high-invasive risk (BRAF mutations and ALK, NTRK, and RET fusions), and 4 (15%) drivers with unreported risk for invasive disease (APC, BLM, and PPM1D mutations and TG-FGFR1 fusion). Based on TBSRTC, 13% (2/15) of AUS/FLUS, 24% (4/17) of FN/SFN, and 31% (4/13) of SM nodules with mutational analysis had driver variants or fusions associated with high risk for invasive disease (shown in Fig. 2).
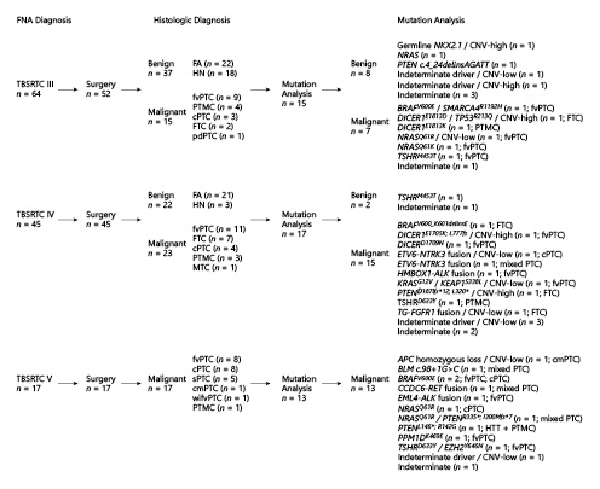
Fig. 2
FNA cytology, histologic diagnosis, and mutation analysis of indeterminate thyroid nodules. FNA, fine-needle aspiration; TBSRTC, The Bethesda System for Reporting Thyroid Cytopathology; FA, follicular adenoma; HN, hyperplastic nodule; PTC, papillary thyroid carcinoma; cPTC, classic variant of PTC; cmPTC, cribriform-morular variant of PTC; fvPTC, follicular variant of PTC; wifvPTC, widely invasive fvPTC; PTMC, papillary microcarcinoma; pdPTC, poorly differentiated PTC; FTC, follicular thyroid carcinoma; MTC, medullary thyroid carcinoma; HTT, hyalinizing trabecular tumor; CNVs, copy number variations. *Mutation analysis is described for patients who pursued surgical resection.
CNVs were detected in 29% (15/51) of indeterminate nodules: 67% (10/15) were CNV-low, and 33% (5/15) were CNV-high. Of the 3 patients with CNVs and benign histology, one was classified as CNV-low, and two were classified as CNV-high. CNV-positive patients with no identifiable driver alteration and benign histology had low (CNV-low; n = 1) and high (CNV-high; n = 1) quantities of chromosomal gains or losses. Of the 12 patients with CNVs and malignant histology, nine were classified as CNV-low, and three were classified as CNV-high. CNV-positive patients with no identifiable driver alteration and malignant histology were all classified as CNV-low (n = 4). The remaining 71% (36/51) had no detected CNVs (CNV-quiet). No observed associations between CNV-quantity and thyroid carcinoma were identified in this cohort.
Discussion
In the current study, we evaluated the correlation between thyroid nodules classified as indeterminate on FNA with clinicopathologic features and mutational analysis. Previous studies investigating molecular profiling in pediatric thyroid lesions are limited by smaller sample size, inclusion of subjects outside of the pediatric age range (>19 years), or segmented review examining a small number of genetic alteration(s) [, -]. In this study, 48% of pediatric patients with indeterminate cytology were ultimately diagnosed with DTC.
The predominant histologic subtypes of DTC in this cohort were fvPTC (51%), followed by classic variant of PTC (28%) and follicular thyroid carcinoma (FTC; 16%). The observation that fvPTC is the most common malignancy among AUS/FLUS, FN/SFN, and SM nodules has previously been reported [-]. Similarly, the majority of FTCs (78%) and solid variant of PTC (100%) were encountered in FN/SFN and SM cytology, respectively, corroborating reports classifying (1) FTC as a rather difficult subtype to diagnose by FNA due to need for histologic examination of capsular and vascular invasion and (2) solid variant of PTC as an aggressive PTC variant associated with TBSRTC categories rendering increased malignancy risk (shown in Table 3) [-]. Interestingly, the majority of DTC patients with indeterminate cytology had a low risk for invasive behavior, with 86% (47/55) limited to the thyroid without locoregional LNM and 96% (53/55) without distant metastasis (shown in Table 4). Overall, 89% (49/55) of patients harboring malignancy were classified as ATA pediatric low risk for persistent disease, with only 6 (11%) patients with FN/SFN or SM cytology classified as ATA pediatric intermediate or high risk (shown in Table 4). When comparing clinicopathologic features of indeterminate nodules, unifocal/unilateral presentation (p = 0.04), the absence of lymphatic invasion (p = 0.02), and the absence of LNM (N0a/N0b; p < 0.01) were significant findings, suggesting these to be informative features to discriminate between TBSRTC indeterminate categories (shown in Table 4).
With the intention to improve the management of pediatric indeterminate FNA specimens, we investigated the utility of molecular profiling to distinguish benign (n = 59) and malignant (n = 55) nodules. Clinically significant driver variants or fusions were more common in pediatric thyroid tumors with malignant histology when compared to those with benign histology. In the current study, 38% (6/16) of benign cases and 77% (27/35) of malignant cases with mutational analysis had detected driver alteration(s), with 82% (27/33) of all driver alteration(s) identified in this cohort to be associated with thyroid cancer. Our results are congruent with several studies demonstrating a strong correlation between molecular marker positivity and thyroid carcinoma. Incidence of malignancy in pediatric driver-positive tumors have been reported to be 89% (74/83) in a prospective study, 97% (32/33) in a retrospective cross-sectional study, and 100% (11/11; 9/9) in two restrospective studies, with all studies investigating BRAF, RAS, RET/PTC, and PAX8/PPARγ [, -].
In our cohort, all driver alteration(s) detected in patients with benign disease were associated with low-invasive behavior and a reduced risk for metastasis [-]. Regarding the thyroid cancer patients with detected driver alteration(s), 52% of drivers possessed low-invasive risk, 33% possessed high-invasive risk, and 15% possessed unreported risk for invasive disease [, , ]. The majority of thyroid cancer patients with drivers associated with low-invasive risk were diagnosed with AUS/FLUS or FN/SFN cytology (71%; 10/14) and presented low-invasive features classified as ATA low risk (93%; 13/14). Conversely, the majority of thyroid cancer patients with high-risk drivers were diagnosed with FN/SFN or SM cytology (89%; 8/9). Interestingly, 67% (6/9) of thyroid cancer patients with high-risk drivers were classified as ATA low risk for persistent disease, demonstrating that not all patients with high-risk drivers present thyroid cancer with invasive behavior. This observation does not discredit the 3 patients in our cohort with high-risk drivers and SM cytology presenting invasive thyroid cancer (shown in Fig. 3; shown in Table 5). The (1) frequency of high-risk drivers increasing with TBSRTC category as well as (2) the number of patients with invasive disease increasing with TBSRTC category reflects the complementary nature of mutational analysis and FNA and underlies the importance of appropriate preoperative counseling for patients with indeterminate cytology (shown in Fig. 2).
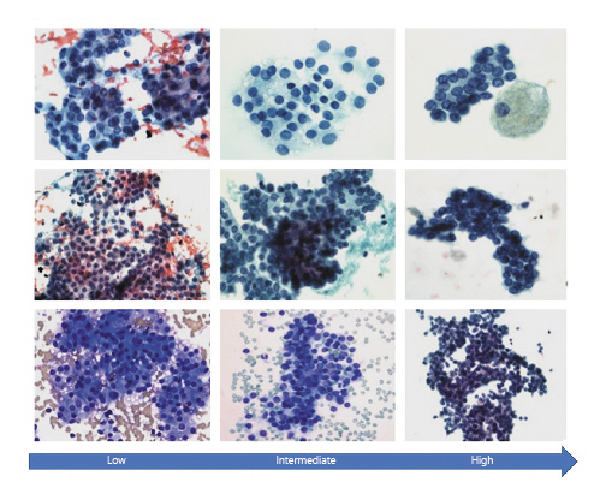
Fig. 3
Representative cytology images of thyroid lesions with indeterminate cytology in our cohort stratified by the American Thyroid Association pediatric thyroid cancer risk levels. Low: DICER1, NRAS, TSHR, PTEN, NRAS, BRAF variants, CNVs, and some fusions in our cohort; monotonous population of follicular cells with round nuclei arranged in cohesive clusters with focal nuclear overlapping and crowding; no nuclear features of PTC are seen. Intermediate to high: CCDC6-RET fusion, TSHRM453T variant, BRAFV600E variant (intermediate), and EML4-ALK fusion (high) in our cohort; monotonous population of follicular cells demonstrating cohesive clusters with nuclear overlapping and crowding. The nuclei are round to oval in shape with thickened nuclear membrane, nuclear chromatin clearing, and rare intranuclear grooves. These features are suspicious but not diagnostic of PTC.
In light of our results, preoperative knowledge of somatic oncogenes in thyroid FNA specimens appears to be an important addition in an effort to optimize management of pediatric patients with indeterminate nodules. For pediatric patients with AUS/FLUS or FN/SFN cytology and a driver oncogenic alteration associated with low-invasive risk, a lobectomy may be adequate to achieve remission secondary to the low risk for extrathyroidal disease. Lobectomy with prophylactic neck dissection may also be suitable for patients with AUS/FLUS or FN/SFN cytology and driver mutation(s) associated with high risk for invasive disease; however, more data are needed to confirm this recommendation. For patients with SM cytology and the presence of an invasive driver alteration, total thyroidectomy is recommended due to the malignancy risk of 100% as well as an increased risk for regional metastasis. This approach may decrease the need for a staged surgery consisting of a diagnostic thyroid lobectomy and subsequent completion thyroidectomy [, , ]. Based on the observation that somatic oncogene testing provides a useful predictor of clinical behavior, pre-surgical analysis of somatic oncogenic alterations in nodules with indeterminate cytology would provide clinicians an opportunity to refine surgical decision-making. Moreover, somatic oncogene analysis using NGS panels may identify driver mutations associated with cancer predisposition syndromes, including PTEN hamartoma tumor syndrome, DICER1 syndrome, and familial adenomatous polyposis, prompting referral to genetic counseling [, ].
Though a positive driver alteration has been confirmed to be more predictive of malignancy, the absence of a driver alteration does not reassure benign thyroid disease. In this study, greater than 20% (8/35) of patients harboring thyroid malignancy did not present with a previously reported thyroid oncogenic driver alteration. This accentuates the importance of appropriate selection of thyroid nodules for FNA to improve preoperative probability for malignancy and demonstrates the value of decreasing the inter- and intra-observer variability in pediatric thyroid cytopathology between institutes. Aligned with ATA guidelines, children and adolescents with AUS/FLUS cytology and a negative genetic test result may benefit from repeat FNA prior to definitive treatment with surgery []. Further investigation is needed to identify additional genetic and genomic markers that may predict both benign and malignant disease in pediatric indeterminate thyroid FNA specimens.
The limitations of this study are its retrospective design, single-center sample size, and the evolving nature of indeterminate nodule management over the past decade. The predicted protein change or DNA variant could not be confirmed for two cases (NRAS and NKX2.1) in our cohort. No conclusions regarding the utility of CNVs to discriminate benign and malignant nodules were drawn due to the small sample size. Since 2010, standardization of care has improved with the formation of an integrated, multidisciplinary Thyroid Center at our institution as well as the inclusion of somatic oncogene testing for pediatric patients undergoing FNA and surgery. In an effort to decrease inter- and intra-observer variability in the evaluation and management of pediatric patients with thyroid nodules, the authors are creating an international Child and Adolescent Thyroid Consortium to increase collaborative efforts between pediatric thyroid centers, which includes exploration of the molecular landscape and tumor microenvironment in an expanded number of samples. Nevertheless, this study describes a large cohort of pediatric patients and presents data that hopefully contribute to the advancement of prognosing and managing children and adolescents with indeterminate thyroid cytology.
Conclusion
Approximately half of pediatric thyroid nodules classified as indeterminate by TBSRTC were malignant on surgical follow-up; however, the majority of malignant cases (89%) were ATA pediatric low risk for persistent disease. Somatic oncogene analysis appears to be complementary to FNA. Preoperative somatic oncogene testing may provide an opportunity to stratify surgical management of nodules with indeterminate cytology, with lobectomy without prophylactic central neck LN dissection for nodules with low-risk oncogenes (DICER1, PTEN, RAS, and TSHR mutations) and lobectomy with prophylactic central LN dissection for nodules with high-risk oncogenes (BRAF or fusions). Based on our findings, we recommend that all pediatric patients with AUS/FLUS or FN/SFN cytology undergo somatic oncogene testing in an effort to optimize stratification of surgery. Comprehensive NGS panels that have been validated in the pediatric population (<19 years) and incorporate an array of somatic variants or fusions, particularly high-risk drivers, should be considered.
Statement of Ethics
This retrospective study involving human subjects was reviewed and approved by the Children’s Hospital of Philadelphia Institutional Review Board (CHOP IRB #17-014224). Written informed consent from the participant and/or participant’s legal guardian was not required per CHOP IRB; a waiver of consent/parental permission has been approved per 45 CRF 46.116(d).
Conflict of Interest Statement
Research was conducted in the absence of commercial or financial conflicts.
Funding Sources
This work was supported by the Children’s Hospital of Philadelphia Frontier Program’s Grant.
Author Contributions
Zubair Baloch and Andrew J. Bauer devised the project. Julia A. Baran collected the data, created the tables and figures, and wrote the manuscript. Julia A. Baran and Stephen Halada perfomed the analysis. Zubair Baloch provided cytology images. Support and critical review was provided by Zubair Balcoh, Andrew J. Bauer, Stephen Halada, Julio C. Ricarte-Filho, Amber Isaza, Lea F. Surrey, Cindy McGrath, Tricia Bhatti, Jalal Jalaly, Sogol Mostoufi-Moab, Aime T. Franco, N. Scott Adzick, Ken Kazahaya, and Anne M. Cahill. All authors discussed the results and provided editorial review of the manuscript.
Data Availability Statement
All data generated and analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. Rossi ED, Pantanowitz L, Faquin WC. The role of molecular testing for the indeterminate thyroid FNA. Genes. 2019;10(10):E736. https://doi.org/10.3390/genes10100736.
- 2. Cramer H. Fine-needle aspiration cytology of the thyroid: an appraisal. Cancer. 2000;90(6):325–9. https://doi.org/10.1002/1097-0142(20001225)90:6<325::aid-cncr1>3.0.co;2-u.
- 3. Gharib H, Papini E, Paschke R. Thyroid nodules: a review of current guidelines, practices, and prospects. Eur J Endocrinol. 2008;159(5):493–505. https://doi.org/10.1530/EJE-08-0135.
- 4. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. https://doi.org/10.1089/thy.2015.0020.
- 5. Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341–6. https://doi.org/10.1089/thy.2017.0500.
- 6. Jia MR, Baran JA, Bauer AJ, Isaza A, Surrey LF, Bhatti T, et al. Utility of fine-needle aspirations to diagnose pediatric thyroid nodules. Horm Res Paediatr. 2021;94(7–8):263–74. https://doi.org/10.1159/000519307.
- 7. Khan TM, Zeiger MA. Thyroid nodule molecular testing: is it ready for prime time?Front Endocrinol. 2020;11:590128. https://doi.org/10.3389/fendo.2020.590128.
- 8. Canberk S, Barroca H, Girao I, Aydin O, Uguz A, Erdogan K, et al. Performance of the Bethesda system for reporting thyroid cytology in multi-institutional large cohort of pediatric thyroid nodules: a detailed analysis. Diagnostics. 2022;12(1):179. https://doi.org/10.3390/diagnostics12010179.
- 9. Mostoufi-Moab S, Labourier E, Sullivan L, LiVolsi V, Li Y, Xiao R, et al. Molecular testing for oncogenic gene alterations in pediatric thyroid lesions. Thyroid. 2018;28(1):60–7. https://doi.org/10.1089/thy.2017.0059.
- 10. Vargas-Salas S, Martinez JR, Urra S, Dominguez JM, Mena N, Uslar T, et al. Genetic testing for indeterminate thyroid cytology: review and meta-analysis. Endocr Relat Cancer. 2018;25(3):R163–77. https://doi.org/10.1530/ERC-17-0405.
- 11. Ferris RL, Baloch Z, Bernet V, Chen A, Fahey TJ 3rd, Ganly I, et al. American Thyroid Association statement on surgical application of molecular profiling for thyroid nodules: current impact on perioperative decision making. Thyroid. 2015;25(7):760–8. https://doi.org/10.1089/thy.2014.0502.
- 12. Lamartina L, Grani G, Arvat E, Nervo A, Zatelli MC, Rossi R, et al. 8th edition of the AJCC/TNM staging system of thyroid cancer: what to expect (ITCO#2). Endocr Relat Cancer. 2018;25(3):L7–11. https://doi.org/10.1530/ERC-17-0453.
- 13. Edge SB, Compton CC. The American Joint Committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. https://doi.org/10.1245/s10434-010-0985-4.
- 14. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716–59. https://doi.org/10.1089/thy.2014.0460.
- 15. Nikiforova MN, Mercurio S, Wald AI, Barbi de Moura M, Callenberg K, Santana-Santos L, et al. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer. 2018;124(8):1682–90. https://doi.org/10.1002/cncr.31245.
- 16. Surrey LF, MacFarland SP, Chang F, Cao K, Rathi KS, Akgumus GT, et al. Clinical utility of custom-designed NGS panel testing in pediatric tumors. Genome Med. 2019;11(1):32. https://doi.org/10.1186/s13073-019-0644-8.
- 17. Chang F, Lin F, Cao K, Surrey LF, Aplenc R, Bagatell R, et al. Development and clinical validation of a large fusion gene panel for pediatric cancers. J Mol Diagn. 2019;21(5):873–83. https://doi.org/10.1016/j.jmoldx.2019.05.006.
- 18. Franco AT, Ricarte-Filho JC, Isaza A, Jones Z, Jain N, Mostoufi-Moab S, et al. Fusion oncogenes are associated with increased metastatic capacity and persistent disease in pediatric thyroid cancers. J Clin Oncol. 2022;40(10):1081–90. https://doi.org/10.1200/JCO.21.01861.
- 19. R: a language and environment for statistical computing. Vienna, Austria; 2021. Available from: https://www.R-project.org/.
- 20. R studio: integrated development environment for R. Boston, MA: PBC; 2021. Available from: http://www.rstudio.com/.
- 21. Wickham H, Averick M, Bryan J, Chang W, McGowan LD, Francois R, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686. https://doi.org/10.21105/joss.01686.
- 22. Kassambara ARstatix: pipe-friendly framework for basic statistical tests. 2021. Available from: https://cran.r-project.org/web/packages/rstatix/index.html.
- 23. Katabi N, Lewis JS. Update from the 4th edition of the World Health Organization classification of head and neck tumours: what is new in the 2017 WHO blue book for tumors and tumor-like lesions of the neck and lymph nodes. Head Neck Pathol. 2017;11(1):48–54. https://doi.org/10.1007/s12105-017-0796-z.
- 24. Buryk MA, Simons JP, Picarsic J, Monaco SE, Ozolek JA, Joyce J, et al. Can malignant thyroid nodules be distinguished from benign thyroid nodules in children and adolescents by clinical characteristics? A review of 89 pediatric patients with thyroid nodules. Thyroid. 2015;25(4):392–400. https://doi.org/10.1089/thy.2014.0312.
- 25. Nikiforov YE, Ohori NP, Hodak SP, Carty SE, LeBeau SO, Ferris RL, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96(11):3390–7. https://doi.org/10.1210/jc.2011-1469.
- 26. Monaco SE, Pantanowitz L, Khalbuss WE, Benkovich VA, Ozolek J, Nikiforova MN, et al. Cytomorphological and molecular genetic findings in pediatric thyroid fine-needle aspiration. Cancer Cytopathol. 2012;120(5):342–50. https://doi.org/10.1002/cncy.21199.
- 27. Valderrabano P, McIver B. Evaluation and management of indeterminate thyroid nodules: the revolution of risk stratification beyond cytological diagnosis. Cancer Control. 2017;24(5):1073274817729231. https://doi.org/10.1177/1073274817729231.
- 28. Rago T, Scutari M, Latrofa F, Loiacono V, Piaggi P, Marchetti I, et al. The large majority of 1520 patients with indeterminate thyroid nodule at cytology have a favorable outcome, and a clinical risk score has a high negative predictive value for a more cumbersome cancer disease. J Clin Endocrinol Metab. 2014;99(10):3700–7. https://doi.org/10.1210/jc.2013-4401.
- 29. Lloyd RV, Erickson LA, Casey MB, Lam KY, Lohse CM, Asa SL, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004;28(10):1336–40. https://doi.org/10.1097/01.pas.0000135519.34847.f6.
- 30. Hirokawa M, Carney JA, Goellner JR, DeLellis RA, Heffess CS, Katoh R, et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol. 2002;26(11):1508–14. https://doi.org/10.1097/00000478-200211000-00014.
- 31. Nath MC, Erickson LA. Aggressive variants of papillary thyroid carcinoma: hobnail, tall cell, columnar, and solid. Adv Anat Pathol. 2018;25(3):172–9. https://doi.org/10.1097/PAP.0000000000000184.
- 32. Depciuch J, Stanek-Widera A, Lange D, Biskup-Fruzynska M, Stanek-Tarkowska J, Czarny W, et al. Spectroscopic analysis of normal and neoplastic (WI-FTC) thyroid tissue. Spectrochim Acta A Mol Biomol Spectrosc. 2018;204:18–24. https://doi.org/10.1016/j.saa.2018.06.010.
- 33. Higuchi M, Hirokawa M, Suzuki A, Takada N, Yamao N, Kuma S, et al. Cytological features of solid variants of papillary thyroid carcinoma: a fine needle aspiration cytology study of 18 cases. Cytopathology. 2017;28(4):268–72. https://doi.org/10.1111/cyt.12399.
- 34. Rossi ED, Faquin WC, Pantanowitz L. Cytologic features of aggressive variants of follicular-derived thyroid carcinoma. Cancer Cytopathol. 2019;127(7):432–46. https://doi.org/10.1002/cncy.22136.
- 35. Khan NE, Bauer AJ, Schultz KAP, Doros L, Decastro RM, Ling A, et al. Quantification of thyroid cancer and multinodular goiter risk in the DICER1 syndrome: a family-based cohort study. J Clin Endocrinol Metab. 2017;102(5):1614–22. https://doi.org/10.1210/jc.2016-2954.
- 36. Wasserman JD, Sabbaghian N, Fahiminiya S, Chami R, Mete O, Acker M, et al. DICER1 mutations are frequent in adolescent-onset papillary thyroid carcinoma. J Clin Endocrinol Metab. 2018;103(5):2009–15. https://doi.org/10.1210/jc.2017-02698.
- 37. Oliver-Petit I, Bertozzi AI, Grunenwald S, Gambart M, Pigeon-Kerchiche P, Sadoul JL, et al. Multinodular goitre is a gateway for molecular testing of DICER1 syndrome. Clin Endocrinol. 2019;91(5):669–75. https://doi.org/10.1111/cen.14074.
- 38. Galuppini F, Vianello F, Censi S, Barollo S, Bertazza L, Carducci S, et al. Differentiated thyroid carcinoma in pediatric age: genetic and clinical scenario. Front Endocrinol. 2019;10:552. https://doi.org/10.3389/fendo.2019.00552.
- 39. Baran JA, Tsai SD, Isaza A, Brodeur GM, MacFarland SP, Zelley K, et al. The clinical spectrum of PTEN hamartoma tumor syndrome: exploring the value of thyroid surveillance. Horm Res Paediatr. 2020;93(11–12):634–42. https://doi.org/10.1159/000515731.
- 40. Jonker LA, Lebbink CA, Jongmans MCJ, Nievelstein RAJ, Merks JHM, Nieveen van Dijkum EJM, et al. Recommendations on surveillance for differentiated thyroid carcinoma in children with PTEN hamartoma tumor syndrome. Eur Thyroid J. 2020;9(5):234–42. https://doi.org/10.1159/000508872.
- 41. Pekova B, Sykorova V, Dvorakova S, Vaclavikova E, Moravcova J, Katra R, et al. RET, NTRK, ALK, BRAF, and MET fusions in a large cohort of pediatric papillary thyroid carcinomas. Thyroid. 2020;30(12):1771–80. https://doi.org/10.1089/thy.2019.0802.
- 42. Rangel-Pozzo A, Sisdelli L, Cordioli MIV, Vaisman F, Caria P, Mai S, et al. Genetic landscape of papillary thyroid carcinoma and nuclear architecture: an overview comparing pediatric and adult populations. Cancers. 2020;12(11):E3146. https://doi.org/10.3390/cancers12113146.
- 43. Bonora E, Tallini G, Romeo G. Genetic predisposition to familial nonmedullary thyroid cancer: an update of molecular findings and state-of-the-art studies. J Oncol. 2010;2010:385206. https://doi.org/10.1155/2010/385206.
- 44. Mester JL, Moore RA, Eng C. PTEN germline mutations in patients initially tested for other hereditary cancer syndromes: would use of risk assessment tools reduce genetic testing?Oncologist. 2013;18(10):1083–90. https://doi.org/10.1634/theoncologist.2013-0174.