Introduction
Whether human semen quality has been declining in recent decades has been the subject of considerable debate. Most of that debate has concerned whether testicular output, as measured by sperm concentration in fresh ejaculates, has declined in recent decades, with strong arguments being made both for and against a substantial decline based on a variety of datasets and analytical procedures (). Evidence in support of the pattern of declining semen quality includes data from meta-analyses of published data, with studies such as , ) concluding that sperm concentration in ejaculates could have fallen by as much as 2.64% per year from 2000 to 2019. However, other meta-analyses of published data, using similar methodologies, have not reached the same conclusion (), thus fueling the controversy. Critics of this meta-analytical approach have suggested that the data simply demonstrate that human sperm count varies across individual men, ecologies, locations, and time periods (), and that those estimates of sperm concentration are unreliable owing to the intrinsic limitations of the published studies on which those meta-analyses rely (). Thus, despite popular media often reporting that semen quality in humans has declined in recent decades, the question is still very much unresolved.
There have been many attempts to investigate changes in the semen quality of men living in Denmark, including studies of men from specific birth cohorts (), men applying to be sperm donors (), men recruited from the general population (; ), and young men being assessed for military service (; ). In each case, although a large number of men have been shown to have poor semen quality (up to 35% in some cases), there is little evidence within each study to suggest that semen quality has declined over the years. Indeed, in the most recent study by , there was no significant decline in the semen quality of 6386 young men (median age 19 years) over a 21-year period from 1996 to 2016.
Here, we used data from a large sperm bank (Cryos International) to examine temporal changes in semen quality of men living in Denmark in 2017–2022, using consistent methodology to assess all samples. Cryos International was established in 1981 and now has a high throughput of men volunteering to be sperm donors () whose motivations are well characterized (). We consider these men to be a reasonable sample of men in the general population because their semen quality prior to application is unknown and they are not providing semen samples because they are concerned about their own fertility. Thus, we hypothesized that, if semen quality had varied in recent years, it might be clearly evident in the populations of men applying to Cryos to be sperm donors.
Materials and methods
Study population
The focal study population consisted of men living in Denmark who were applying to become sperm donors (henceforth “donor candidates”) at Cryos International sperm bank from 2017 to 2022. Donor candidates lived in or close to one of the four largest cities of Denmark (Copenhagen, Aarhus, Odense, and Aalborg). In each of those cities, Cryos has a facility where semen samples were produced and analyzed. The recruitment process at Cryos has recently been described by . To be considered as a donor candidate and be invited to produce a semen sample for analysis, men had to be between the ages of 18 and 45 years old. We also examined data from all donor candidates who were then accepted to be donors (henceforth “accepted donors”), each of whom provided multiple subsequent semen samples. The analysis of repeated samples from accepted donors allowed us to check for variation that might have been caused by undocumented changes or drift in analytical techniques and to examine patterns within individual men.
Semen analysis
All semen samples were obtained by masturbation in a private room at each donation site and collected into a sterile plastic cup (Sarstedt, Hounisen Laboratory equipment A/S, Skanderborg, Denmark) after a recommended 2–5 days of sexual abstinence. Ejaculates were initially kept at room temperature (21 °C) to allow liquefaction and then weighed to estimate ejaculate volume (1.0 g/ml) as recommended by the World Health Organization (, ). An aliquot of each ejaculate was then examined by computer-assisted semen analysis (CASA) 30–60 min after production to determine sperm concentration and motility. The CASA system used was a Sperm Class Analyzer® (SCA® CASA, Microptic, Barcelona, Spain) with semen samples observed using a CX-41 upright light microscope (OLYMPUS, Microptic, Barcelona, Spain) and a Makler Counting Chamber (Sefi Medical Instruments Ltd, Haifa, Israel). Grades of sperm motility were classified according to the definitions in : grade a (rapidly progressive, ≥25 µm/s), grade b (slowly progressive, 5 to <25 µm/s), grade c (nonprogressive, >0 to <5 µm/s), and grade d (immotile). All equipment and protocols were identical at each site throughout the study period.
Data collection and data analysis
Data on semen quality from all donor candidate (Supplementary Data File S1) and accepted donor (Supplementary Data File S2) ejaculates collected between 5 January 2017 and 30 December 2022 were extracted retrospectively from the sperm bank database at Cryos International and anonymized before analysis. No samples were collected from 15 March to 31 May 2020 when Cryos was temporarily closed at all sites owing to the coronavirus disease 2019 (COVID-19) pandemic. All data were checked for anomalies and those with impossible values were assumed to be data entry errors and were removed from the datasets before statistical analyses (Supplementary Materials and Methods).
For each ejaculate, the dataset included the date and city where the sample was produced as well as the man’s age, ejaculate volume (ml), sperm concentration (106/ml), and the concentrations of grade a and grade b sperm (millions of spermatozoa per ml). We calculated total sperm count, total motile sperm count (TMSC), and the total counts of grades a and b sperm as the product of ejaculate volume and sperm concentrations. Motile sperm are defined by as comprising grade a and grade b sperm, thus the TMSC is the number of grades a and b sperm in an ejaculate and motile sperm concentration is the total number of grades a and b sperm per ml. Monthly mean temperatures (average of daily highs, means, and lows; Supplementary Data File S3) were obtained from the Danish Meteorological Institute (Copenhagen, Denmark) and were used to control statistically for variation in ambient temperature as this is known to affect sperm quality (; ; ). Danish law does not require ethical approval for the secondary analysis of anonymized data, but ethical approval for this study was obtained from The University of Manchester (ref: 2023-18428-31578).
Statistical analysis
Data analysis was performed using R 4.3.3 (). For regression models to predict ejaculate volume, total sperm count, sperm concentration, and measures of sperm motility (motile sperm concentration and TMSC), we entered the man’s age, monthly mean high temperature, city of donation, and date of semen production as predictors. Exploratory analyses indicated that all these variables influenced semen quality and that monthly average high temperature was most often the best single temperature variable to use as a predictor (Supplementary Table S1). The use of monthly mean or low temperatures had no effect on our conclusions as these three temperature variables are highly correlated (r > 0.84 for each city across all years). For the analyses of donor candidate data, we used linear models; for the accepted donor data we used linear mixed models (with random slopes and intercepts) with anonymized male identity as a random effect to control statistically for multiple measures per accepted donor (Supplementary Materials and Methods).
In all models, age was entered as a categorical variable as there was some nonlinearity in its relationship to response variables. All measures of semen quality were log10-transformed to normalize residuals and better satisfy other statistical assumptions (Supplementary Figs S1 and S2 and Table S1). From each statistical model, the semen quality measures for different years were compared using the Tukey post hoc test analyzed with the emmeans function in the emmeans package (version 1.8.5). Thus, we usually report the marginal means of sperm quality measures, calculated from the regression models. Marginal means are the mean values adjusted statistically for city, age, and monthly mean high temperature in each statistical model and thus are comparable to one another (Supplementary Materials and Methods).
Results
Study populations
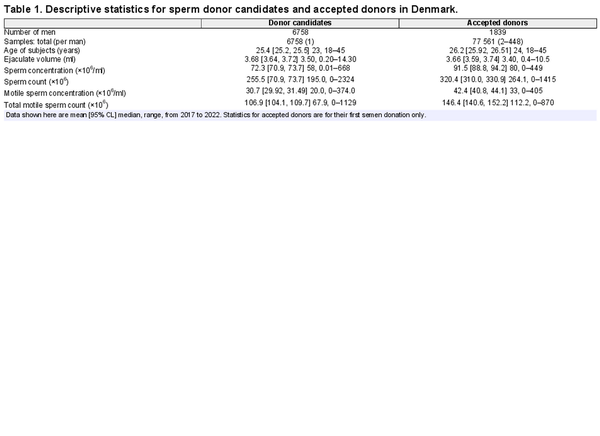
The donor candidates (n = 6758 men) were a sample from the populations of men living near each of the four cities in Denmark, whereas the accepted donor population (n = 1839 men) was a selected subset of donor candidates who had applied to be sperm donors, sometimes prior to 2017 (Supplementary Table S2). As a result, the means of semen quality measures are different between these two samples (Table 1, Fig. 1) by design (Materials and Methods). Donor candidates also averaged ∼9 months younger than accepted donors (at their first donation after 1 January 2017; Table 1), largely due to a lower proportion of men ≤25 years old being accepted to be sperm donors (Fig. 2D). Median age was 23 years in donor candidates and 24 years in accepted donors, meaning that about half of the semen samples were from men in the 18–24 years age group with the other half of the samples gradually decreasing in numbers for each age up to 45 years (Fig. 2D).
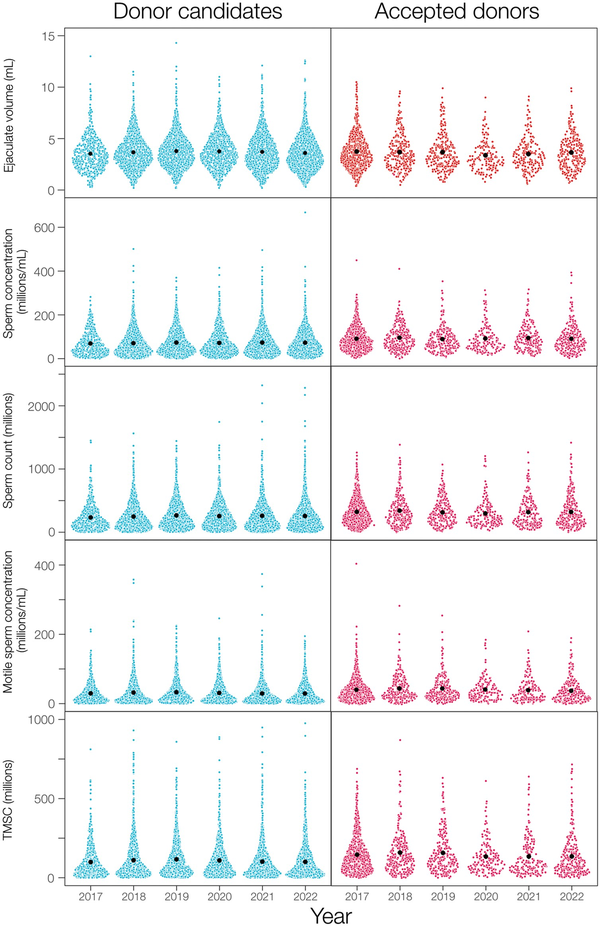
Figure 1
Measurements of semen quality in ejaculates from sperm banks in four cities in Denmark. Raw data are shown here for the single semen samples of all donor candidates (n = 6758), and for the first sample (n = 1839) provided by each accepted donor after 1 January 2017 (right), with mean values (black dots) calculated from the raw data. TMSC, total motile sperm count.
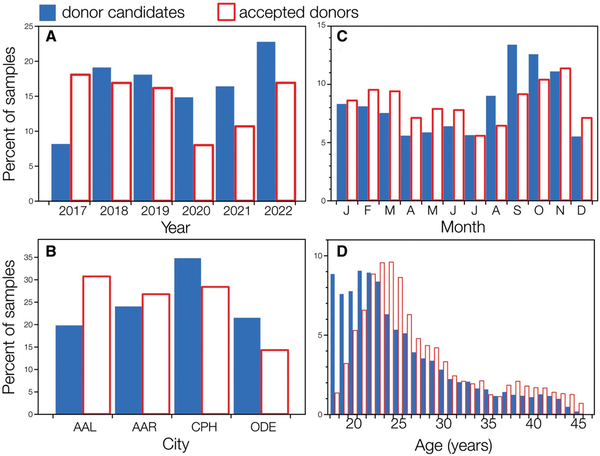
Figure 2
Frequency distributions showing the percentage of semen samples provided by donor candidates and accepted donors. (A) Per year, (B) in each Danish city (AAL = Aalborg, AAR = Aarhus, CPH = Copenhagen, ODE = Odense), (C) in each month across all years, and (D) across the mens’ ages on the day that semen samples were produced.
All semen samples were collected and analyzed across all six years of our study and within each month of the year (Fig. 2A and C, Supplementary Fig. S3). The 1839 accepted donors provided 77 561 ejaculates (Table 1) with the number of samples distributed approximately as for the donor candidates across years, cities, months of the year, and male ages (Fig. 2). About half of the accepted donors (882/1839) provided ejaculates in only one year of our study; only 14 accepted donors provided semen samples in all six years.
Donor candidates
Ejaculate volume, sperm count, and sperm concentration
Among the 6758 donor candidates, there was relatively little variation in average ejaculate volume, sperm concentration, and the sperm count per ejaculate across years (Fig. 3). Marginal mean ejaculate volume increased 7% from 2017 to 2019 (Tukey post hoc test, t = 2.84, P = 0.05) then declined 6% from 2019 to 2022 (t = 3.04, P = 0.03; Supplementary Table S3 and Fig. S4).
Figure 3
Year-to-year variation (2017–2022) in the semen parameters of ejaculates from donor candidates and accepted donors. (A) Ejaculate volume, (B) sperm concentration, and (C) total sperm count. Marginal means ± 95% CL are plotted here for donor candidates (n = 6758) and accepted donors (n = 1839), calculated from models that accounted for the man’s age, monthly mean high temperature, and Danish city (see also Supplementary Tables S3, S4, and S5).
Neither the marginal means of sperm concentration (F = 0.99, P = 0.42) or sperm count (F = 2.15, P = 0.06) of donor candidates varied significantly across years, changing 1–12% from one year to the next, with no clear patterns (Fig. 3B and C; Supplementary Tables S4 and S5 and Fig. S5). From 2019 to 2022, the marginal mean sperm concentration of donor candidates decreased by only 3.3%, from 52.1 to 50.4 million/ml, while the marginal mean sperm count per ejaculate declined 9%, from 180.9 to 164.7 million (Fig. 3B and C).
Motile sperm concentration and count
In contrast to overall sperm concentration and total sperm count (Fig. 3), the marginal means of motile sperm concentration and TMSC in donor candidates declined steeply and significantly from 2019 to 2022 (Fig. 4). Motile sperm concentration declined 16%, from 18.4 [95% CL: 17.0, 20.0] million/ml in 2019 to 15.5 [14.4, 16.7] million/ml in 2022 (Tukey post hoc test, t = 3.53, P = 0.006). This decline was independent of variation between cities, ages, and monthly mean high temperatures, all of which were adjusted for in the linear model (Supplementary Table S6).
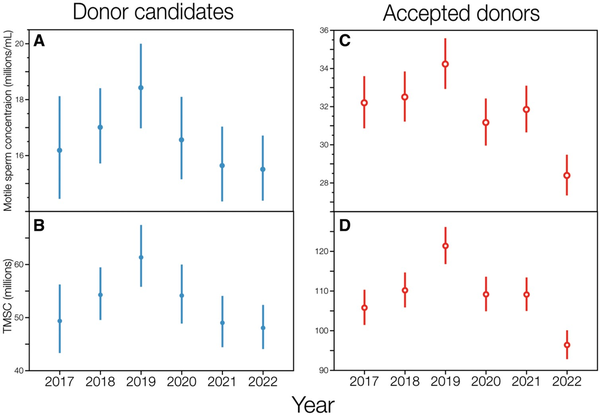
Figure 4
Motile sperm concentration and total motile sperm count in ejaculates from donor candidates and accepted donors from 2017 to 2022. Marginal means ±95% CL are shown here for donor candidates (A, B) and accepted donors (C, D), from linear models accounting for age, Danish city, and monthly mean high temperature (see also Supplementary Tables S6 and S7). TMSC, total motile sperm count.
Marginal mean TMSC declined 22%, from 61.4 [55.8, 67.5] million per ejaculate in 2019 to 48.1 [44.1, 52.4] million in 2022 (Tukey post hoc test, t = 4.33, P = 0.0002; Fig. 4B). During this same interval, the marginal mean ejaculate volume decreased by only 5.4% (Fig. 3A), which would have had a small effect on TMSC if motile sperm concentration had not also declined by 16% (Fig. 4B). Note also that the marginal mean TMSC per ejaculate increased significantly by 24% from 2017 to 2019 (Tukey post hoc test, t = 2.87, P = 0.05; Fig. 4B, Supplementary Table S7) due to a 9% increase in ejaculate volume (Fig. 3A) and a 14% increase in motile sperm concentration (Fig. 4A) during that 2-year period.
The patterns of declining sperm motility were the same in the concentrations of both grade a and grade b sperm per ejaculate, analyzed separately (Fig. 5A and C; Supplementary Tables S8 and S9), suggesting that there was a general decline in motility and not simply a decline in the concentration of one type of sperm. The concentration of grade a sperm, in particular, declined steeply, by 30% from 2019 to 2022 in donor candidates (Fig. 5A). Correlations between the concentrations of grade a and b sperm within semen samples were strong and statistically significant (all r ≥ 0.72) for all measures of sperm concentration and sperm count in all years for each city (Supplementary Table S10). Thus, changes in the concentrations of grade a sperm were usually paralleled by changes in the concentration of grade b sperm within ejaculates.
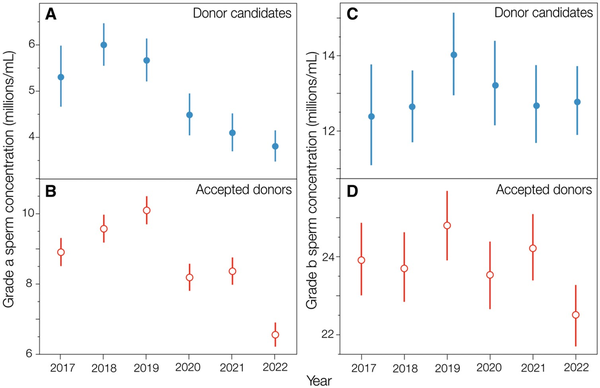
Figure 5
Concentration of grades a and b sperm in ejaculates from donor candidates and accepted donors from 2017 to 2022. Marginal means ±95% CL are shown here from linear models accounting for age, Danish city, and monthly mean high temperature (see also Supplementary Tables S8, S9, S18, and S19).
We also looked at the year-to-year patterns within each quartile of TMSC to see if the general pattern of decline from 2019 to 2022 across all donor candidates might be driven by a subset of men with particularly low or high semen quality. In all quartiles, TMSC declined from 2019 to 2022 (Supplementary Fig. S6 and Table S11), a pattern that was clearest and most pronounced in the lowest three quartiles.
Accepted donors
Semen quality
All measures of semen quality in accepted donors showed the same patterns as for the donor candidate population (Figs 3, 4, and 5), albeit with larger ejaculate volumes, higher overall sperm concentrations and counts, and higher motile sperm concentrations and counts. Such differences are expected because accepted donors were selected from an applicant pool (i.e. donor candidates) that included many men with lower semen quality and response to cryopreservation (see ).
For accepted donors, ejaculate volume varied significantly across years (Type II Wald chi-square = 329.6, P < 0.0001), increasing 8% from 2017 to 2019, then decreasing 3% from 2019 to 2022 (analysis of marginal means, Supplementary Table S12). Sperm concentrations and counts in accepted donors also varied significantly across years (Supplementary Tables S13, S14, and S15), with a 9% decline in sperm count, a 6% decline in sperm concentration, and a 17% decline in motile sperm concentration between 2019 and 2022.
The TMSC of accepted donors decreased by 21% from 2019 to 2022 (Fig. 4D), from 121.4 to 96.4 millions per ejaculate (marginal means controlling statistically for city, monthly mean high temperature, and multiple measures per accepted donor). TMSC of accepted donors declined significantly from 2019 to 2020 and from 2021 to 2022, but not from 2020 to 2021 (Fig. 4D; Tukey post hoc tests, Supplementary Table S16).
Individual accepted donors
Ejaculate quality (motile sperm parameters) measured for the first donation of each accepted donor showed the same pattern as for the single ejaculates from donor candidates (Supplementary Table S17). Thus, the marginal mean TMSC of the first donations made after 1 January 2019 declined from 2019 to 2022 by 23%, from 111.1 [95% CL: 102.6, 120.2] million per ejaculate in 2019 to 85.2 [76.0, 95.6] million in 2022. As with donor candidates, the concentrations of both grades a and b sperm showed similar patterns of decline in accepted donors from 2019 to 2022 (Fig. 5B and D; Supplementary Tables S18 and S19).
Of the accepted donors who made at least eight donations in the 2019–2022 period, the estimates (slopes) for the effect of date on those parameters were positive for 425 accepted donors, and negative for 587 (Supplementary Fig. S7). Thus, significantly more of them had a linear reduction in sperm quality over time rather than a linear increase (binomial test, P < 0.0001). Looking at just the significant estimates of changes with time (Fig. 6), shows the same pattern, as significantly more were negative (85) than positive (42), with 67% being negative (binomial test (P = 0.0008). We conclude that the average decline in the concentration of motile sperm in accepted donors during the study period was caused by two factors: a decline in the motile sperm concentration in their initial semen sample; and a decline in the motile sperm concentration in the successive ejaculates of a larger proportion of individual accepted donors over time.
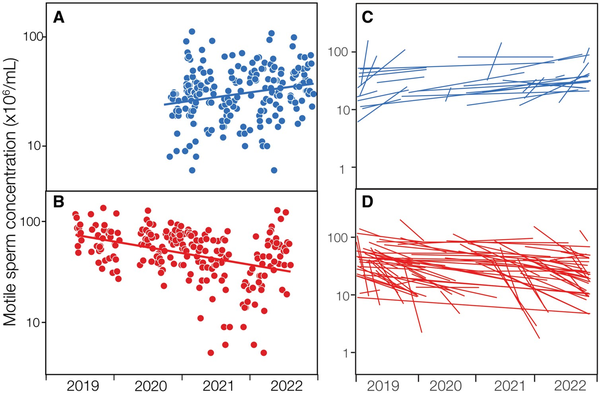
Figure 6
Significantly positive and negative relationships between motile sperm concentration in the ejaculates from accepted donors in 2019–2022. Graphs show (A, B) examples from two different accepted donors, and (C, D) all accepted donors who made eight or more sperm donations during that period and in two or more years. C and D show only significantly positive (blue; n = 42) and negative (red; n = 85) regressions.
Discussion
We investigated the semen quality of young men who were applying to be sperm donors at Cryos International in Denmark from early 2017 to late 2022. We consider this donor candidate sample to be representative of the populations of men living near those cities in Denmark each year, but we have no way of knowing how random this sample is with respect to those source populations. Whilst there was relatively little change in semen volume, sperm concentration or total sperm count in an ejaculate (Fig. 3), the measures of sperm motility—TMSC and motile sperm concentration (millions per ml)—both declined significantly, by 22% and 16%, respectively, from 2019 through 2022 (Fig. 4).
While there was a significant decline in the ejaculate volume of donor candidates from 2019 to 2022, this decline was not accompanied by significant declines in either sperm concentration or total sperm count (Fig. 3). Indeed, there was no significant variation across years in either sperm concentration or total sperm count of donor candidates during the study period from 2017 to 2022 (Supplementary Tables S4 and S5), despite the large sample size and high statistical power to detect even small year-to-year changes. We conclude that there is no evidence for a change in sperm concentration or total sperm count in the donor candidate population but that there is strong evidence for a decline in sperm quality from 2019 to 2022 as shown by the changes in means of both TMSC and motile sperm concentration in donor candidates. The decline in TMSC from 2019 to 2022, for example, represents a decline of 7.5% per year.
It is also worth noting that, while we did find a significant decline in average sperm motility (TMSC) in donor candidates, our statistical model can account for only 3% of the variation in TMSC (Supplementary Table S7) due to year-to-year variation, the age of donor candidates, and monthly mean high temperatures. Thus 97% of the variation in TMSC is caused by factors that are unknown and therefore not included in our statistical model. Indeed, none of the models of measures of semen quality that we report in this study accounted for more than 9% of the variation in those measures. Thus, even though we have uncovered some significant year-to-year variation, the distributions of each sperm parameter almost completely overlap from year to year (Fig. 1). This means that even large declines in the average motility (or other sperm parameters) are not accompanied by year-to-year declines in the sperm quality of all donor candidates (or accepted donors; Fig. 6).
Although Cryos International was established in 1981, more than 40 years ago, we studied only the data collected since early 2017 as, prior to this date, all semen analyses at Cryos were performed manually. Manual measurements of sperm concentration () and motility () can vary over time owing to variation in techniques and equipment and may thus be unreliable for longitudinal studies semen quality. From early 2017 onward, Cryos used the same protocols and CASA system at all its donation sites in Denmark, providing a high level of consistency to data collection from year to year. The Sperm Class Analyzer® (SCA® CASA, MICROPTIC, Barcelona, Spain) used by Cryos is validated annually for the measurement of sperm concentration and has been shown to have less measurement error than manual methods, as well as correlating well with data from the Improved Neubauer chamber (considered to be the Gold Standard method of semen analysis; ). CASA has also been shown to give reliable measurements of sperm motility, reduce operator subjectivity, and allow a higher number of sperm to be examined with less measurement error than the manual equivalent ().
We are, therefore, confident in the accuracy of the sperm motility measurements obtained in this study and have concluded that the decline in sperm motility observed from 2019 to 2022 is not an artifact of changes to analytical methods. Initially, we planned to look only at the single ejaculate from the 6758 men who applied to be a sperm donor (i.e. donor candidates). However, to rule out whether or not there was a long-term change in the sperm motility of individuals, we also examined the 77 561 ejaculates of the 1839 accepted donors, men who were accepted onto the program and donated regularly during 2017–2022. In this accepted donor subpopulation, the TMSC in their first donations (i.e. not the semen samples they provided as donor candidates) declined by 21% from 2019 to 2022. In addition, the motile sperm concentration in 58% of 1012 accepted donors declined during 2019–2022, though only 8% significantly so. Thus, our working hypothesis is that between 2019 and 2022 there was a gradual reduction in the baseline motile sperm concentrations and motile sperm count (TMSC) in both the donor candidate and accepted donor populations as well as a gradual decline in these variables within about half of the accepted donors. Moreover, because this decline was observed in both grade a and b sperm, it was an overall decline in the population of motile sperm in ejaculates rather than a reduction in sperm swimming speed that simply reclassified sperm from grade a to grade b.
Such a sharp decline in sperm motility to occur over a relatively short length of time suggests that external factors were likely to have played a role. In this context, we note that the observed decline in sperm motility roughly corresponds to the onset of the worldwide COVID-19 pandemic. Whilst it had been established that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is not present in semen (), there is some evidence that SARS-CoV-2 can temporarily reduce sperm motility in men after infection (). However, it is an unlikely explanation for our observations since Cryos was closed and did not process any semen samples for 11 weeks in the spring of 2020. Moreover, once Cryos reopened, men were asked to report and defer providing semen samples for at least 30 days if they had symptoms or were diagnosed with SARS-CoV-2. As SARS-CoV2-2 infections were rare in Denmark (typically <5% of the population; ), very few men were asked to defer. We should be mindful that many other aspects of life also altered around this time. For example, widespread lockdowns may have led to changes in working patterns, diet, and levels of physical activity. Several studies have shown that each of these factors can impact sperm motility (; ; ).
Reductions in sperm motility over time (defined in several different ways) have been documented in other accepted sperm donor populations, although often in association with concomitant changes in sperm concentration and other measures of semen quality. For example, examined 1927 sperm donor candidates in the Copenhagen area between 1977 and 1995 and found that whilst the percentage sperm motility decreased significantly during that time, sperm counts increased. In the USA, examined 489 men who applied to be a sperm donor in the Boston, MA, metropolitan area over a 10-year period (2003–2013) and found a decline in TMSC as well as sperm concentration and count, with no significant change in semen volume. Finally, in China, examined the semen quality of over 30 000 young men who applied to be sperm donors in Hunan province over a 15-year period (2001–2015) and found decreases in the progressively motile sperm count (grades a + b sperm), sperm concentration, and the percentage of sperm with normal morphology. It is clear from these studies, as well as the present study, that reductions in sperm motility in populations of accepted donors and donor candidates do occur from time to time. However, it is unclear whether these changes are permanent, caused by transient external factors (disease, changes in lifestyle), or simply part of natural year-to-year variation.
Although we saw dramatic changes in sperm motility from 2019 to 2022, we did not find any evidence for changes in overall sperm concentration in donor candidates. This is contrary to what might be predicted from the meta-analysis published by who proposed that sperm concentration has declined by as much as 2.64% per year in unselected men (which we consider the donor candidates to be) since the year 2000. In our donor candidate population this would translate to a 7.6% decline in sperm concentration from 2019 to 2022, whereas we found a (nonsignificant) decline of only 3% in the sperm concentration of donor candidates over that period (Fig. 3B). In contrast, our results for the donor candidates are consistent with the meta-analysis conducted by who concluded that no statistical changes in sperm concentration had occurred between 1993 and 2018. Interestingly, our results for sperm concentration are similar to other reports in Danish populations, the most recent of which () examined the ejaculate quality of 6386 young men (median age 19) over a 21-year period between 1996 and 2016 and found there was no significant decline in sperm concentration (although a significant number had sperm concentrations below reference ranges). This does, however, illustrate the need for ongoing prospective monitoring of well-defined populations if we are to truly describe and understand any temporal changes in semen quality.
Although there was no evidence for changes in sperm concentration and total sperm count of donor candidates in 2017–2022 (Fig. 3B and C), this was not true for the accepted donor population where many of the pairwise differences between years were significant (Supplementary Tables S13 and S14). Similarly, while all the significant changes in TMSC and grade a sperm concentration in donor candidates were declines (Fig. 5A), there were some significantly positive increases in those sperm parameters during 2017–2019 in accepted donors. We have no explanation for those increases, especially in the absence of lifestyle information on those accepted donors and no obvious changes in Danish society during that period. Accepted donors, by design, had higher levels of sperm motility than donor candidates and thus might be expected to show greater year-to-year variation in average sperm motility.
The major strength of this study is the high number of ejaculates examined (>84 000): 6758 from donor candidates and 77 561 from 1839 accepted donors. This means we have the statistical power to detect small changes in average values of ejaculate quality. Also, because Cryos recruited men from four cities in Denmark, we are able to include a diversity of individuals, rather than men from just one city or region. In our analyses, controlling statistically for age, city, and local temperature, and the use of CASA, means that we have robust measures of sperm motility. Conversely, study weaknesses include the relatively short window of 6 years over which the data was collected. We also lacked information on lifestyle, occupation, and other factors (e.g. the precise abstinence period) about individuals, which could have impacted their semen quality. Data on sperm morphology were not examined as this is not used by Cryos as a criterion for sperm donor selection.
In conclusion, we have described a significant reduction in sperm motility (motile sperm concentration and TMSC) in a population of young men who were applying to be or were accepted as sperm donors in Denmark between 2017 and 2022. This decline in sperm motility was far greater than changes to other semen parameters (e.g. ejaculate volume, total sperm count, sperm concentration). Such a change in semen quality has implications for sperm donor recruitment because motile sperm concentration is an essential selection criterion (). It also has potential implications for human fertility because sperm motility is correlated with the probability of conception (; ). We propose that men applying to be sperm donors are a useful population in which to monitor changes in human semen quality over time that might help identify external causal factors for any decline in sperm quality. This in turn could lead to bespoke treatments or lifestyle changes personalized for each donor in order to optimize ejaculate quality and maximize sperm donor recruitment.
Acknowledgements
The authors acknowledge the contributions of all laboratory technicians of Cryos International sperm bank for pertinent semen analysis, donor candidate assessment, cryopreservation, and registration of all semen samples.
References
- Ata B, Vermeulen N, Mocanu E, Gianaroli L, Lundin K, Rautakallio-Hokkanen S, Tapanainen JS, Veiga A. SARS-CoV-2, fertility and assisted reproduction. Hum Reprod Update 2023;29:177–196.
- Auger J, Eustache F, Chevrier C, Jégou B. Spatiotemporal trends in human semen quality. Nat Rev Urol 2022;19:597–626.
- Bonde JP, Ernst E, Jensen TK, Hjollund NH, Kolstad H, Henriksen TB, Scheike T, Giwercman A, Olsen J, Skakkebaek NE. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet 1998;352:1172–1177.
- Bonde JP, Ramlau-Hansen CH, Olsen J. Trends in sperm counts: the saga continues. Epidemiology 2011;22:617–619.
- Boulicault M, Perret M, Galka J, Borsa A, Gompers A, Reiches M, Richardson S. The future of sperm: a biovariability framework for understanding global sperm count trends. Hum Fertil (Camb) 2022;25:888–902.
- Burke CA, Skytte AB, Kasiri S, Howell D, Patel ZP, Trolice MP, Parekattil SJ, Michael SF, Paul LM. A cohort study of men infected with COVID-19 for presence of SARS-CoV-2 virus in their semen. J Assist Reprod Genet 2021;38:785–789.
- Carlsen E, Swan SH, Petersen JH, Skakkebaek NE. Longitudinal changes in semen parameters in young Danish men from the Copenhagen area. Hum Reprod 2005;20:942–949.
- Centola GM, Blanchard A, Demick J, Li S, Eisenberg ML. Decline in sperm count and motility in young adult men from 2003 to 2013: observations from a U.S. sperm bank. Andrology 2016;4:270–276.
- Cipriani S, Ricci E, Chiaffarino F, Esposito G, Dalmartello M, La Vecchia C, Negri E, Parazzini F. Trend of change of sperm count and concentration over the last two decades: a systematic review and meta-regression analysis. Andrology 2023;11:997–1008.
- Cooper TG, Yeung CH. Computer-aided evaluation of assessment of “grade a” spermatozoa by experienced technicians. Fertil Steril 2006;85:220–224.
- Dearing C, Jayasena C, Lindsay K. Can the Sperm Class Analyser (SCA) CASA-Mot system for human sperm motility analysis reduce imprecision and operator subjectivity and improve semen analysis? Hum Fertil (Camb) 2021;24:208–218.
- Dearing CG, Kilburn S, Lindsay KS. Validation of the sperm class analyser CASA system for sperm counting in a busy diagnostic semen analysis laboratory. Hum Fertil (Camb) 2014;17:37–44.
- Espenhain L, Tribler S, Sværke Jørgensen C, Holm Hansen C, Wolff Sönksen U, Ethelberg S. Prevalence of SARS-CoV-2 antibodies in Denmark: nationwide, population-based seroepidemiological study. Eur J Epidemiol 2021;36:715–725.
- Gyllenborg J, Skakkebaek NE, Nielsen NC, Keiding N, Giwercman A. Secular and seasonal changes in semen quality among young Danish men: a statistical analysis of semen samples from 1927 donor candidates during 1977-1995. Int J Androl 1999;22:28–36.
- Hoang-Thi AP, Dang-Thi AT, Phan-Van S, Nguyen-Ba T, Truong-Thi PL, Le-Minh T, Nguyen-Vu QH, Nguyen-Thanh T. The impact of high ambient temperature on human sperm parameters: a meta-analysis. Iran J Public Health 2022;51:710–723.
- Huang C, Li B, Xu K, Liu D, Hu J, Yang Y, Nie H, Fan L, Zhu W. Decline in semen quality among 30,636 young Chinese men from 2001 to 2015. Fertil Steril 2017;107:83–88.e2.
- Jørgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, Olesen IA, Juul A, Andersson AM, Carlsen E, Petersen JH et al Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open 2012;2:e000990.
- Jørgensen N, Lamb DJ, Levine H, Pastuszak AW, Sigalos JT, Swan SH, Eisenberg ML. Are worldwide sperm counts declining? Fertil Steril 2021;116:1457–1463.
- Larsen L, Scheike T, Jensen TK, Bonde JP, Ernst E, Hjollund NH, Zhou Y, Skakkebaek NE, Giwercman A. Computer-assisted semen analysis parameters as predictors for fertility of men from the general population. The Danish First Pregnancy Planner Study Team. Hum Reprod 2000;15:1562–1567.
- Lassen E, Pacey A, Skytte A-B, Montgomerie R. Data and code for recent decline in sperm motility among donor candidates at a sperm bank in Denmark. Borealis 2024. https://doi.org/10.5683/SP3/TMOA1A.
- Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Jolles M, Pinotti R, Swan SH. Temporal trends in sperm count: a systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum Reprod Update 2023;29:157–176.
- Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update 2017;23:646–659.
- Pacey AA. Are sperm counts declining? Or did we just change our spectacles? Asian J Androl 2013;15:187–190.
- Pacey AA, Pennings G, Mocanu E, Rothmar J, Pinborg A, Adrian SW, Burke C, Skytte AB. An analysis of the outcome of 11 712 men applying to be sperm donors in Denmark and the USA. Hum Reprod 2023;38:352–358.
- Pennings G, Mocanu E, Herrmann JR, Skytte AB, Burke C, Pacey A. Attitudes of sperm donors towards offspring, identity release and extended genetic screening. Reprod Biomed Online 2021;43:700–707.
- Povey AC, Clyma JA, McNamee R, Moore HD, Baillie H, Pacey AA, Cherry NM; Participating Centres of Chaps-UK. Modifiable and non-modifiable risk factors for poor semen quality: a case-referent study. Hum Reprod 2012;27:2799–2806.
- Priskorn L, Nordkap L, Bang AK, Krause M, Holmboe SA, Egeberg Palme DL, Winge SB, Mørup N, Carlsen E, Joensen UN et al Average sperm count remains unchanged despite reduction in maternal smoking: results from a large cross-sectional study with annual investigations over 21 years. Hum Reprod 2018;33:998–1008.
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna (Austria): R Foundation for Statistical Computing, 2024. https://www.R-project.org/
- Rasmussen PE, Erb K, Westergaard LG, Laursen SB. No evidence for decreasing semen quality in four birth cohorts of 1,055 Danish men born between 1950 and 1970. Fertil Steril 1997;68:1059–1064.
- Sun B, Messerlian C, Sun ZH, Duan P, Chen HG, Chen YJ, Wang P, Wang L, Meng TQ, Wang Q et al Physical activity and sedentary time in relation to semen quality in healthy men screened as potential sperm donors. Hum Reprod 2019;34:2330–2339.
- Tang F, Jiang Z, Jin M, Sheng H, Feng L, Chen J, Li Y, Huang J, Xu L, Lou J. Association of occupations with decreased semen quality in eastern China: a cross-sectional study of 12 301 semen donors. BMJ Open 2022;12:e061354.
- Wang X, Tian X, Ye B, Zhang Y, Zhang X, Huang S, Li C, Wu S, Li R, Zou Y et al The association between ambient temperature and sperm quality in Wuhan, China. Environ Health 2020;19:44.
- WHO [World Health Organization]. WHO Laboratory Manual for the Examination and Processing of Human Semen. Geneva: World Health Organization, 2010.
- WHO [World Health Organization]. WHO Laboratory Manual for the Examination and Processing of Human Semen. Geneva: World Health Organization, 2021.
- Yun Z, Tianqing M, Li W, Yonggang D, Guo L, Chunxiang S, Hai Z, Zhe P, Chuangang F, Jixuan M et al Association between ambient temperature and semen quality: a longitudinal study of 10 802 men in China. Environ Int 2020;135:105364.