Introduction
Ivabradine is a channel blocker that acts on the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel, consisting of a compound of benzocyclobutane that exhibits a unique specificity. It is an approved medication that has garnered significant attention in the field of heart failure, cardiology, and cardiovascular medicine. It is being used and studied in conditions like acute myocardial infarction and is also considered an adjuvant treatment for chronic heart failure. It is often used in combination with other medications such as ACE inhibitors, β-blockers, and MRA. This medication stands out as a unique and innovative approach to managing heart-related conditions, particularly those involving the regulation of heart rate.
The SA node is often referred to as the “natural pacemaker” of the heart. Within the SA node are specific ion channels known as “funny current” or If channels. These channels allow sodium and potassium ions to flow in and out of the SA node cells, which is crucial for the generation of electrical impulses responsible for initiating heartbeats. Ivabradine selectively inhibits the funny current (If) channels in the sinoatrial node, decreasing heart rate. It primarily targets the heart rate without significantly altering the inotropy, lusitropy or the repolarization of the ventricles. Indeed, ivabradine is distinct from many other heart rate-lowering medications like β-blockers because it does not affect myocardial contraction, relaxation, or ventricular repolarization. Ivabradine exerts its effects through a unique target, the HCN-gated channels, which share a tetrameric structure similar to cyclic nucleotide-modulated voltage-gated potassium channels. It attaches itself to the HCN4 receptors, specifically targeting residues Y506, F509 and other components. It binds to a location within the channel pore from the intracellular side, leading to the disruption of If ion current flow. Within the family of four known HCN isoforms, HCN4 is more represented predominantly on the membrane of cardiac atrial sinus node cells. Notably, this isoform is subject to modulation by cAMP, and the binding of cAMP to HCN channels induces a protein conformational change that enhances the likelihood of the channel opening during hyperpolarization., This disruption causes an extension of the diastolic depolarization phase in cardiac cells, ultimately resulting in a reduction in heart rate., As a result, the SA node cells generate electrical impulses more slowly and less frequently. This slower heart rate is beneficial in certain medical conditions, such as angina or heart failure. This review aims to provide an overview of Ivabradine and its clinical intervention, exploring its molecular mechanism of action, efficacy, safety profile and future prospects for the management of various cardiovascular conditions.
Molecular Aspect of Ivabradine
Ivabradine is metabolized in the liver, with over 50% of the drug being metabolized by CYP3A4-mediated liver metabolism with an elimination half-life of two hours and excreted through the kidneys. Ivabradine’s mechanism of action is intricately tied to its interaction with ion channels in cardiac cells. Ivabradine works by inhibiting these channels, reducing the flow of sodium ions into the cardiac cells during the diastolic phase. Consequently, it lengthens the time it takes for the SA node to depolarize and generate the next action potential, effectively slowing down the heart rate without impacting other cardiac functions such as contractility or blood pressure. This unique molecular action offers a more selective approach compared to traditional β-blockers, which affect a broader range of adrenergic receptors. Ivabradine attaches itself to specific amino acid residues within the HCN4 channel, particularly residues Y506 and F509, providing crucial binding interaction for its inhibitory effect Ivabradine is a selective inhibitor of the If ion channels in the heart’s sinoatrial node. Ivabradine enters the ion channel from the intracellular side of the SA node cells. This location is significant because it allows the drug to interfere with the flow of ions through the channel. Once inside the channel, ivabradine disrupts the flow of sodium and potassium ions which are essential for the generation of electrical impulses in the SA node. By inhibiting the movement of these ions, ivabradine slows down the depolarization phase of the SA node cells. The disruption caused by ivabradine prolongs the diastolic depolarization phase, which is the time when the SA node cells are repolarizing and preparing to generate the next electrical impulse. The SA node produces electrical impulses at a slower pace as a result of the extended depolarization. Due to the decreased rate of electrical signal generation in the SA node, the heart contracts at a slower pace, leading to a decrease in heart rate without affecting contractility or blood pressure. This decrease in heart rate represents the primary therapeutic outcome of ivabradine and makes it a promising heart rate-controlling agent.
Ivabradine also inhibits the retinal current (Ih), sharing resemblances with If ion channels in its characteristics. Ih is involved in the temporal resolution of the visual system, moderating retinal responses to intense light stimuli. In specific situations, like sudden and significant shifts in luminosity, the partial inhibition of Ih may be responsible for the visual phenomena known as phosphenes, which some patients experience. This dual action of Ivabradine underscores its potential to affect not only the heart but also the visual responses, providing a multifaceted perspective on its pharmacological influence.,
Clinical Intervention of Ivabradine
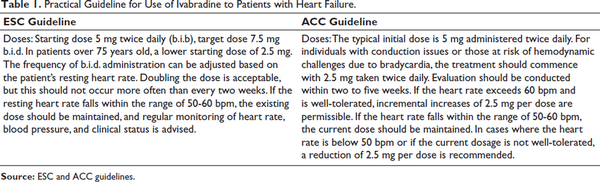
Ivabradine is a therapeutic agent primarily used to manage certain cardiovascular conditions by selectively reducing heart rate, such as chronic stable angina, heart failure with reduced ejection fraction (HFrEF), inappropriate sinus tachycardia, and in certain cases, atrial fibrillation (AF). Its unique mechanism of action involves selective inhibition of the If channels in the sinoatrial node, leading to heart rate reduction without significant impact on myocardial contractility or blood pressure (Table 1).
Figure 1 shows a schematic diagram showing the mechanism of action of Ivabradine. Ivabradine specifically targets and blocks the If channels in the SA node, resulting in a decreased rate of spontaneous depolarization, which reduces the cardiac output and thus helps in angina or heart failure.
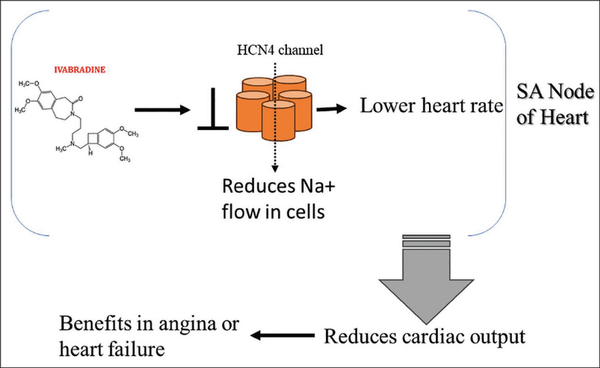
Mechanism of Action of Ivabradine.
A unique and clinically significant feature of ivabradine is its use dependence, which means its potency increases as the heart rate rises. This property is rooted in its selective inhibition of the If channels in the sinoatrial node, which are more active at higher heart rates. As heart rate increases, the If channels spend more time in an open state, providing greater access for ivabradine to bind and exert its effect. Consequently, the drug demonstrates enhanced heart rate reduction during tachycardia while exerting minimal effects at lower heart rates. This use-dependent action ensures that ivabradine reduces heart rate effectively in situations where it is most needed, such as during exercise or periods of heightened sympathetic activity, without causing excessive bradycardia at rest. This makes ivabradine particularly valuable in conditions like chronic stable angina and HFrEF, where controlling elevated heart rates is critical for reducing myocardial oxygen demand and improving cardiac efficiency. Furthermore, this property contributes to its favorable safety profile, as it reduces the risk of severe bradycardia, especially in patients with already low resting heart rates
Chronic Stable Angina and Heart Failure
In patients with chronic stable angina, Ivabradine reduces heart rate, alleviating chest pain, improving exercise tolerance, and enhancing overall quality of life., For those with heart failure, especially HFrEF, it serves as an adjunct to standard therapy, including ACE inhibitors, β-blockers, and mineralocorticoid receptor antagonists. In patients with symptomatic heart failure and a left ventricular ejection fraction of 35% or lower, ivabradine has been shown to reduce the risk of hospitalization for worsening heart failure. The SHIFT trial demonstrated that ivabradine decreased the relative risk of cardiovascular death or hospital admissions for worsening heart failure by 18% compared with placebo.
Atrial Fibrillation
Ivabradine’s role in AF is nuanced. While it is not a first-line treatment for rhythm or rate control in AF, it may have utility in certain contexts, such as managing inappropriate sinus tachycardia or heart failure in patients who also exhibit episodes of AF. In these cases, Ivabradine can be used cautiously, as it primarily targets the sinoatrial node and has no direct effect on atrial conduction or rhythm regularization. However, its use in AF patients requires careful consideration and close monitoring to avoid the exacerbation of arrhythmias or bradycardia. A recent study investigated ivabradine for controlling heart rate in permanent AF. The study found that ivabradine produced a moderate rate of reduction in patients with permanent AF, primarily through inhibition of the funny current in the atrioventricular node. However, compared with digoxin, ivabradine was less effective in reducing heart rate but was better tolerated, with a similar rate of serious adverse events. A prospective study indicated that ivabradine can cause AF regardless of baseline left atrial size, suggesting the need for careful patient selection and monitoring.
Ivabradine serves as an alternative or adjunct for patients who cannot tolerate β-blockers due to comorbidities such as asthma, diabetes, or peripheral vascular disease. Unlike β-blockers, Ivabradine does not adversely affect bronchial function or glucose metabolism, making it a preferable option for such individuals. Ivabradine is generally well-tolerated, with the most common side effects being visual disturbances (phosphenes) and bradycardia. Phosphenes are typically transient and dose-dependent, while bradycardia may occasionally necessitate dose adjustment or discontinuation. Patients with AF must be closely monitored, as Ivabradine may be less effective or even contraindicated depending on the clinical context.,
Ivabradine in Disease Manifestations
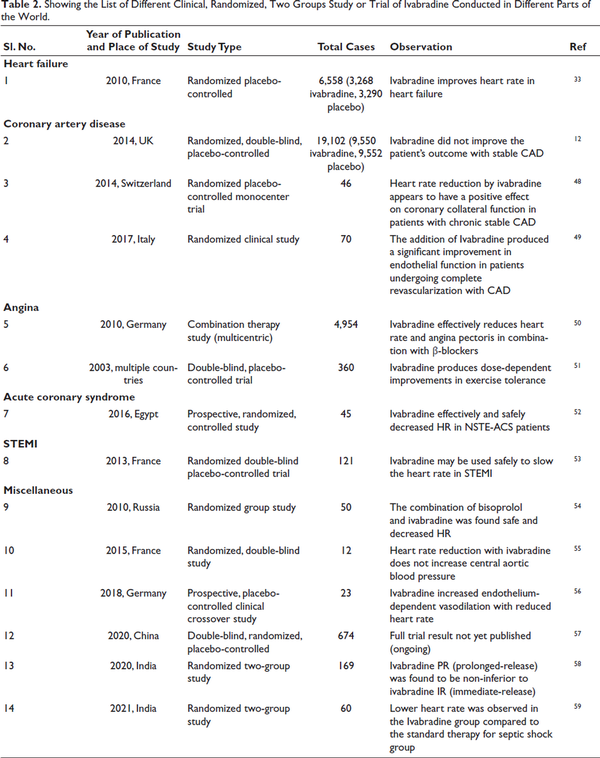
Ivabradine is used for stable angina and chronic heart failure, particularly in patients with reduced ejection fraction who remain symptomatic despite standard therapy. It lowers heart rate without affecting contractility or blood pressure, unlike β-blockers. In the INITIATIVE study, Ivabradine (7.5 mg twice daily) and Atenolol (100 mg daily) showed similar benefits in exercise duration and angina reduction, with a slight difference in weekly angina attacks (2.2 ± 4.3 vs. 2.7 ± 12.3) (Table 2).,
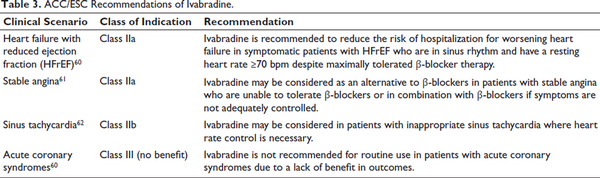
The European Society of Cardiology (ESC) and the American College of Cardiology (ACC) provide key guidelines for ivabradine use in HFrEF. The ESC recommends ivabradine to lower heart failure hospitalization and cardiovascular death in symptomatic patients with normal sinus rhythm, HR ≥70 bpm, despite optimal β-blocker, ACE inhibitor/ARB, and MRA therapy. The ACC 2022 guidelines suggest ivabradine for NYHA class II-III HFrEF patients (LVEF ≤35%) on guideline-directed medical therapy (GDMT) to reduce hospitalizations and mortality (Table 3).
Ivabradine in Heart Failure
Ivabradine is essential in managing chronic heart conditions like angina and HFrEF. By lowering heart rate, it improves diastolic filling, stroke volume, and myocardial efficiency, reducing oxygen demand in angina patients. Elevated resting heart rates predict worse outcomes in heart failure due to increased sympathetic activity.– While β-blockers improve survival in HFrEF, heart rate reduction correlates with better outcomes., Combining ivabradine with β-blockers enhances treatment efficacy, improving quality of life in chronic heart failure patients.
Ivabradine in Coronary Artery Disease
Ivabradine plays a key role in CAD management by reducing heart rate without affecting blood pressure. Research shows it improves hyperemic coronary flow velocity and CFR in stable CAD patients. Skalidis et al. demonstrated its ability to enhance coronary blood flow and endothelial function., While initial trials in CAD with left ventricular dysfunction showed no clear benefits, post hoc analyses indicated improved outcomes in patients with a resting heart rate ≥70 bpm, especially those with angina. Another study confirmed benefits in stable CAD without heart failure, though its impact varies by patient subgroup.
Ivabradine in AF
AF is a common arrhythmia, but ivabradine’s role in its management is limited. It is primarily used in sinus rhythm-dependent conditions, particularly HFrEF and a resting heart rate ≥70 bpm despite guideline-directed therapy., Ivabradine is ineffective for ventricular rate control in AF and is not a first-line treatment. Studies suggest an increased AF risk with ivabradine use (RR: 1.15-1.24)., While beneficial in select patients with preserved sinus rhythm, careful evaluation of its risks and further research are needed.
Ivabradine in Angina
Lowering heart rate is essential in managing chronic stable angina. Ivabradine, a well-tolerated anti-anginal drug, reduces myocardial oxygen demand by selectively inhibiting If channels, effectively alleviating symptoms without causing tolerance., Studies show it significantly decreases angina attacks and nitrate use while reducing hospitalization rates for cardiovascular events.– While some meta-analyses found no significant impact on angina episodes, ivabradine remains a safe and effective option across diverse patient groups, improving quality of life regardless of age, comorbidities, or β-blocker use. Clinical studies from various regions are summarized in Table 2.
Limitations
Ivabradine, while a valuable therapeutic option in cardiovascular medicine, is not without controversy. Its association with an increased risk of AF, as noted in trials like SHIFT, raises concerns about its routine use, particularly in patients predisposed to arrhythmias. Moreover, ivabradine is effective only in patients with sinus rhythm, limiting its applicability in conditions like chronic AF. Compared to β-blockers, which remain the first-line treatment for conditions such as heart failure and angina, ivabradine serves primarily as an adjunct or alternative in patients who cannot tolerate β-blockers. Additionally, its benefits in heart failure, such as reducing hospitalizations, are more pronounced in specific subgroups, such as those with a heart rate >70 bpm, which has led to debates about the generalizability of trial findings. Visual disturbances (phosphenes) and bradycardia also contribute to adherence challenges, while its off-label use in conditions like inappropriate sinus tachycardia sparks ongoing debates about safety and efficacy. Despite these controversies, ivabradine remains a useful option in carefully selected patients, emphasizing the importance of personalized medicine.
Conclusion and Future Prospects
Ivabradine is a medication that can be beneficial in certain cardiac disorders, particularly when heart rate reduction is indicated. However, its use should be carefully monitored by a healthcare provider, and it may not be suitable for all patients, depending on their specific medical history and conditions. The dosage of Ivabradine can vary depending on the specific condition. Ivabradine has shown effectiveness in reducing angina symptoms and improving exercise tolerance in angina patients. Common side effects of Ivabradine may include visual disturbances (phosphenes), bradycardia (slow heart rate), and AF.
Currently, Ivabradine is approved for use in patients with chronic stable angina and heart failure. Its ability to reduce heart rate without affecting blood pressure makes it an attractive option for patients who cannot tolerate traditional β-blockers. Current research and clinical trials suggest that ivabradine may have broader applications in cardiovascular medicine. Additionally, its potential to improve exercise tolerance, reduce symptoms, and enhance the quality of life in heart failure patients offers hope for better outcomes in the management of cardiac disease conditions. Its ability to reduce heart rate without impacting blood pressure makes it a valuable tool for addressing a range of cardiovascular issues, and promising studies using omics, bioinformatics, and NGS for personalized medicine trials are likely to unveil more potential applications.
As research continues, ivabradine could become an integral part of the evolving landscape of cardiovascular disease management, offering innovative approaches to enhancing patient care and outcomes. Currently, Ivabradine remains primarily indicated for chronic stable angina and heart failure, with ongoing studies exploring additional potential uses.
Declaration of Conflicting Interests The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The authors received no financial support for the research, authorship, and/or publication of this article.
- 1. Andrikopoulos G, Dasopoulou C, Sakellariou D, . Ivabradine: a selective If current inhibitor in the treatment of stable angina. Recent Pat Cardiovasc Drug Discov 2006;1(3):277–282. doi: 10.2174/157489006778777052
- 2. Henri C, O’Meara E, De Denus S, Elzir L, Tardif JC. Ivabradine for the treatment of chronic heart failure. Expert Rev Cardiovasc Ther 2016;14(5):553–561. doi: 10.1586/14779072.2016.1165092
- 3. Benstoem C, Kalvelage C, Breuer T, . Ivabradine as adjuvant treatment for chronic heart failure. Cochrane Database Syst Rev 2020;11(11):CD013004. doi: 10.1002/14651858.CD013004.pub2
- 4. Koruth JS, Lala A, Pinney S, Reddy VY, Dukkipati SR. The clinical use of ivabradine. J Am Coll Cardiol 2017;70(14):1777–1784. doi: 10.1016/j.jacc.2017.08.038
- 5. Scicchitano P, Carbonara S, Ricci G, . HCN channels and heart rate. Molecules 2012;17(4):4225–4235. doi:10.3390/molecules17044225
- 6. Scicchitano P, Cortese F, Ricci G, . Ivabradine, coronary artery disease, and heart failure: beyond rhythm control. Drug Des Devel Ther 2014;8:689–700. doi: 10.2147/DDDT.S60591
- 7. Tse S, Mazzola N. Ivabradine (Corlanor) for heart failure: the first selective and specific IF inhibitor. Pharm Ther. 2015;40(12):810.
- 8. Ide T, Ohtani K, Higo T, Tanaka M, Kawasaki Y, Tsutsui H. Ivabradine for the treatment of cardiovascular diseases. Circ J 2019;83(2):252–260. doi: 10.1253/circj.CJ-18-1184
- 9. Di YM, Li CG, Xue CC, Zhou SF. Clinical drugs that interact with St. John’s wort and implication in drug development. Curr Pharm Des 2008;14(17):1723–1742. doi: 10.2174/138161208784746798
- 10. Sulfi S, Timmis A. Ivabradine—the first selective sinus node if channel inhibitor in the treatment of stable angina. Int J Clin Pract. 2006;60(2):222–228.
- 11. Gordan R, Gwathmey JK, Xie L-H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015;7(4):204.
- 12. Fox K, Ford I, Ferrari R. Ivabradine in stable coronary artery disease. New Engl J Med. 2014;371(25):2435.
- 13. Urbanek I, Kaczmarek K, Cygankiewicz I, Ptaszynski P. Risk-benefit assessment of ivabradine in the treatment of chronic heart failure. Drug Healthc Patient Saf 2014;6:47–54. doi: 10.2147/DHPS.S43275
- 14. Sathyamurthy I, Newale S. Ivabradine: evidence and current role in cardiovascular diseases and other emerging indications. Indian Heart J. 2018;70:S435–S441.
- 15. Volterrani M, Iellamo F. Complementary and synergic role of combined beta-blockers and ivabradine in patients with chronic heart failure and depressed systolic function: a new therapeutic option? Card Fail Rev. 2016;2(2):130.
- 16. Kaski JC, Gloekler S, Ferrari R, . Role of ivabradine in management of stable angina in patients with different clinical profiles. Open Heart. 2018;5(1):e000725. Published 2018 Mar 9. doi:10.1136/openhrt-2017-000725
- 17. Bouabdallaoui N, O’Meara E, Bernier V, . Beneficial effects of ivabradine in patients with heart failure, low ejection fraction, and heart rate above 77 b.p.m. ESC Heart Fail. 2019;6(6):1199–1207. doi:10.1002/ehf2.12513
- 18. Bryan Richard S, Huang B, Liu G, Yang Y, Luo S. Impact of ivabradine on the cardiac function of chronic heart failure reduced ejection fraction: meta-analysis of randomized controlled trials. Clin Cardiol 2021;44(4):463–471. doi: 10.1002/clc.23581
- 19. Qiu S, Shi S, Ping H, Zhou S, Wang H, Yang B. Efficacy of Ivabradine versus β-Blockers for Heart Rate Reduction during computed tomography coronary angiography: a meta-analysis of randomized controlled trials. Cardiology 2016;135(3):133–140. doi:10.1159/000447236
- 20. Davis K, Dietrich E. STEPS: ivabradine (Corlanor) for heart failure. Am Fam Phys. 2016;93(8):682.
- 21. Saha M, Marber MS. If at first you don’t succeed try … a new target in the treatment of angina. Eur Heart J. 2005;26(23):2482–2483.
- 22. Heidenreich PA, Bozkurt B, Aguilar D, . AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145(18):e876–e894. doi: 10.1161/CIR.0000000000001062
- 23. Das D, Savarese G, Dahlström U, . Ivabradine in heart failure: the representativeness of SHIFT (Systolic Heart Failure Treatment With the IF Inhibitor Ivabradine Trial) in a broad population of patients with chronic heart failure. Circ Heart Fail 2017;10(9):e004112. doi: 10.1161/CIRCHEARTFAILURE.117.004112
- 24. Badu-Boateng C, Jennings R, Hammersley D. The therapeutic role of ivabradine in heart failure. Ther Adv Chronic Dis. 2018;9(11):199–207.
- 25. Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham study. Am Heart J 1987;113(6):1489–1494. doi: 10.1016/0002-8703(87)90666-1
- 26. Fox K, Borer JS, Camm AJ, . Resting heart rate in cardiovascular disease. J Am Coll Cardiol 2007;50(9):823–830. doi: 10.1016/j.jacc.2007.04.079
- 27. Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol 1995;26(5):1257–1263. doi: 10.1016/0735-1097(95)00332-0
- 28. Custodis F, Reil JC, Laufs U, Böhm M. Heart rate: a global target for cardiovascular disease and therapy along the cardiovascular disease continuum. J Cardiol 2013;62(3):183–187. doi: 10.1016/j.jjcc.2013.02.018
- 29. Bui AL, Grau-Sepulveda MV, Hernandez AF, . Admission heart rate and in-hospital outcomes in patients hospitalized for heart failure in sinus rhythm and in atrial fibrillation. Am Heart J 2013;165(4):567–574.e6. doi: 10.1016/j.ahj.2013.01.007
- 30. Lechat P, Hulot JS, Escolano S, . Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial. Circulation 2001;103(10):1428–1433. doi: 10.1161/01.cir.103.10.1428
- 31. McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med 2009;150(11):784–794. doi: 10.7326/0003-4819-150-11-200906020-00006
- 32. Skalidis EI, Hamilos MI, Chlouverakis G, Zacharis EA, Vardas PE. Ivabradine improves coronary flow reserve in patients with stable coronary artery disease. Atherosclerosis 2011;215(1):160–165. doi:10.1016/j.atherosclerosis.2010.11.035
- 33. Swedberg K, Komajda M, Böhm M, . Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study [published correction appears in Lancet. 2010 Dec 11;376(9757):1988. Lajnscak, M [corrected to Lainscak, M]; Rabanedo, I Roldan [corrected to Rabadán, I Roldan]; Leva, M [corrected to Ieva, M]]. Lancet 2010;376(9744):875–885. doi:10.1016/S0140-6736(10)61198-1
- 34. Fox K, Ford I, Steg PG, Tendera M, Ferrari R. BEAUTIFUL Investigators. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372(9641):807–816. doi:10.1016/S0140-6736(08)61170-8
- 35. Fox K, Ford I, Steg PG, . Relationship between ivabradine treatment and cardiovascular outcomes in patients with stable coronary artery disease and left ventricular systolic dysfunction with limiting angina: a subgroup analysis of the randomized, controlled BEAUTIFUL trial. Eur Heart J 2009;30(19):2337–2345. doi:10.1093/eurheartj/ehp358
- 36. Ponikowski P, Voors AA, Anker SD, . Wytyczne ESC dotyczące diagnostyki i leczenia ostrej i przewlekłej niewydolności serca w 2016 roku [2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure]. Kardiol Pol 2016;74(10):1037–1147. doi: 10.5603/KP.2016.0141
- 37. Abdelnabi M, Ahmed A, Almaghraby A, Saleh Y, Badran H. Ivabradine and AF: coincidence, correlation or a new treatment?. Arrhythm Electrophysiol Rev 2020;8(4):300–303. doi: 10.15420/aer.2019.30.2
- 38. Dimza M, Kurup V, Canha C, . Pharmacological therapy optimization for heart failure: a practical guide for the internist. Am J Med 2023;136(8):745–752. doi: 10.1016/j.amjmed.2023.04.033
- 39. Martin RI, Pogoryelova O, Koref MS, Bourke JP, Teare MD, Keavney BD. Atrial fibrillation associated with ivabradine treatment: meta-analysis of randomised controlled trials. Heart 2014;100(19):1506–1510. doi: 10.1136/heartjnl-2014-305482
- 40. Tanboğa İH, Topçu S, Aksakal E, . The risk of atrial fibrillation with ivabradine treatment: a meta-analysis with trial sequential analysis of more than 40000 patients. Clin Cardiol 2016;39(10):615–620. doi: 10.1002/clc.22578
- 41. Giavarini A, de Silva R. The role of ivabradine in the management of angina pectoris. Cardiovasc Drugs Ther. 2016;30:407–417.
- 42. Tardif JC, Ford I, Tendera M, Bourassa MG, Fox K. INITIATIVE Investigators. Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J 2005;26(23):2529–2536. doi: 10.1093/eurheartj/ehi586
- 43. Ruzyllo W, Tendera M, Ford I, Fox KM. Antianginal efficacy and safety of ivabradine compared with amlodipine in patients with stable effort angina pectoris: a 3-month randomised, double-blind, multicentre, noninferiority trial. Drugs 2007;67(3):393–405. doi: 10.2165/00003495-200767030-00005
- 44. Hartmann C, Bosch NL, de Aragão Miguita L, Tierie E, Zytinski L, Baena CP. The effect of ivabradine therapy on heart failure patients with reduced ejection fraction: a systematic review and meta-analysis. Int J Clin Pharm 2018;40(6):1443–1453. doi: 10.1007/s11096-018-0715-8
- 45. López-Bescós L, Filipova S, Martos R. Long-term safety and efficacy of ivabradine in patients with chronic stable angina. Cardiology. 2007;108(4):387–396.
- 46. Werdan K, Perings S, Köster R, . Effectiveness of ivabradine treatment in different subpopulations with stable angina in clinical practice: a pooled analysis of observational studies. Cardiology 2016;135(3):141–150. doi: 10.1159/000447443
- 47. Kalvelage C, Stoppe C, Marx N, Marx G, Benstoem C. Ivabradine for the Therapy of Chronic Stable Angina Pectoris: a Systematic Review and Meta-Analysis. Korean Circ J 2020;50(9):773–786. doi: 10.4070/kcj.2020.0031
- 48. Gloekler S, Traupe T, Stoller M, . The effect of heart rate reduction by ivabradine on collateral function in patients with chronic stable coronary artery disease. Heart 2014;100(2):160–166. doi: 10.1136/heartjnl-2013-304880
- 49. Mangiacapra F, Colaiori I, Ricottini E, . Heart Rate reduction by IVabradine for improvement of ENDothELial function in patients with coronary artery disease: the RIVENDEL study. Clin Res Cardiol 2017;106(1):69–75. doi: 10.1007/s00392-016-1024-7
- 50. Koester R, Kaehler J, Ebelt H, Soeffker G, Werdan K, Meinertz T. Ivabradine in combination with beta-blocker therapy for the treatment of stable angina pectoris in every day clinical practice. Clin Res Cardiol 2010;99(10):665–672. doi: 10.1007/s00392-010-0172-4
- 51. Borer JS, Fox K, Jaillon P, Lerebours G. Ivabradine investigators group. Antianginal and antiischemic effects of ivabradine, an I(f) inhibitor, in stable angina: a randomized, double-blind, multicentered, placebo-controlled trial. Circulation 2003;107(6):817–823. doi: 10.1161/01.cir.0000048143.25023.87
- 52. Adel M, Mansour S, Sabri NA, Badary OA, Saleh MA. A clinical study evaluating the effect of ivabradine on inflammation in patients with non st-segment elevation acute coronary syndromes. Int J Pharm Sci Res 2016; 7(4):1441–1449. doi: 10.13040/IJPSR.0975
- 53. Steg P, Lopez-de-Sà E, Schiele F, . Safety of intravenous ivabradine in acute ST-segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention: a randomized, placebo-controlled, double-blind, pilot study. Eur Heart J Acute Cardiovasc Care 2013;2(3):270–279. doi: 10.1177/2048872613489305
- 54. Ageev FT, Makarova GV, Patrusheva IF, Orlova IaA. The efficacy and safety of the combination of β-blocker bisoprolol and if inhibitor I (f) ivabradine in patients with stable angina and chronic obstructive pulmonary disease. Kardiologiia. 2010;50(10):22–26.
- 55. Dillinger JG, Maher V, Vitale C, . Impact of ivabradine on central aortic blood pressure and myocardial perfusion in patients with stable coronary artery disease. Hypertension 2015;66(6):1138–1144. doi: 10.1161/HYPERTENSIONAHA.115.06091
- 56. Hohneck AL, Fries P, Ströder J, . Effects of heart rate reduction with ivabradine on vascular stiffness and endothelial function in chronic stable coronary artery disease. J Hypertens 2019;37(5):1023–1031. doi: 10.1097/HJH.0000000000001984
- 57. Su Y, Ma T, Wang Z, . Efficacy of early initiation of ivabradine treatment in patients with acute heart failure: rationale and design of SHIFT-AHF trial. ESC Heart Fail 2020;7(6):4465–4471. doi: 10.1002/ehf2.12997
- 58. Mullasari A. Efficacy and safety of ivabradine once-daily prolonged-release versus twice-daily immediate-release formulation in patients with stable chronic heart failure with systolic dysfunction: a randomized, double-blind, phase 3 non-inferiority (PROFICIENT) Study. Cardiol Ther. 2020;9:505–521.
- 59. Datta PK, . Effectiveness of enteral ivabradine for heart rate control in septic shock: a randomised controlled trial. Anaesth Intensive Care. 2021;49(5):366–378.
- 60. Ponikowski P, Voors AA, Anker SD, . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37(27):2129–2200.
- 61. Knuuti J, Wijns W, Saraste A, . 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477.
- 62. January CT, Wann LS, Calkins H, . 2019 AHA/ACC/HRS focused update on atrial fibrillation: management of patients with atrial fibrillation. Circulation. 2019;140(2):e125–e151.