INTRODUCTION
Kidney disease is quietly becoming a hidden global epidemic, growing at the third fastest rate among global causes of death, and is projected to become the fifth leading cause of death by 2040. Acute kidney injury (AKI) occurs in about 10%-15% of hospitalized patients and up to 50% in the intensive care unit. With its rapid onset and high mortality rate, AKI is an acute and critical condition of kidney disease.[] Chronic kidney disease (CKD) is a progressive condition characterized by changes in the structure and function of the kidneys due to a variety of causes. The global burden of CKD is enormous and growing: approximately 10% of the world’s adult population is affected by some form of CKD, resulting in 1.2 million deaths and 28.0 million life expectancy lost each year.[] Quercetin, a widely consumed flavonoid,[] has been reported to play an inhibitory role in AKI[,] and chronicrenal fibrosis.[,] It is now clear that the renal protective effects of quercetin are attributed to its antioxidant, anti-inflammatory, and anti-apoptotic properties, making it a potential therapeutic drug for various kidney-related diseases.[] Over the past decade, the renoprotective effect of quercetin on AKI has been studied in a wide range of experimental models, including ischemic AKI,[] cisplatin-induced AKI,[] and coronavirus disease 2019 (COVID-19) related AKI.[,] Mechanistic studies have shown that quercetin effectively attenuates AKI by inhibiting renal inflammation, ferroptosis, and cell apoptosis. Quercetin has also been reported to be renoprotective in a number of CKD cases, including ureteral obstruction,[] diabetic nephropathy,[,] cyclosporine A nephropathy,[] and ferric nitrilotriacetate nephropathy.[] The anti-inflammatory properties of quercetin are attributed to its ability to inhibit epithelial-to-mesenchymal transition (EMT), inflammation, and cellular senescence.[] However, quercetin has poor water solubility, low absorption, and limited bioavailability. In human studies, its oral bioavailability is about 24%, which is further reduced in rats by around 16%.[,] When taken in capsule form, the bioavailability in humans can be as low as 1%.[,] Despite these challenges, quercetin is well tolerated and safe. Human studies have shown that the use of doses up to 1000 mg/day does not cause adverse effects on blood parameters, liver and kidney function, hematology, or serum electrolytes, even when it is taken for several months.[,] Current advances in nanotechnology have resulted in the formulation of quercetin nanoparticles that have overcome their poor water solubility and improved their therapeutic efficacy on kidney disease.[]
QUERCETIN IS THE MAIN INGREDIENT IN MANY HERBS AND REMEDIES FOR TREATING KIDNEY DISEASE
According to traditional Chinese medicine (TCM) theory, the kidney is the foundation of innate nature and is the storage place of the body’s “innate essence”. The kidney is also the foundation of five organs and is the place where “vital energy”, the driving force of human life activities, is produced and stored. Thus, the kidney has many functional activities, such as storing essence, mastering growth, development, reproduction, mastering water, and holding the “qi”, Kidney diseases may occur due to the internalization of excessive pathogenic factors based on the deficiency of the “qi” and the imbalance of “the yin and yang”. Excessive pathogenic factors can be either exogenous or endogenous and mainly include retention of phlegm and morbid fluid, retention of dampness, and blood stasis. Therefore, the treatment of kidney diseases is based on tonifying the kidney and eliminating pathogenic factors, especially clearing “heat” (inflammation), draining dampness, purging turbidity, and activating blood (increasing blood circulation).
Quercetin is a main active ingredient in a variety of heat-clearing, detoxifying, and dampness-relieving herbs, such as Rhizoma Coptidis,[] Smilax glabra Roxb,[] Abelmoschus manihot (Linn.) Medicus (A. Manihot),[] and Lobeliae Chinensis Herba.[] It is also the main active ingredient in many kidney tonic herbs, such as Astragali Radix,[] Semen Cuscutae,[] and Cornus officinalis.[] In addition, quercetin is the main active component of a variety of TCM formulas for the clinical treatment of kidney disease. Liuwei Dihuanng pill (LWDH) is a popular Chinese medical prescription that has proven to be effective in the treatment of CKD. Xie et al. used Cytoscape to construct a drug-ingredient-target network to clarify the active ingredients of LWDH. The results showed that quercetin is the central component of LWDH.[] Quercetin is also an effective component of the Fufang Shenhua tablet, which is a TCM preparation with a long-term therapeutic effect on CKD.[] Again, quercetin is a main active ingredient in HuangZhi YiShen capsule, a Chinese patent herbal drug with therapeutic effects on diabetic kidney disease.[] Yishen Qingli Heluo Granule (YQHG) is a TCM compound for the clinical treatment of CKD, and quercetin is considered the main active compound of YQHG based on compound-target network interactions.[]
THERAPEUTIC EFFECTS AND MECHANISMS OF QUERCETIN ON AKI
AKI is a nephrotic syndrome characterized by a rapid decline in glomerular filtration, which may mostly be induced by sepsis, ischemia-reperfusion injury, nephrotoxic drugs, and even SARS-CoV-2 infection.[,] Quercetin may exert its therapeutic effects on AKI by inactivating pro-inflammatory macrophages and blocking cell death pathways [Figure 1]. A previous study reported that treatment with quercetin strikingly improved renal dysfunction and ameliorated tubular injury caused by lipopolysaccharide (LPS) in mice. Quercetin pretreatment obviously restrains LPS-triggered cell apoptosis and inflammation by upregulating Sirt1 while suppressing nuclear factor kappa-B (NF-κB) activation.[] Quercetin can inhibit ferroptosis by reducing the levels of activation transcription factor 3 (ATF3) and lipid reactive oxygen species and by increasing the levels of glutathione peroxidase 4, thereby ameliorating AKI induced by ischemia-reperfusion or folic acid.[] Quercetin can also inhibit renal ischemia-reperfusion injury by reducing macrophage infiltration and inducible nitric oxide synthase activity.[] Quercetin can significantly reduce the serum levels of creatinine, blood urea nitrogen, interleukin 1β (IL-1β), IL-6, and tumor necrosis factor-α in a cisplatin-induced AKI model.[] Further studies have also revealed that quercetin exerts its renal protective effects on AKI by suppressing the activation of pro-inflammatory macrophages through a Mincle-dependent mechanism.[] AKI is also common in critically ill COVID-19 patient.[] In SARS-CoV-2 N protein-induced AKI, treatment with quercetin significantly inhibits the release of a damage-associated molecular pattern molecule high-mobility group protein 1 and inactivates M1 pro-inflammatory macrophages while promoting reparative M2 macrophage responses by suppressing Mincle-Syk/NF-κB signaling in vivo and in vitro [Figure 1].[] Using network pharmacology and molecular docking, quercetin can interact with SARS-CoV-2 proteins to exert its potential therapeutic effect on COVID-19.[] This finding suggests that quercetin may directly interact with SARS-CoV-2 to protect against COVID-19 AKI. Indeed, quercetin may serve as a SARS-CoV-2 inhibitor by binding to the active sites of SARS-CoV-2 main protease 3CL and ACE2, thus suppressing the viral life cycle.[] Our other study demonstrated that treatment with quercetin can effectively inhibit SARS-CoV-2 N-induced AKI in diabetic db/db mice by blocking the binding of SARS-CoV-2 N protein to Smad3, thus inhibiting SARS-CoV-2 N-induced tubular epithelial cell (TEC) death through the Smad3-p16-dependent G1 cell-cycle arrest mechanism [Figure 1].[] Taken together, quercetin is a therapeutic agent for SARS-CoV-2 N protein-induced AKI in db/db mice and may inhibit AKI by switching M1 to M2 macrophage activation, which may be associated with the inactivation of Mincle signaling [Figure 1].[] These novel findings may well explain the efficacy and mechanisms of TCM-based therapies for COVID-19 patients.
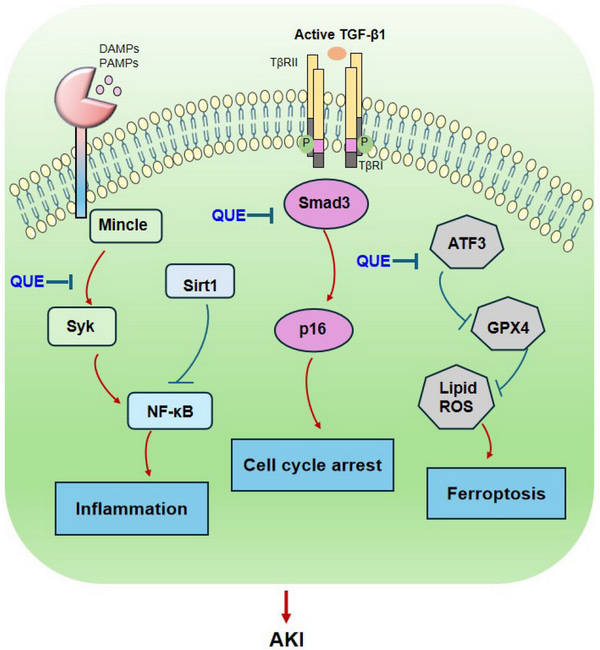
Figure 1.
Therapeutic mechanisms of quercetin in AKI. Quercetin exerts its therapeutic effect on AKI by inactivating pro-inflammatory macrophages and inhibiting renal inflammation through the Mincle-Sky/NFκB-dependent mechanisms and by blocking the cell death pathways, including TGF-β/Smad3-mediated G1 cell-cycle arrest and ATF3-dependent ferroptosis. Red arrows represent the pathogenic or positive regulatory mechanisms, while blue lines indicate the protective or negative regulatory pathways during AKI. AKI, acute kidney injury; TGF-β, transforming growth factor-β; ATF3, activation transcription factor 3; NF-κB, nuclear factor kappa B.
THERAPEUTIC EFFECTS AND MECHANISMS OF QUERCETIN ON RENAL FIBROSIS
Inhibition of EMT
The burden of CKD on health, society, and the economy is substantial worldwide. CKD is characterized by a gradual and irreversible decline in kidney function, indicated by a progressive reduction in the glomerular filtration rate (GFR).[] Renal fibrosis represents the end stage of CKD, encompassing conditions such as chronic glomerulonephritis, obstructive nephropathy, and diabetic nephropathy, and it is also a primary cause of progressive renal failure.[] Renal fibrosis is characterized by tissue sclerosis or scar formation resulting from excessive extracellular matrix (ECM) deposition during a chronic inflammatory response triggered by various stimuli.[] The ECM is a complex mixture of cellular and non-cellular components present in tissues and organs, forming a three-dimensional structural network. The ECM contains nearly a thousand proteins, with its main components being structural proteins, such as collagen, elastin, fibronectin, and laminin.[]
During fibrogenesis, EMT occurring in renal TEC can trigger the process of renal fibrosis. EMT is described as a biological process in which epithelial cells lose their characteristic phenotypes, such as E-cadherin expression, while expressing mesenchymal cell markers, such as α-smooth muscle actin.[] Transforming growth factor-β (TGF-β) signaling is a key pathway leading to progressive renal fibrosis.[] Increasing evidence demonstrates that quercetin exerts its anti-fibrotic effect on CKD by inhibiting the fibrotic pathways, such as TGF-β, Sonic Hedgehog, and mTOR signaling, while activating the SIRT1-PINK1/Parkin pathway [Figure 2]. It has been reported that treatment with quercetin can inhibit renal fibrosis in a mouse model of unilateral ureteral obstruction (UUO) by suppressing the EMT process via the TGF-β-dependent mechanism.[,] Quercetin can also effectively suppress EMT, ECM deposition, and cellular proliferation by inhibiting the hyperactive Hedgehog pathway or amphiregulin (ARGR)/epidermal growth factor receptor (EGFR) signaling.[,] In diabetic nephropathy, quercetin reveals its suppressive effect on EMT and renal fibrosis by inactivating the mTORC1/p70S6K pathway.[]
TGF-β is a key mediator in renal fibrosis.[] TGF-β, produced by various cells in an inactive form, is responsible for regulating ECM remodeling, which is a central pathway of fibrosis. Inhibition of TGF-β1 or its downstream signaling pathways substantially limits renal fibrosis in a wide range of disease models.[] Increasing evidence shows that quercetin exerts its anti-fibrotic effect on CKD by targeting TGF-β signaling [Figure 2]. A study showed that quercetin inhibits asymmetric dimethylarginine (ADMA)-induced TGF-β expression via the PERK-CHOP- and IRE1-JNK-mediated pathways, thereby preventing renal fibrosis.[] Quercetin also significantly inhibits TGF-β-induced EMT and ameliorates renal fibrosis in vivo and in vitro.[,] A recent study demonstrated that quercetin has anti-fibrotic effects on CKD patients by suppressing the TGF-β1-induced expression of miR-21.[] The anti-fibrotic effects of quercetin on CKD by targeting TGF-β signaling are also found in a variety of animal models, including glomerulosclerosis and diabetic nephropathy.[,] In addition, quercetin treatment can ameliorate diabetic renal damage by inhibiting CTGF expression.[]
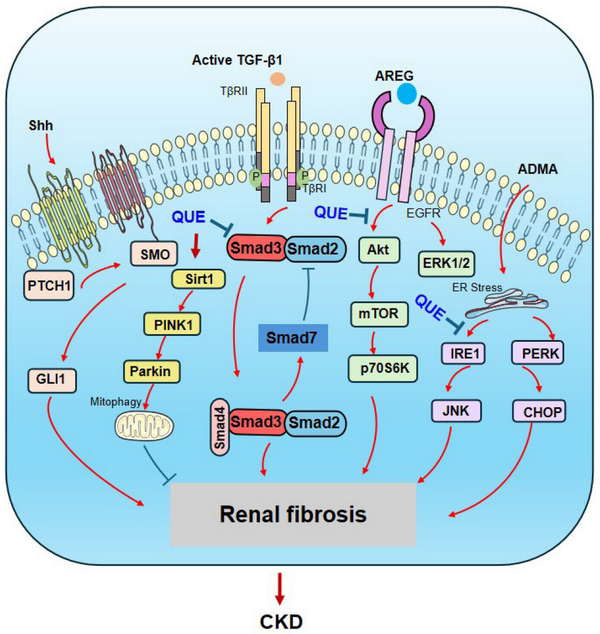
Figure 2.
Anti-fibrotic mechanisms of quercetin in renal fibrosis. Quercetin exerts its anti-fibrotic effect by inhibiting the fibrotic pathways, such as TGF-β/Smad, Sonic Hedgehog, ARGR/EGFR, and ADMA signaling, while activating the SIRT1-PINK1/Parkin pathway. Red arrows represent the pathogenic or positive regulatory pathways, while blue lines indicate the protective or negative regulatory pathways during renal fibrosis. EGFR, epidermal growth factor receptor; ADMA, asymmetric dimethylarginine; ARGR, amphiregulin.
Anti-inflammation
Inflammation occurs throughout the fibrotic process. Renal injuries promote the recruitment of inflammatory cells and the release of related cytokines, chemokines, and ROS.[,,] It has been reported that quercetin can attenuate tubulointerstitial inflammation and fibrosis in vivo and in vitro by inhibiting Hsp70- or Hsp90-mediated exosome release and intercellular communication.[] Quercetin can also inhibit M1 macrophage polarization and reduce the excessive accumulation of ECM to improve interstitial fibrosis in the kidneys via the NF-κB/IRF5 signaling pathway and the TGF-β/Smad pathway.[] In lupus nephritis, quercetin can ameliorate pathological deterioration by reversing IL-33-induced inflammation and fibrosis.[] In diabetic nephropathy, histopathological findings revealed that treatment with quercetin significantly reduced inflammatory cell infiltration and renal fibrosis by suppressing the expression of COX-2 protein in STZ-induced diabetic rats.[] Moreover, quercetin serves as a potent inhibitor of YY1. Its capacity to mitigate tissue inflammation is associated with the inhibition of the YY1-mediated IL-6/STAT-3 pathway, resulting in an improvement in renal fibrosis associated with diabetic nephropathy.[]
Anti-cell senescence
Increasing evidence shows that cellular senescence may trigger renal fibrosis in CKD patients.[] Quercetin can effectively attenuate cellular senescence and renal interstitial fibrosis by enhancing mitophagy through the activation of the SIRT1-PINK1/Parkin pathway in vivo and in vitro.[] Treatment with quercetin and Dasatinib alleviates renal senescence and reduces the progression of renal fibrosis in unilateral ischemia-reperfusion injury and multiple cisplatin-treatment murine models.[]
Inhibition of macrophage-myofibroblast transition (MMT)
TGF-β regulates renal fibrosis through canonical and noncanonical TGF-β signaling.[] MMT has recently been discovered as a newly identified pathway for ECM-producing myofibroblasts through the TGF-β/Smad3-dependent mechanism.[,] Further studies have demonstrated that Src and Pou4f1, which serve as direct Smad3 target genes, are essential for MMT-driven fibrotic diseases.[,] Treatment with clopidogrel, a P2Y12 inhibitor, is capable of inhibiting TGF-β/Smad3-mediated MMT and progressive renal fibrosis in vivo and in vitro.[] This finding suggests that quercetin may act as a Smad3 inhibitor capable of inhibiting renal fibrosis by blocking the MMT process, which may provide new insight into quercetin in the treatment of renal fibrosis.
CONCLUSION AND FUTURE PERSPECTIVES
Quercetin has anti-inflammatory, anti-fibrotic, anti-aging, antioxidant, and anti-apoptotic properties. Current advances in research on the efficacy of quercetin have provided insight into the mechanism of action of quercetin as an anti-inflammatory and anti-cell death agent in AKI. In terms of renal fibrosis, quercetin blocks fibrotic pathways and ameliorates inflammation and cellular senescence. TGF-β/Smad3 can mediate MMT and progressive renal fibrosis. Quercetin has been shown to be an inhibitor of Smad3 and may have a blocking effect on the MMT process, which needs to be confirmed by further studies. In addition, the mechanisms of the AKI-CKD transition have received increasing attention in recent years. However, whether quercetin can inhibit the AKI-CKD transition requires further investigation. Although numerous studies have confirmed that quercetin can effectively treat a variety of kidney diseases, the mechanism has not been well studied, and more research is needed to identify specific targets. Currently, the therapeutic effects of quercetin are mostly experimental, and clinical trials are required to validate these experimental notions. Furthermore, quercetin has poor water solubility and low oral bioavailability, which limit its clinical therapeutic effect. Thus, there is an urgent need to improve the properties and delivery systems of quercetin to make it a suitable therapeutic agent for clinical use.
Financial support and sponsorship
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 82304910, No. 82374384) and the Natural Science Foundation of Hubei Province, China (Grant No. 2024AFB925, No. 2022CFD021).
Author contributions
Wang WB: Conceptualization, Writing—Original draft preparation. Wu WJ: Conceptualization, Writing— Reviewing and Editing. All authors have reviewed and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interest.
Data availability statement
Not applicable.
How to cite: Wang WB, Wu WJ. Therapeutic Effects of Quercetin on Renal Fibrosis and Injury. Integr Med Nephrol Androl. 2025;12:e24-00051. doi: 10.1097/IMNA-D-24-00051
REFERENCES
1.
Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394:1949-1964.2.
Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. Lancet. 2021;398:786-802.3.
Manach C, Morand C, Crespy V, et al. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett. 1998;426:331-336.4.
Wang Y, Quan F, Cao Q, et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res. 2021;28:231-243.5.
Zeng YF, Li JY, Wei XY, et al. Preclinical evidence of reno-protective effect of quercetin on acute kidney injury: a meta-analysis of animal studies. Front Pharmacol. 2023;14:1310023.6.
Hu Q, Noor M, Wong YF, et al. In vitro anti-fibrotic activities of herbal compounds and herbs. Nephrol Dial Transplant. 2009;24:3033-3041.7.
Tan RZ, Wang C, Deng C, et al. Quercetin protects against cisplatin-induced acute kidney injury by inhibiting Mincle/Syk/NF-κB signaling maintained macrophage inflammation. Phytother Res. 2020;34:139-152.8.
Chen YQ, Chen HY, Tang QQ, et al. Protective effect of quercetin on kidney diseases: From chemistry to herbal medicines. Front Pharmacol. 2022;13:968226.9.
Singh D, Chander V, Chopra K. The effect of quercetin, a bioflavonoid on ischemia/reperfusion induced renal injury in rats. Arch Med Res. 2004;35:484-494.10.
Wu W, Wang W, Liang L, et al. SARS-CoV-2 N protein induced acute kidney injury in diabetic db/db mice is associated with a Mincle-dependent M1 macrophage activation. Front Immunol. 2023;14:1264447.11.
Wu W, Wang W, Liang L, et al. Treatment with quercetin inhibits SARS-CoV-2 N protein-induced acute kidney injury by blocking Smad3-dependent G1 cell-cycle arrest. Mol Ther. 2023;31:344-361.12.
Jones EA, Shahed A, Shoskes DA. Modulation of apoptotic and inflammatory genes by bioflavonoids and angiotensin II inhibition in ureteral obstruction. Urology. 2000;56(2):346-351.13.
Anjaneyulu M, Chopra K. Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2004;31(4):244-248.14.
Gao F, He X, Liang S, et al. Quercetin ameliorates podocyte injury via inhibition of oxidative stress and the TGF-β1/Smad pathway in DN rats. RSC Adv. 2018;8(70):35413-35421.15.
Mostafavi-Pour Z, Zal F, Monabati A, et al. Protective effects of a combination of quercetin and vitamin E against cyclosporine A-induced oxidative stress and hepatotoxicity in rats. Hepatol Res. 2008;38(4):385-392.16.
Singh D, Chander V, Chopra K. Quercetin, a bioflavonoid, attenuates ferric nitrilotriacetate-induced oxidative renal injury in rats. Drug Chem Toxicol. 2004;27(2):145-156.17.
Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol. 2019;15(3):144-158.18.
Nijveldt RJ, van Nood E, van Hoorn DE, et al. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74(4):418-425.19.
Hernandez-Ortega LD, Alcantar-Diaz BE, Ruiz-Corro LA, et al. Quercetin improves hepatic fibrosis reducing hepatic stellate cells and regulating pro-fibrogenic/anti-fibrogenic molecules balance. J Gastroenterol Hepatol. 2012;27(12):1865-1872.20.
Verma R, Kushwah L, Gohel D, et al. Evaluating the Ameliorative Potential of Quercetin against the Bleomycin-Induced Pulmonary Fibrosis in Wistar Rats. Pulm Med. 2013;921724.21.
Hollman PC, van Trijp JM, Mengelers MJ, et al. Bioavailability of the dietary antioxidant flavonol quercetin in man. Cancer Lett. 1997;114(1-2):139-140.22.
Khaled KA, El-Sayed YM, Al-Hadiya BM. Disposition of the flavonoid quercetin in rats after single intravenous and oral doses. Drug Dev Ind Pharm. 2003;29(4):397-403.23.
Gugler R, Leschik M, Dengler HJ. Disposition of quercetin in man after single oral and intravenous doses. Eur J Clin Pharmacol. 1975;9(2-3):229-234.24.
Martinez FJ. Double trouble: French colonialism in Morocco and the early history of the Pasteur Institutes of Tangier and Casablanca (1895-1932). Dynamis. 2016;36(2):317-339.25.
Heinz SA, Henson DA, Austin MD, et al. Quercetin supplementation and upper respiratory tract infection: A randomized community clinical trial. Pharmacol Res. 2010;62(3):237-242.26.
Shanely RA, Knab AM, Nieman DC, et al. Quercetin supplementation does not alter antioxidant status in humans. Free Radic Res. 2010;44(2):224-231.27.
Sanchez-Jaramillo EA, Gasca-Lozano LE, Vera-Cruz JM, et al. Nanoparticles formulation improves the antifibrogenic effect of quercetin on an adenine-induced model of chronic kidney disease. Int J Mol Sci. 2022;23(10):5392.28.
Zheng Y, Shi X, Hou J, et al. Integrating metabolomics and network pharmacology to explore Rhizoma Coptidis extracts against sepsis-associated acute kidney injury. J Chromatogr B. 2021;1164:122495.29.
Zhao M, Xu J, Qian D, et al. Identification of astilbin metabolites produced by human intestinal bacteria using UPLC-Q-TOF/MS. Biomed Chromatogr. 2014;28(7):1024-1029.30.
Cai H-D, Su S-L, Qian D-W, et al. Renal protective effect and action mechanism of Huangkui capsule and its main five flavonoids. J Ethnopharmacol. 2017;206:152-159.31.
Wang X, Xiang J, Huang G, et al. Inhibition of podocytes DPP4 activity is a potential mechanism of Lobeliae chinensis herba in treating diabetic kidney disease. Front Pharmacol. 2021;12:779652.32.
Wang Q, Zhen W, Lippi G, et al. The effect of Astragali radix-Radix angelica sinensis on acute kidney injury: a network pharmacology and molecular docking study. Transl Androl Urol. 2024;13(1):91-103.33.
Yang Y, Wei Q, An R, et al. Anti-osteoporosis effect of Semen cuscutae in ovariectomized mice through inhibition of bone resorption by osteoclasts. J Ethnopharmacol. 2022;285:114834.34.
Ma W, Wang K-J, Cheng CS, et al. Bioactive compounds from Cornus officinalis fruits and their effects on diabetic nephropathy. J Ethnopharmacol. 2014;153(3):840-845.35.
Xie X, Lou H, Shi Y, et al. A network pharmacological-based study of the mechanism of Liuwei Dihuang pill in the treatment of chronic kidney disease. Medicine. 2023;102(20):e35419.36.
Li R, Shi C, Wei C, et al. Fufang Shenhua tablet inhibits renal fibrosis by inhibiting PI3K/AKT. Phytomedicine. 2023;116:154873.37.
Zhou XF, Zhou WE, Liu WJ, et al. A network pharmacology approach to explore the mechanism of HuangZhi YiShen capsule for treatment of diabetic kidney disease. J Transl Intern Med. 2021;9(2):98-113.38.
Sun X, Huang Y, Zhu S, et al. Yishen Qingli Heluo Granule in the treatment of chronic kidney disease: network pharmacology analysis and experimental validation. Drug Des Devel Ther. 2022;16:769-787.39.
Song N, Thaiss F, Guo L. NFkappaB and kidney injury. Front Immunol. 2019;10:815.40.
Wang W, Chen J, Hu D, et al. SARS-CoV-2 N protein induces acute kidney injury via Smad3-dependent G1 cell cycle arrest mechanism. Adv Sci (Weinh). 2022;9(11):e2103248.41.
Lu S, Zhou S, Chen J, et al. Quercetin nanoparticle ameliorates lipopolysaccharide-triggered renal inflammatory impairment by regulation of Sirt1/NF-κB Pathway. J Biomed Nanotechnol. 2021;17(2):230-241.42.
Li ZL, Hu J, Li YL, et al. The effect of hyperoside on the functional recovery of the ischemic/reperfused isolated rat heart: potential involvement of the extracellular signal-regulated kinase 1/2 signaling pathway. Free Radic Biol Med. 2013;57:132-140.43.
Yang G, Liu Y, Hou J, et al. COVID-19 and kidney involvement: a systematic review. Integr Med Nephrol Androl. 2021;8(1):p4.44.
Zhong Y, He JC. COVID-19 acute kidney injury: current knowledge and barriers of research. Integr Med Nephrol Androl. 2021;8(1):p6.45.
Fu Y, Dong Z. Immune response in COVID-19-associated acute kidney injury and maladaptive kidney repair. Integr Med Nephrol Androl. 2023;10(1):e00022.46.
Gu YY, Zhang M, Cen H, et al. Quercetin as a potential treatment for COVID-19-induced acute kidney injury: based on network pharmacology and molecular docking study. PLoS One. 2021;16(1):e0245209.47.
Yuan Q, Tang B, Zhang C. Signaling pathways of chronic kidney diseases: implications for therapeutics. Signal Transduct Target Ther. 2022;7(1):182.48.
Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69(2):213-217.49.
Humphreys BD. Mechanisms of renal fibrosis. Annu Rev Physiol. 2018;80:309-326.50.
Song L, Zhang W, Tang SY, et al. Natural products in traditional Chinese medicine: molecular mechanisms and therapeutic targets of renal fibrosis and state-of-the-art drug delivery systems. Biomed Pharmacother. 2024;170:116039.51.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420-1428.52.
Lan HY. The Yin and Yang role of transforming growth factor-β in kidney disease. Integr Med Nephrol Androl. 2021;8(1):p1.53.
Wang Q, Wang F, Li X, Ma Z, Jiang D. Quercetin inhibits the amphiregulin/EGFR signaling-mediated renal tubular epithelial-mesenchymal transition and renal fibrosis in obstructive nephropathy. Phytother Res. 2023;37(1):111-123.54.
Liu X, Sun N, Mo N, et al. Quercetin inhibits kidney fibrosis and the epithelial to mesenchymal transition of the renal tubular system involving suppression of the Sonic Hedgehog signaling pathway. Food Funct. 2019;10(6):3782-3797.55.
Lu Q, Ji XJ, Zhou YX, et al. Quercetin inhibits the mTORC1/p70S6K signaling-mediated renal tubular epithelial-mesenchymal transition and renal fibrosis in diabetic nephropathy. Pharmacol Res. 2015;99:237-247.56.
Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325-338.57.
Guo W, Ding J, Zhang A, et al. The inhibitory effect of quercetin on asymmetric dimethylarginine-induced apoptosis is mediated by the endoplasmic reticulum stress pathway in glomerular endothelial cells. Int J Mol Sci. 2014;15(3):484-503.58.
Cao Y, Hu J, Sui J, et al. Quercetin is able to alleviate TGF-beta-induced fibrosis in renal tubular epithelial cells by suppressing miR-21. Exp Ther Med. 2018;16(3):2442-2448.59.
Elbe H, Vardi N, Esrefoglu M, et al. Amelioration of streptozotocin-induced diabetic nephropathy by melatonin, quercetin, and resveratrol in rats. Hum Exp Toxicol. 2015;34(1):100-113.60.
Lai PB, Zhang L, Yang LY. Quercetin ameliorates diabetic nephropathy by reducing the expressions of transforming growth factor-beta1 and connective tissue growth factor in streptozotocin-induced diabetic rats. Ren Fail. 2012;34(1):83-87.61.
Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7(12):684-696.62.
Chung AC, Lan HY. Chemokines in renal injury. J Am Soc Nephrol. 2011;22(5):802-809.63.
Yin D, Cao JY, Yang Y, et al. Quercetin alleviates tubulointerstitial inflammation by inhibiting exosomes-mediated crosstalk between tubular epithelial cells and macrophages. Inflamm Res. 2023;72(5):1051-1067.64.
Lu H, Wu L, Liu L, et al. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization. Biochem Pharmacol. 2018;154:203-212.65.
Chen HY, Chiang YF, Hong YH, et al. Quercetin ameliorates renal injury and pyroptosis in lupus nephritis through inhibiting IL-33/ST2 pathway in vitro and in vivo. Antioxidants (Basel). 2022;11(11):2238.66.
Rahmani AH, Alsahli MA, Khan AA, Almatroodi SA. Quercetin, a plant flavonol, attenuates diabetic complications, renal tissue damage, renal oxidative stress, and inflammation in streptozotocin-induced diabetic rats. Metabolites. 2023;13(1):130.67.
Yang T, Hu Y, Jiang W, et al. YY1 was indispensable for the alleviation of quercetin on diabetic nephropathy-associated tubulointerstitial inflammation. Phytomedicine. 2023;111:154659.68.
Docherty MH, Baird DP, Hughes J, Ferenbach DA. Cellular senescence and senotherapies in the kidney: current evidence and future directions. Front Pharmacol. 2020;11:755.69.
Liu T, Yang Q, Zhang X, et al. Quercetin alleviates kidney fibrosis by reducing renal tubular epithelial cell senescence through the SIRT1/PINK1/mitophagy axis. Life Sci. 2020;257:118116.70.
Li C, Shen Y, Huang L, Liu C, Wang J. Senolytic therapy ameliorates renal fibrosis post-acute kidney injury by alleviating renal senescence. FASEB J. 2021;35(9):e21229.71.
Gu YY, Liu XS, Huang XR, Yu XQ, Lan HY. TGF-β in renal fibrosis: triumphs and challenges. Future Med Chem. 2020;12(9):853-866.72.
Lan HY. Macrophage-myofibroblast transition in kidney disease. Integr Med Nephrol Androl. 2022;9(1):p12.73.
Nikolic-Paterson DJ, Wang S, Lan HY. Macrophages promote renal fibrosis through direct and indirect mechanisms. Kidney Int Suppl (2011). 2014;4(1):34-38.74.
Meng XM, Wang S, Huang XR, et al. Inflammatory macrophages can transdifferentiate into myofibroblasts during renal fibrosis. Cell Death Dis. 2016;7(12):e2495.75.
Tang PC, Chung JY, Xue VW, et al. Smad3 promotes cancer-associated fibroblasts generation via macrophage-myofibroblast transition. Adv Sci (Weinh). 2022;9(3):e2101235.76.
Tang PM, Zhou S, Li CJ, et al. The proto-oncogene tyrosine protein kinase Src is essential for macrophage-myofibroblast transition during renal scarring. Kidney Int. 2018;93(1):173-187.77.
Tang PM, Zhang YY, Xiao J, et al. Neural transcription factor Pou4f1 promotes renal fibrosis via macrophage-myofibroblast transition. Proc Natl Acad Sci USA. 2020;117(33):20741-20752.78.
Chen J, Tang Y, Zhong Y, et al. P2Y12 inhibitor clopidogrel inhibits renal fibrosis by blocking macrophage-to-myofibroblast transition. Mol Ther. 2022;30(6):3017-3033.
