IMPACT STATEMENT
Fentanyl use is increasing in the United States. We evaluated the performance of a simple rapid urine fentanyl test that would allow clinicians to test patients in a variety of point-of-care testing settings.
INTRODUCTION
Fentanyl is a synthetic opioid that is FDA approved to treat pain related to surgery and complex pain conditions (). Over the past decade, fentanyl that is illegally manufactured and distributed has been increasingly found in the drug supply (). People knowingly and unknowingly consume fentanyl and synthetic opioids when they are mixed with other drugs, such as heroin, cocaine, or counterfeit pills. Since fentanyl is 50 to 100 times more potent than morphine, using a drug that is contaminated or replaced with fentanyl can increase the risk of an overdose (). More than a million people have died since 1999 from drug overdose (). Deaths from overdose are on the rise, and 106 699 people overdosed in the US in 2021 (). Opioids, mainly synthetic opioids other than methadone, have been implicated in 75% of all drug overdose deaths in 2021 (, ). Fentanyl is one of the top 5 most frequently identified drugs from the National Forensic Laboratory Information System ().
With the increasing prevalence of fentanyl in cities across the United States, it is important to rapidly detect and treat patients who abuse street drugs or are accidentally exposed to fentanyl-contaminated drugs. While mass spectrometry remains the gold standard for confirming the presence of fentanyl and its metabolites in urine, mass spectrometry assays for fentanyl are not widely available in all clinical laboratories. Qualitative immunoassays that can run on automated chemistry analyzers can produce a presumptive result in about 10 minutes. However, samples from community clinics and healthcare facilities, like pharmacy clinics, must be transported to a clinical laboratory for analysis, delaying test result turnaround time. The recent availability of a simple 5-minute test strip that is “waived” under federal CLIA regulations can provide rapid test results while the patient is still being seen by a clinician. We propose to evaluate the performance of this test compared to mass spectrometry and 2 urine drug screen immunoassays.
MATERIALS AND METHODS
Urine specimens submitted to the laboratory for send-out fentanyl reference laboratory testing and specimens from high-risk patients flagged by clinicians were aliquoted and frozen at <−70°C for this study. All samples were deidentified after clinical analysis before aliquoting for this study. Samples were collected from inpatient medical units, emergency rooms, psychiatric hospitals, rehabilitation clinics, and other community clinic patients. The Vanderbilt Human Research Protections Program Institutional Review Board determined this study (IRB #220218) posed minimal risk to participants and met 45 CFR 46.104 (d) category (4)(ii) for Exempt Review.
Samples were thawed at room temperature, centrifuged, and analyzed by the Rapid Drug Test Device (RDTD) for fentanyl in urine (CLIA Waived, Inc.). The RDTD is a competitive binding, lateral flow immunochromatographic assay for the qualitative detection of fentanyl in urine. Test strips are visually read as the absence of a detection line in the test zone at 5 minutes after application of the sample. Comparison immunoassays were the ARK Fentanyl II assay (ARK Diagnostics Inc.) and Immunalysis SEFRIA Fentanyl assay (Immunalysis Corporation) conducted on the Abbott Alinity c analyzer (Abbott Laboratories). Mass spectrometry analysis was performed at ARUP Laboratories to quantitate the amount of fentanyl and its metabolite, norfentanyl, in each sample. Assay sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and efficiency were calculated using a 1 ng/mL positive cutoff for all tests. Assay-specific quality control was provided by each manufacturer and analyzed with every batch of deidentified patient samples.
RESULTS
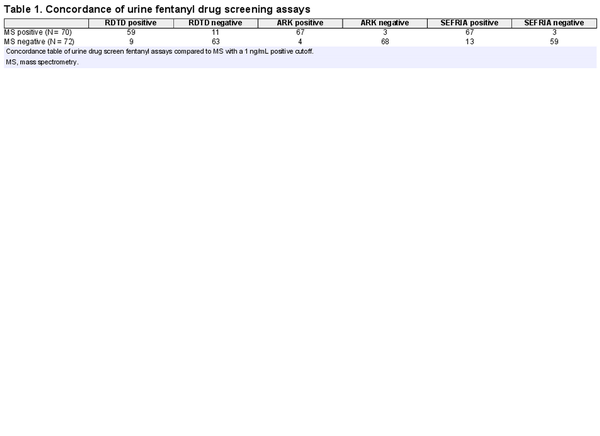
A total of 142 urine samples were analyzed with 70 positive and 72 negative samples by mass spectrometry. Positive and negative quality control precision (N = 10) was 3.0% CV and 9.6% CV for the ARK Fentanyl II assay and 10.2% CV and 15.9% CV for the Immunalysis SEFRIA assays, respectively. Qualitative controls were analyzed with each batch of samples on the RDTD to document reagent stability and appropriate result interpretation by staff.
The ARK Fentanyl II assay showed identical sensitivity to the Immunalysis SEFRIA assay but had higher specificity and overall efficiency for the samples from our patient population. A concordance table is shown in Table 1. The ARK Fentanyl II sensitivity = 95.7%, specificity = 94.4%, PPV = 94.4%, NPV = 95.8%, and efficiency = 95.1% compared to the Immunalysis SEFRIA Fentanyl sensitivity = 95.7%, specificity = 81.9%, PPV = 83.8%, NPV = 95.2%, and efficiency = 88.7%. The RDTD showed lower sensitivity = 84.3% with specificity = 87.5%, PPV = 86.8%, NPV = 85.1%, and efficiency = 85.9%.
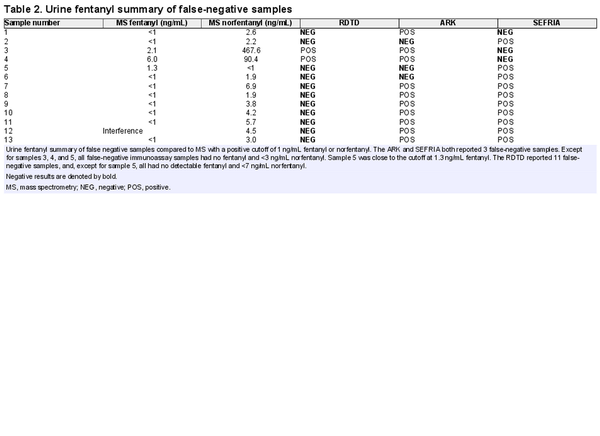
We noted differences in the number of false negatives between assays (Table 2). A total of 6 false negatives were reported by the ARK Fentanyl II and Immunalysis SEFRIA assays, 3 by each test. However, 4 out of 6 of these samples had combined fentanyl and norfentanyl concentrations of <3 ng/mL by mass spectrometry. Both immunoassays showed less cross-reactivity to norfentanyl, consistent with manufacturer package inserts (ARK Fentanyl II 7% cross-reactivity and Immunalysis SEFRIA 0.0001% cross-reactivity with norfentanyl). Specimens 3 and 4 would be expected to be positive with the Immunalysis SEFRIA method based on a cutoff of 1 ng/mL fentanyl mass spectrometry quantitation in those samples. The manufacturer package inserts and published literature suggest that the Immunalysis SEFRIA assay may show better sensitivity to fentanyl derivatives, compared to the ARK Fentanyl II method, but only fentanyl and norfentanyl were analyzed by mass spectrometry in this evaluation. The presence of fentanyl derivatives may explain the ARK Fentanyl II and Immunalysis SEFRIA result positivity for specimens 7 to 13, despite no detectable fentanyl and low levels <7 ng/mL of norfentanyl in these samples. The RDTD also demonstrated less cross-reactivity to norfentanyl with 11 false-negative samples that all had combined fentanyl and norfentanyl concentrations <7 ng/mL; however, the manufacturer package insert provides no cross-reactivity data for norfentanyl or other fentanyl derivatives. Concurrent medications prescribed to patients at the time of urine collection were reviewed for potential false positivity with the assays, but no consistent pattern of medication was noted for each assay’s false positives.
DISCUSSION
The availability of rapid drug screening tests can assist clinicians in monitoring compliance with prescribed medications as well as the intentional or unintentional use of illicit drugs (). These tests should be simple and waived under federal CLIA regulations, which allow for implementation in a wide variety of settings with minimal federal and state oversight. However, a quality management system and use of good laboratory practice are recommended to ensure the reliability of test results (, ). This includes regular analysis of positive and negative quality controls and may also include operator training, periodic competency evaluation, and enrollment in a proficiency testing program (). Evaluation of the analytical performance of rapid tests is important to determine a test’s ability to meet medical needs prior to introducing the test for patient care. While test evaluation optimally should be conducted by the staff who would ultimately be performing the testing on patient samples, this evaluation was limited to laboratory staff using leftover samples. However, samples were selected from the variety of clinical settings that routinely order urine fentanyl testing in our healthcare enterprise. Thus the elements of a quality management system will need to be emphasized before implementing fentanyl drug screening at our point-of-care testing sites.
The RDTD assay demonstrated acceptable performance in the detection of urine fentanyl in our patient populations. The RTDT showed >85% efficiency compared to the Immunalysis SEFRIA (88.7% efficiency) and ARK Fentanyl II (95.1% efficiency) methods. Although less sensitive and specific than the urine fentanyl immunoassays in a central laboratory, the ability of the RDTD to provide a rapid result at the point-of-care allows clinicians to counsel the patient while still in the clinic. The risk of a false negative and missing the presence of fentanyl presents less risk of harm to an alert patient in a clinic setting compared to an acute presentation in an emergency room where overdose patients will be treated symptomatically with naloxone while waiting for drug test results. Clinicians should be aware of the potential for false positives as well as false negatives, ensure their drug test methods and result turnaround times meet patient needs, and follow up with mass spectrometry confirmation as clinically indicated. This study emphasizes the need to assess the acceptability of drug screen analytic performance using samples from the institution’s patient populations.
References
- 1. Schug SA, Ting S. Fentanyl formulations in the management of pain: an update. Drugs2017;77:747–63.
- 2. Palamar JJ, Ciccarone D, Rutherford C, Keyes KM, Carr TH, Cottler LB. Trends in seizures of powders and pills containing illicit fentanyl in the United States, 2018 through 2021. Drug Alcohol Depend2022;234:109398.
- 3. National Institute on Drug Abuse. Fentanyl highlights. December 2021. https://nida.nih.gov/research-topics/fentanyl#references (Accessed May 2024).
- 4. Centers for Disease Control and Prevention. Wide-ranging online data for epidemiologic research (WONDER). Atlanta (GA): CDC, National Center for Health Statistics; 2022. http://wonder.cdc.gov (Accessed May 2024).
- 5. Centers for Disease Control and Prevention. Drug overdose deaths in the United States, 2002–2022. https://www.cdc.gov/nchs/products/databriefs/db491.htm (Accessed May 2024).
- 6. US Department of Justice, National Forensic Laboratory Information System. NFLIS-drug 2022 annual report. Springfield (VA): Drug Enforcement Administration, Diversion Control Division; 2023.
- 7. Wiencek JR, Colby JM, Nichols JH. Rapid assessment of drugs of abuse. In: Makowski GS, editor. Advances in clinical chemistry. Vol. 80. Cambridge (MA): Academic Press; 2017. p. 193–225.
- 8. Centers for Disease Control and Prevention. Ready, set, test! patient testing is important. Get the right results. Atlanta (GA): CDC; 2015. https://www.cdc.gov/clia/docs/waived-tests/ready-set-test-booklet.pdf (Accessed May 2024).
- 9. Abel G, Brugnara C, Das S, David K, Deaton-Mohney EB, Halverson K, et al Point-of-care testing: a “how-to” guide for the non-laboratorian. Washington (DC): Am Assoc Clin Chem2022. https://www.myadlm.org/science-and-research/poct-how-to-guide-for-non-laboratorians(Accessed May 2024).
- 10. Nichols JH, Alter D, Chen Y, Isbell TS, Jacobs E, Moore N, Shajani-Yi S. AACC guidance document on management of point-of-care testing. J Appl Lab Med2020;5:762–87.