Introduction
The availability of highly effective ART has transformed HIV from a terminal illness into a chronic condition requiring long-term clinical management. Thus, a major contemporary goal for the care of people with HIV is to ensure that they live long and healthy lives. Healthcare providers have advocated for the addition of good health-related quality of life to the UNAIDS treatment targets for people with HIV, including the management of comorbidities, mental health, self-perceived quality of life, fatigue and addressing HIV-associated stigma.
Maintaining optimal cardiometabolic health and body composition is important to achieving good health. Cardiometabolic health encompasses a spectrum of parameters (e.g. blood pressure, body weight, lipids) that influence an individual’s likelihood of developing cardiometabolic disease. Body composition comprises fat, lean mass, and bone quantity and quality, and affects multiple domains of metabolic health. Metabolic diseases are multifactorial and are linked to anthropometric changes, in particular ectopic fat accumulation., In clinical settings, weight and body composition assessment provides an opportunity to discuss metabolic health status.
Traditional and HIV-associated risk factors contribute to the higher burden of comorbidities in people with HIV, including those related to metabolic health and body composition. For example, a systematic review and meta-analysis (N = 8 848 569) reported that people with HIV had a 1.8-fold increased risk of heart failure compared with the general population. In a population-based matched cohort study in the UK, people with HIV had a 1.5-fold greater risk of cardiovascular disease (CVD) and a 1.3-fold greater risk of type 2 diabetes compared with people without HIV. An analysis of the TREAT Asia HIV Observational Database projected that incidence of CVD will double in people with HIV in Asia between 2019 and 2028.
Numerous studies have evaluated cardiometabolic health and body composition in people with HIV, and clinical guidelines have been issued. However, given the rapid evolution of data and development of ART, comprehensive practical guidance aimed at improving cardiometabolic outcomes in this population is needed. Here, on the basis of a literature review and professional experience, an international panel of experts developed consensus data statements and identified data gaps, culminating in a set of clinical recommendations to support the management of cardiometabolic health in people with HIV.
Methods
Targeted literature review
A targeted literature review was performed using PubMed to search for articles evaluating cardiometabolic health and body composition in people with HIV published between January 2016 and April 2022. This date range was selected to include contemporary data on metabolic health in people with HIV. Topics included weight change; metabolic syndrome and lipids; diabetes, insulin resistance, glucose and fatty liver disease; cardiac health and bone health. Articles were filtered by title and abstract text for relevance and study quality. Overall, 281 reports formed the basis of the targeted literature review (see Supplementary Methods (available as Supplementary data at JAC Online) for additional details). This targeted literature review was used to draft a set of data statements to initiate the consensus process.
Consensus process
An international panel of 16 HIV and cardiometabolic health experts agreed to engage in the consensus process (Table 1). The panel was selected based on publication records, geographic representation, and availability. A modified Delphi method composed of online surveys and virtual workshops was used to achieve consensus (Figure 1). A decision tree composed of three questions was used to evaluate consensus for each statement (Figure 2). Feedback from all members of the panel was evaluated and votes from each member were treated equally. To reduce the risk of bias, all survey responses were anonymous.
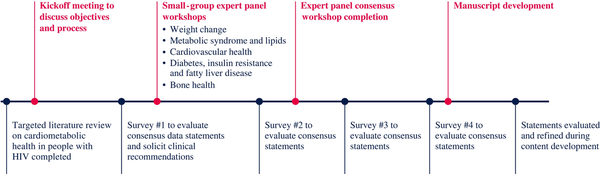
Figure 1
Timeline of the modified Delphi process to achieve consensus. Initially, a targeted literature review on cardiometabolic health in people with HIV was completed, which provided the foundation for initial draft consensus data statements. The expert panel evaluated the draft statements and proposed additional clinical recommendations in the first online survey and a series of small-group workshops. Revised consensus data statements and clinical recommendations were then evaluated in a second online survey and a subsequent workshop that included the complete expert panel. Two additional surveys (surveys 3 and 4) were conducted to evaluate the consensus statements. Statements were further evaluated and revised during content (poster and manuscript) development. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
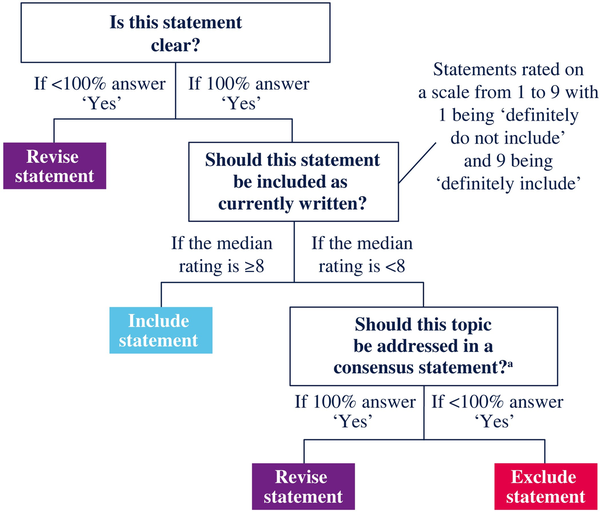
Figure 2
Decision tree for evaluating consensus statements. Question 1 assessed whether a statement was clear (understandable) and was rated as yes or no. If any expert responded no to Question 1, the statement was revised for clarity. Question 2 assessed whether a statement should be included as currently written and was rated on a scale from 1 to 9. A statement had to receive a median rating ≥8 to be included as currently written. A third question asked whether the topic should be addressed in a consensus statement. If any expert responded that the topic should not be addressed, then the statement was excluded. If 100% of experts voted to address the topic, the statement was revised based on their feedback and resurveyed. The experts could also provide their feedback and propose new statements in response to free-form questions in each survey. aIf a topic was evaluated by 100% of experts to address in a consensus statement, then the topic was no longer polled. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Initially, a set of consensus data statements and data gaps was generated based on the targeted literature review. In the first online survey, the expert panel evaluated the draft consensus statements, provided feedback for the data statements, and proposed additional data statements and a set of clinical recommendations. Subsequently, revised data statements and clinical recommendations were evaluated in a series of small-group virtual workshops addressing cardiometabolic health topics, each including four to five experts (Table S1). Additional data statements and clinical recommendations were also proposed during each workshop. The draft consensus data statements and clinical recommendations from the small-group workshops were subsequently evaluated in a second online survey. After survey 2, statements were reorganized and those addressing similar topics were grouped together where appropriate. The results of the second survey were then evaluated by the entire panel in a large virtual workshop. After the large workshop, the panel evaluated data statements and clinical recommendations in two additional online surveys. All online surveys were provided to all members of the panel. All statements that had achieved consensus through survey 4 were included. Statements that did not achieve consensus by survey 4 were revised on the basis of panel feedback and incorporated into the manuscript text but not considered to have achieved consensus. Additional feedback on the wording of statements was obtained during manuscript development. All members of the panel reviewed and approved the manuscript.
To ensure that the consensus statements were not limited by the parameters used for the initial targeted literature review, additional references were provided by the panel and additional literature searches were conducted to inform the evolving consensus statements.
Role of the study sponsor
ViiV Healthcare was the consensus sponsor. ViiV Healthcare identified the expert panel and hosted the workshop meetings. To reduce the risk of bias, a third party (MedThink SciCom, Raleigh, NC, USA) managed the surveys and guided the workshop discussions on the content of the statements. One member of the panel became an employee of ViiV Healthcare during the consensus process; their input was treated equally to that of the other members. The study sponsor reviewed the manuscript but did not have any influence on the consensus statements.
Consensus statements
Overview
Overall, 10 data statements, five data gaps and 14 clinical recommendations reached consensus. Four proposed statements did not achieve consensus, with each receiving a median rating of 7 (Table S2). Survey completion, workshop attendance, and statement evolution are presented in Tables S3–S5. Notably, the expert panel rejected all statements related to metabolic syndrome due to a concern that metabolic syndrome as a composite endpoint does not provide value beyond its individual components.
Consensus data statements and summary of evidence
Consensus data statements are listed in Table 2. In the data statements, the panel describes increased risk of cardiometabolic health concerns in people with HIV compared with the general population, known risk factors that contribute to cardiometabolic health outcomes, and the potential impact of ART on cardiometabolic health.
Weight change
Overweight and obesity are major causes of worsened health globally and have become more prevalent over time in both people with HIV and the general population
Summary of evidence: Globally, obesity has nearly tripled between 1975 and 2021. Obesity increases the risk of developing multiple life-limiting chronic conditions, and between 1990 and 2017, global deaths attributable to high BMI have more than doubled. Among people with HIV, obesity has increased at a rate comparable to that of the general population.,
Weight gain is expected in some people with HIV after antiretroviral therapy initiation, in part, due to a ‘return to health’
Summary of evidence: Untreated HIV is associated with weight loss, and after ART initiation, weight gain may occur due to a ‘return to health’ resulting from immune reconstitution and viral suppression. Greater increases in weight, lean mass and fat mass have been reported in people with HIV initiating ART with low CD4+ cell count or high viral load. Weight gain of 4.5 to 6.8 kg (10–15 pounds) after ART initiation in people with HIV with normal baseline BMI was associated with reduced mortality.
Weight gain is more pronounced after initiation of tenofovir alafenamide and second-generation integrase strand transfer inhibitors when compared with older antiretroviral agents (mainly tenofovir disoproxil fumarate and efavirenz)
Summary of evidence: In large cohort studies of treatment-naive people with HIV initiating ART, second-generation integrase strand transfer inhibitors (INSTIs) were associated with greater weight gain compared with cobicistat-boosted elvitegravir and NNRTIs. Similarly, studies of ART-naive people with HIV initiating treatment have reported greater weight gain with use of tenofovir alafenamide versus lamivudine, tenofovir disoproxil fumarate or abacavir.,, It was noted that efavirenz and tenofovir disoproxil fumarate have been independently associated with weight suppression, although this statement did not reach consensus.
Demographic characteristics including female sex and, in US studies, black race have been associated with more pronounced weight gain thus far
Summary of evidence: In large cohort studies of ART-naive people with HIV initiating treatment, greater weight gain was observed in women and, in the USA, black people.,,,
Cardiovascular health
Cardiovascular disease risk is higher in people with HIV compared with the general population
Summary of evidence: Results from meta-analyses support greater CVD risk in people with HIV compared with the general population., In large cohort studies, people with HIV had a higher risk of cardiovascular events including myocardial infarction, stroke, ischaemia, heart failure and sudden cardiac death compared with people without HIV.,
Traditional risk factors for cardiovascular disease apply to people with HIV
Summary of evidence: Traditional risk factors for CVD including smoking, excess adiposity, diabetes, dyslipidaemia, hypertension, and male sex apply to people with HIV and some, such as smoking, are more prevalent.,, Non-traditional risk factors for CVD, including alcohol use disorders, depression, and hepatitis C virus co-infection, are more common in people with HIV. More recently, hepatic steatosis was also associated with higher cardiovascular risk in individuals with BMI <25 kg/m2 (versus ≥25 kg/m2), those with BMI <30 kg/m2 (versus ≥30 kg/m2) and those aged <60 years (versus ≥60 years).,
Untreated HIV and some antiretroviral agents (abacavir, ritonavir-boosted darunavir and lopinavir) have been associated with increased cardiovascular disease risk
Summary of evidence: Untreated HIV is associated with increased risk of CVD.,, Untreated HIV can lead to CD4+ T-cell depletion, greater intestinal permeability, microbial translocation and cholesterol metabolism alterations, which can promote inflammation and atherogenesis. Low CD4+ cell count, particularly <200 cells/mm3, has been associated with increased risk of clinical CVD., Furthermore, in cohort studies that included large numbers of women, low CD4+ cell count was associated with atherosclerosis.,
Antiretroviral agents including abacavir, ritonavir-boosted darunavir and lopinavir have been associated with a modest increase in risk of CVD. Large cohort studies and systematic reviews and meta-analyses have reported an association between abacavir exposure and increased risk of CVD, including myocardial infarction. Risk of CVD from recent abacavir exposure ranged from an incidence rate ratio of 1.40 (95% CI, 1.20–1.64) to a relative risk of 1.71 (95% CI, 1.39–2.10)., A pooled analysis of people with HIV enrolled in clinical trials reported a lower incidence of cardiovascular events among individuals on abacavir (relative rate, 0.62 per 1000 person-years; 95% CI, 0.39–0.98). Cumulative exposure to ritonavir-boosted lopinavir was associated with higher risk of myocardial infarction in a study of the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) cohort (relative rate, 1.13; 95% CI, 1.05–1.21) and a systematic review and meta-analysis (relative risk, 1.19; 95% CI, 1.03–1.39)., Exposure to ritonavir-boosted darunavir was associated with increased incidence of cardiovascular events in the D:A:D cohort (adjusted incidence rate ratio, 1.59 per 5 years; 95% CI, 1.33–1.91).
Type 2 diabetes, insulin resistance, non-diabetic hyperglycaemia and metabolic-associated fatty liver disease
Short-term data suggest that the prevalence of type 2 diabetes is similar or greater in people with HIV compared with individuals without HIV
Summary of evidence: Large cohort studies have reported a higher prevalence of type 2 diabetes in people with versus without HIV. However, a large cohort study and a systematic review and meta-analysis reported no significant differences in incidence and odds of type 2 diabetes, respectively, in people with versus without HIV., Two large cohort studies reported numerically higher prevalence or incidence of type 2 diabetes in people with versus without HIV.,
Risk factors for insulin resistance and type 2 diabetes are multifactorial and include traditional and non-traditional factors
Summary of evidence: Several studies reported older age as a risk factor for type 2 diabetes in people with HIV. People with HIV of black race or with a family history of type 2 diabetes had a higher incidence of prediabetes or type 2 diabetes. Cardiometabolic health characteristics including increased waist circumference and higher BMI have been associated with increased risk of developing insulin resistance or type 2 diabetes in people with HIV., In the general population, unhealthy diet and lack of physical activity have been associated with increased risk of type 2 diabetes. Hepatitis C virus co-infection and HIV-related inflammation and lipodystrophy are non-traditional risk factors that can increase the risk of insulin resistance and diabetes in people with HIV.
Metabolic-associated fatty liver disease is an important component of metabolic health in people with HIV
Summary of evidence: The expert panel supported recently published diagnostic criteria for metabolic-associated fatty liver disease (MAFLD),, which are evidence of hepatic steatosis plus any of the following: overweight or obesity, type 2 diabetes or metabolic dysregulation. MAFLD is caused by systemic metabolic dysfunction that affects the liver downstream. Although data are currently limited, approximately one-third of people with HIV met criteria for MAFLD in two studies.,
Male sex, physical inactivity, higher body mass index and type 2 diabetes have been identified as being associated with higher likelihood of hepatic steatosis and older age with higher likelihood of hepatic fibrosis
Summary of evidence: Identified risk factors associated with a higher likelihood of hepatic steatosis include physical inactivity, type 2 diabetes, and male sex. Across five studies, higher BMI was associated with increased likelihood of hepatic steatosis, including in one study that enrolled only women with HIV.,, Three large cohort studies reported an association between older age and higher likelihood of hepatic fibrosis.,,
Bone health
Abnormal bone health is a potential concern for all people with HIV: compared with individuals without HIV, people with HIV have greater bone loss as reflected by decreased bone mineral density and increased risk of fractures
Summary of evidence: Compared with people without HIV, people with HIV have a greater risk of bone loss, including decreased bone mineral density (BMD), an important risk factor for fractures, and increased fracture risk. A meta-analysis reported that people with HIV had a higher prevalence and incidence of fractures compared with people without HIV. Another meta-analysis reported that people with HIV had higher odds of developing osteopenia or osteoporosis at the lumbar spine and hip compared with people without HIV. Compared with women without HIV, women with HIV had lower BMD in a cross-sectional study and greater bone loss in a prospective study.
Identified risk factors associated with reduced bone mineral density in people with HIV are traditional and HIV-specific
Summary of evidence: Traditional risk factors for reduced BMD and fractures apply to people with HIV and include demographic characteristics such as older age, and female sex,,,, vitamin D deficiency,,, and hypogonadal states (e.g. lower testosterone levels in men,, and menopause in women,,). Notably, one study reported greater risk of BMD loss in people with versus without HIV who transitioned from pre- to post-menopause. Modifiable risk factors associated with lower BMD include tobacco use,,, and heavy alcohol consumption., Additional factors or conditions associated with reduced BMD and/or increased fracture risk in people with HIV and the general population include adrenal insufficiency, haemophilia, gastrointestinal malabsorption, glucocorticoid use, emphysema, opiate use, dietary calcium deficiency, primary hyperparathyroidism, Cushing’s syndrome, renal phosphate wasting, idiopathic hypercalciuria, celiac sprue, multiple myeloma and mastocytosis.,,
HIV-related parameters and co-infections contribute to lower BMD in people with HIV. Lower nadir CD4+ cell count is associated with decreased BMD.,, Chronic hepatitis C virus co-infection has been associated with reduced BMD and increased risk of fractures.,,,
Initiating antiretroviral therapy is associated with decreases in bone mineral density regardless of antiretroviral therapy type
Summary of evidence: A meta-analysis reported that ART-experienced versus ART-naive people with HIV had higher odds of osteopenia and osteoporosis at the lumbar spine and total hip. In the START study, immediate versus deferred initiation of PI- or NNRTI-based ART (83.7% on tenofovir disoproxil fumarate) was associated with decreases in BMD. Other studies reported lower trabecular bone score in ART-experienced versus ART-naive people with HIV and an association between longer ART duration and lower BMD.
Across antiretroviral agents, studies have generally reported a decrease in BMD after ART initiation. People with HIV naive to ART initiating tenofovir disoproxil fumarate and individuals without HIV using tenofovir disoproxil fumarate for pre-exposure prophylaxis (PrEP) had decreases in BMD.,,,, Initiation of NNRTIs or boosted PIs in ART-naive people with HIV has been associated with decreased BMD. Initiation of second-generation INSTIs in ART-naive people with HIV has been associated with significantly decreased BMD, numerically decreased BMD or numerically increased BMD depending on study and site investigated (e.g. hip, spine or femoral neck).,
Vitamin D supplementation attenuates decreases in bone mineral density after antiretroviral therapy initiation
Summary of evidence: A systematic review and meta-analysis of people with and without HIV using tenofovir disoproxil fumarate as part of ART or PrEP, respectively, reported that vitamin D supplementation was correlated with improved BMD. A randomized, double-blind, placebo-controlled study assessing vitamin D and calcium supplementation in ART-naive people with HIV initiating efavirenz/emtricitabine/tenofovir disoproxil fumarate reported smaller declines in total hip and lumbar spine BMD in participants receiving high-dose vitamin D and calcium. An analysis of people with HIV taking dolutegravir-based ART in the prospective observational SCOLTA Project found that vitamin D supplementation was associated with improved BMD.
Compared with other antiretroviral agents, use of tenofovir disoproxil fumarate is associated with greater decreases in bone mineral density, which can be exacerbated by concomitant use of a boosted protease inhibitor
Summary of evidence: A systematic review and meta-analysis reported greater decreases in lumbar spine, total hip, and femoral neck BMD with tenofovir disoproxil fumarate-containing ART versus ART without tenofovir disoproxil fumarate. In a prospective study and a sub-study of a phase 3, randomized, open-label trial, treatment-naive people with HIV who initiated tenofovir disoproxil fumarate-containing ART had decreases in BMD at the hip and spine., In studies of people without HIV taking PrEP, use of tenofovir disoproxil fumarate-containing PrEP was associated with decreased BMD at the hip and lumbar spine and with a statistically significant increase in the incidence of osteoporosis or osteopenia.,,, Regimens containing pharmacokinetic enhancers can intensify the negative effect of ART, including tenofovir disoproxil fumarate, on bone health. A meta-analysis of clinical trial data found that when tenofovir disoproxil fumarate versus tenofovir alafenamide was administered as part of a boosted regimen, tenofovir disoproxil fumarate was associated with decreased BMD and higher risk of fractures; however, differences were attenuated in comparisons in which both agents were included in an unboosted regimen.
Switching from a regimen that includes tenofovir disoproxil fumarate to one that does not is associated with improved bone mineral density
Summary of evidence: Several studies reported improvements in hip and spine BMD after a switch from a regimen with tenofovir disoproxil fumarate to one without, although there is a lack of data suggesting improvement in bone quality.
Data gaps
The expert panel identified key cardiometabolic health data gaps (Table 3). For weight change, data gaps include the need for more studies investigating the causative effects mediating weight gain with tenofovir alafenamide and second-generation INSTIs, studies evaluating weight change in people with HIV using newer ART regimens compared with weight change in the general population and studies assessing the consequences of weight change on cardiometabolic health. With respect to cardiovascular health, the expert panel recommended additional research into the pathogenesis of atherosclerotic CVD in people with HIV and studies on risk stratification and characterizing and optimizing prevention and treatment of CVD in people with HIV. The panel identified the need for more studies evaluating the impact of contemporary ART regimens on insulin resistance in relation to weight gain and the effect on MAFLD. Regarding bone health, the panel recommended more studies investigating the impact of HIV and/or ART on bone health and studies in people with HIV investigating behavioural and biomedical interventions supportive of improved bone health in the general population, such as bone-loading physical activity, smoking cessation and dietary changes. The panel noted that additional research is needed investigating cardiometabolic health and body composition in important groups of people with HIV including cisgender women initiating hormone replacement therapy, transgender individuals initiating gender-affirming hormonal therapy and transgender men transitioning into menopause.
Clinical recommendations
The expert panel developed clinical recommendations to provide supportive advice to healthcare providers treating people with HIV (Table 4). The panel acknowledged that some recommendations may not be feasible to implement in all care settings and recommended that they should be implemented if at all possible and in alignment with local treatment guidelines. For example, some low- or middle-income countries may not have access to some assays or a network of healthcare providers who are able to provide comprehensive care. Nonetheless, the goal was to outline a gold standard for the management of cardiometabolic health and body composition in people with HIV.
General cardiometabolic care
The expert panel agreed that comprehensive care should be provided to people with HIV, including appropriate referral pathways and/or guidance to provide adequate care for clinically relevant weight changes. During interactions with patients, healthcare providers should discuss weight and lifestyle in a non-stigmatizing and empathetic manner while being mindful of the perceptions that people with HIV have regarding their weight. Supportive advice related to healthy eating, physical activity, stress management, substance use management and improving sleep quality should be provided. Healthcare providers should also seek to understand barriers to healthy eating and adequate physical activity.
Cardiometabolic health assessments
To monitor cardiometabolic health, the expert panel recommended that healthcare providers assess weight, BMI, waist-to-height or waist-to-hip ratio, blood pressure and fasting lipids and glucose (if possible) at the first visit, annually and after ART initiation or switch. They also recommended assessing and discussing alcohol and substance use and smoking.
The panel underscored the importance of MAFLD as a cardiometabolic health concern in people with HIV. Elevated alanine transaminase levels may suggest MAFLD, especially among individuals with type 2 diabetes or visceral adiposity. Elastography can aid in further evaluating liver health, including assessing fibrosis and steatosis, if available.
The panel stressed that fragility and sarcopenia are concerns in adults ageing with HIV and recommended screening for sarcopenia using handgrip strength and walking speed. Sarcopenia is diagnosed by the documentation of low muscle mass and either low muscle strength or low physical performance., Specifically, individuals with walking speed <0.8 m/s and low handgrip strength should undergo muscle mass testing. Many factors contribute to sarcopenia, with lack of physical activity considered one of the most important. Cardiometabolic disorders including diabetes and obesity may also adversely affect skeletal muscle, potentially worsening sarcopenia.
ART and cardiometabolic health
The impact of ART on cardiometabolic health is one aspect that should be considered in the context of individualized treatment. Before initiating or switching ART, people with HIV should be counselled on the possibility of weight change. Although the statement did not achieve consensus, the expert panel noted that when initiating ART, in addition to traditional considerations including those related to HIV and drug–drug interactions, the choice of ART should account for cardiometabolic factors such as risk of cardiometabolic disorders and patient concerns, awareness and engagement regarding weight. Furthermore, the impact of ART on weight should be contextualized for each individual considering known parameters including ART history; HIV markers such as high viral load and low CD4+ cell count; family history of obesity and/or other cardiometabolic diseases; concurrent metabolic conditions; concomitant medications that may affect weight; substance use disorders; mental health disorders; socioeconomic, cultural and lifestyle parameters and food access. ART-naive people with HIV with low baseline CD4+ cell count and high baseline viral load may experience greater weight gain after ART initiation.,,
Understanding the potential for drug–drug interactions between antiretroviral agents and medications prescribed for the treatment or prevention of cardiometabolic-related disorders is critical for the optimal management of both HIV and cardiometabolic health. For example, statins, which decrease low-density-lipoprotein cholesterol, have the potential for drug–drug interactions when used with boosted antiretroviral agents and older NNRTIs. It is necessary to titrate statin dosing and monitor closely for side effects when co-prescribing statins with boosted antiretroviral agents. Insufficient lipid control with co-administration of PIs and statins has been reported in people with HIV. Antidiabetic glucagon-like peptide 1 (GLP-1) receptor agonists (RAs) decrease HbA1c and weight, including in people with HIV., Because they inhibit gastric secretion, GLP-1 RAs have the potential to reduce ART absorption. Thus, monitoring of viral load after initiation of GLP-1 RAs is important. For details on drug–drug interactions with therapies for metabolic disorders, please see the review by Gutierrez et al. Drug–drug interactions with antiretroviral agents can also be queried using the University of Liverpool HIV drug interaction checker (https://www.hiv-druginteractions.org/).
Cardiovascular health
Considering the increased risk in people with HIV, cardiovascular health should be assessed routinely and management individualized accordingly. Risk calculators for cardiovascular health in the general population are recommended for use in people with HIV; however, they tend to underestimate risk in this population.,
Although the statement did not reach consensus, the expert panel noted that there may be an elevated risk of CVD in groups of people with HIV not normally considered at high risk in the general population, including those who are younger with BMI <30 kg/m2,, transgender,, perimenopausal, have long-standing HIV or who have substance use disorders.
Results from the phase 3 randomized REPRIEVE study of pitavastatin calcium versus placebo in people with HIV on stable ART with low-to-moderate risk of atherosclerotic CVD were noted as important, although the study was published after the consensus process was completed. Pitavastatin calcium versus placebo was associated with reduced risk of major adverse cardiovascular events (hazard ratio, 0.65; 95% CI, 0.48–0.90; P = 0.002).
Bone health
The expert panel emphasized the importance of screening for osteoporosis in women with HIV who are post-menopausal and men with HIV aged >50 years given the increased risk of metabolic bone disease. Osteoporosis is asymptomatic until fractures occur. Bone health should be assessed in people with HIV using fracture risk assessment tools at a frequency recommended by available HIV guidelines. If available and feasible to use, dual-energy X-ray absorptiometry is the gold standard for evaluating bone health. As certain antiretroviral agents can negatively affect bone health, it is recommended that healthcare providers review the impact of ART on bone health and modify when appropriate.
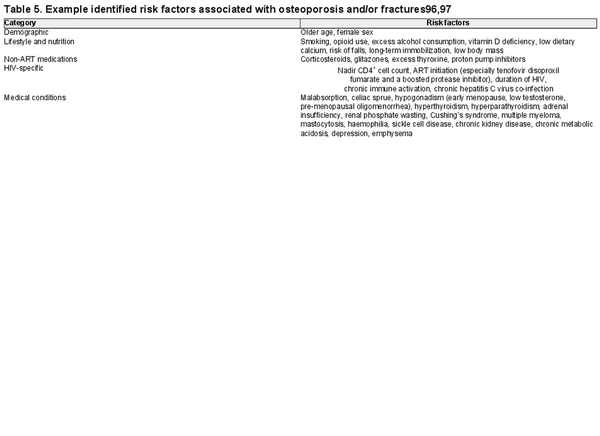
The expert panel noted that assessing risk factors for bone health is important, although this statement did not achieve consensus. Key risk factors for osteoporosis and/or fractures in people with HIV include demographics, HIV-specific factors, lifestyle characteristics and some medical conditions and medications (Table 5)., For a comprehensive list of risk factors for bone health in the general population, please see LeBoff et al.
Lifestyle interventions that can improve bone health are encouraged in all people with HIV, including smoking cessation, decreased alcohol consumption, weight-bearing and resistance physical activity, a balanced diet with adequate calcium intake and vitamin D supplementation. In people with HIV with decreased BMD or history of fracture, create an individual management plan, aiming to reduce pain and risk of falls, prevent disability and improve quality of life. In the event of fracture or established osteoporosis, refer to available guidelines for additional details on management.
Although the statement did not achieve consensus, the expert panel noted the importance of routinely discussing concerns about falls with individuals at risk of falls. Falls are a major cause of fractures, disability, hospitalization and injuries, including fatal injuries, in adults aged ≥65 years. It was remarked that assessing risk of falls is particularly important in people with HIV because of the increased risk of bone loss, accelerated process of ageing and impact of polypharmacy on fall risk.,
Discussion
Maintaining optimal cardiometabolic health and body composition are important to achieving good health and well-being in people with HIV. Although a large body of research has investigated cardiometabolic health and clinical guidelines have been developed, there remained a need for comprehensive practical guidance to support cardiometabolic health in people with HIV. Using a modified Delphi method, an international panel of HIV and cardiometabolic health experts drafted a set of consensus data statements, identified data gaps and developed clinical recommendations.
Professional experience supported by scientific evidence resulted in a set of consensus data statements that confirmed the increased risk of cardiometabolic health and body composition concerns in people with HIV compared with the general population, the role of traditional and HIV-specific risk factors in this increased risk, and the importance of choice of ART regimen. These statements provided a foundation for evidence-based clinical recommendations. Identified data gaps provide a guide for future research that will yield fruitful data supportive of improved cardiometabolic health and body composition in people with HIV.
The expert panel’s clinical recommendations emphasize the importance of providing comprehensive care to people with HIV as part of a multidisciplinary team; assessing cardiometabolic health at an appropriate frequency; considering the impact of ART, including ART adherence, on cardiometabolic health and providing care with the awareness that people with HIV have an increased risk of cardiometabolic health concerns. Given the impact of HIV-specific risk factors and ART regimen on cardiometabolic health and body composition, it is essential that healthcare providers consider these factors when providing care for people with HIV. Additionally, care of people with HIV should be individualized, considering unique patient characteristics and needs., People with HIV who are ageing have a heightened risk of non-communicable diseases and comorbidities (e.g. frailty); therefore, it is especially important that this group receives comprehensive and multidisciplinary care that incorporates cardiometabolic health and body composition. Healthcare providers are encouraged to be mindful of these recommendations and existing clinical guidelines to ensure appropriate cardiometabolic health management for people with HIV. Given the complex interplay between HIV, traditional risk factors, impact of ART regimen and cardiometabolic health and body composition, a multidisciplinary care model (e.g. working together with endocrinologists and cardiologists) is strongly encouraged for the care of people with HIV, where possible. Implementing these recommendations requires a flexible approach that considers context and available resources.
There are some limitations to acknowledge. Few experts from low- or middle-income countries were represented in the panel, and some of the clinical recommendations may not be feasible to implement outside high-income countries. Participation was <75% for two surveys and one of the small-group workshops; however, all members of the panel attended a consensus workshop to review workshop outputs, in addition to reviewing and approving the final manuscript. Although the consensus process was designed to reduce risk of bias from the study sponsor, some of the statements discuss antiretroviral agents developed by the study sponsor and its competitors.
Conclusions
The guidance proposed here highlights the importance of reshaping the model of care for people with HIV towards a holistic and comprehensive approach involving a multidisciplinary team of care providers. As new high-quality data emerge to address key data gaps and yield novel insights into cardiometabolic health and body composition in people with HIV, it is critical that healthcare providers remain informed of study findings and that clinical guidelines are shaped by emerging information. The clinical management of people with HIV remains an evolving process combining high-quality research, professional experience from a multidisciplinary team, and consideration of unique patient characteristics to maximize health and well-being.
Acknowledgements
Data included in this manuscript have previously been presented in part at the European Meeting on HIV & Hepatitis 2023; 7–9 June 2023; Rome, Italy; Poster 85.
References
- 1. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet2013; 382: 1525–33. https://doi.org/10.1016/S0140-6736(13)61809-7
- 2. World Health Organization. HIV and AIDS: Key Facts. https://www.who.int/news-room/fact-sheets/detail/hiv-aids.
- 3. Lazarus JV, Safreed-Harmon K, Barton SE, et al Beyond viral suppression of HIV—the new quality of life frontier. BMC Med2016; 14: 94. https://doi.org/10.1186/s12916-016-0640-4
- 4. Vincent GE, Jay SM, Sargent C, et al Improving cardiometabolic health with diet, physical activity, and breaking up sitting: what about sleep? Front Physiol 2017; 8: 865. https://doi.org/10.3389/fphys.2017.00865
- 5. Longo M, Zatterale F, Naderi J, et al Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci2019; 20: 2358. https://doi.org/10.3390/ijms20092358
- 6. Godfrey C, Bremer A, Alba D, et al Obesity and fat metabolism in human immunodeficiency virus-infected individuals: immunopathogenic mechanisms and clinical implications. J Infect Dis2019; 220: 420–31. https://doi.org/10.1093/infdis/jiz118
- 7. Martínez-Sanz J, Serrano-Villar S, Vivancos MJ, et al Management of comorbidities in treated HIV infection: a long way to go: HIV, comorbidities and aging. Int J Antimicrob Agents2022; 59: 106493. https://doi.org/10.1016/j.ijantimicag.2021.106493
- 8. Chen Y, Gao Y, Zhou Y, et al Human immunodeficiency virus infection and incident heart failure: a meta-analysis of prospective studies. J Acquir Immune Defic Syndr2021; 87: 741–9. https://doi.org/10.1097/QAI.0000000000002629
- 9. Gooden TE, Gardner M, Wang J, et al Incidence of cardiometabolic diseases in people with and without human immunodeficiency virus in the United Kingdom: a population-based matched cohort study. J Infect Dis2022; 225: 1348–56. https://doi.org/10.1093/infdis/jiab420
- 10. Bijker R, Kumarasamy N, Kiertiburanakul S, et al Cardiovascular disease incidence projections in the TREAT Asia HIV Observational Database (TAHOD). Antivir Ther2019; 24: 271–9. https://doi.org/10.3851/IMP3298
- 11. Hsu C-C, Sandford BA. The Delphi technique: making sense of consensus. Pract Assess Res Eval2007; 12: 10. https://doi.org/10.7275/PDZ9-TH90
- 12. World Health Organization. Obesity and Overweight: Key Facts. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 13. Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism2019; 92: 6–10. https://doi.org/10.1016/j.metabol.2018.09.005
- 14. Dai H, Alsalhe TA, Chalghaf N, et al The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: an analysis of the Global Burden of Disease Study. PLoS Med2020; 17: e1003198. https://doi.org/10.1371/journal.pmed.1003198
- 15. Koethe JR, Jenkins CA, Lau B, et al Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses2016; 32: 50–8. https://doi.org/10.1089/aid.2015.0147
- 16. Yuh B, Tate J, Butt AA, et al Weight change after antiretroviral therapy and mortality. Clin Infect Dis2015; 60: 1852–9. https://doi.org/10.1093/cid/civ192
- 17. Wood BR, Huhn GD. Excess weight gain with integrase inhibitors and tenofovir alafenamide: what is the mechanism and does it matter?Open Forum Infect Dis2021; 8: ofab542. https://doi.org/10.1093/ofid/ofab542
- 18. Lakey W, Yang L-Y, Yancy W, et al Short communication: from wasting to obesity: initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retroviruses2013; 29: 435–40. https://doi.org/10.1089/aid.2012.0234
- 19. Grant PM, Kitch D, McComsey GA, et al Long-term body composition changes in antiretroviral-treated HIV-infected individuals. AIDS2016; 30: 2805–13. https://doi.org/10.1097/QAD.0000000000001248
- 20. McComsey GA, Moser C, Currier J, et al Body composition changes after initiation of raltegravir or protease inhibitors: ACTG A5260s. Clin Infect Dis2016; 62: 853–62. https://doi.org/10.1093/cid/ciw017
- 21. Bourgi K, Rebeiro PF, Turner M, et al Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis2020; 70: 1267–74. https://doi.org/10.1093/cid/ciz407
- 22. Ruderman SA, Crane HM, Nance RM, et al Brief report: weight gain following ART initiation in ART-naïve people living with HIV in the current treatment era. J Acquir Immune Defic Syndr2021; 86: 339–43. https://doi.org/10.1097/QAI.0000000000002556
- 23. Venter WDF, Sokhela S, Simmons B, et al Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV2020; 7: e666–76. https://doi.org/10.1016/S2352-3018(20)30241-1
- 24. Bansi-Matharu L, Phillips A, Oprea C, et al Contemporary antiretrovirals and body-mass index: a prospective study of the RESPOND cohort consortium. Lancet HIV2021; 8: e711–22. https://doi.org/10.1016/S2352-3018(21)00163-6
- 25. Sax PE, Erlandson KM, Lake JE, et al Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis2020; 71: 1379–89. https://doi.org/10.1093/cid/ciz999
- 26. Bourgi K, Ofner S, Musick B, et al Weight gain among treatment-naïve persons with HIV receiving dolutegravir in Kenya. J Acquir Immune Defic Syndr2022; 91: 490–6. https://doi.org/10.1097/QAI.0000000000003087
- 27. Martínez-Sanz J, Blanco J-R, Muriel A, et al Weight changes after antiretroviral therapy initiation in CoRIS (Spain): a prospective multicentre cohort study. J Int AIDS Soc2021; 24: e25732. https://doi.org/10.1002/jia2.25732
- 28. Lake JE, Wu K, Bares SH, et al Risk factors for weight gain following switch to integrase inhibitor-based antiretroviral therapy. Clin Infect Dis2020; 71: e471–7. https://doi.org/10.1093/cid/ciaa177
- 29. Coelho LE, Jenkins CA, Shepherd BE, et al Weight gain post-ART in HIV+ Latinos/as differs in the USA, Haiti, and Latin America. Lancet Reg Health Am2022; 8: 100173. https://doi.org/10.1016/j.lana.2021.100173
- 30. Erqou S, Lodebo BT, Masri A, et al Cardiac dysfunction among people living with HIV: a systematic review and meta-analysis. JACC Heart Fail2019; 7: 98–108. https://doi.org/10.1016/j.jchf.2018.10.006
- 31. Gutierrez J, Albuquerque ALA, Falzon L. HIV infection as vascular risk: a systematic review of the literature and meta-analysis. PLoS ONE2017; 12: e0176686. https://doi.org/10.1371/journal.pone.0176686
- 32. Eyawo O, Brockman G, Goldsmith CH, et al Risk of myocardial infarction among people living with HIV: an updated systematic review and meta-analysis. BMJ Open2019; 9: e025874. https://doi.org/10.1136/bmjopen-2018-025874
- 33. Jones BI, Freedman A, Thomas MJ, et al Comorbid diseases and conditions in people with HIV in the UK. Curr Med Res Opin2022; 38: 277–85. https://doi.org/10.1080/03007995.2021.2003671
- 34. Puhr R, Petoumenos K, Huang R, et al Cardiovascular disease and diabetes in HIV-positive and HIV-negative gay and bisexual men over the age of 55 years in Australia: insights from the Australian Positive & Peers Longevity Evaluation Study. HIV Med2019; 20: 121–30. https://doi.org/10.1111/hiv.12689
- 35. Lai Y-J, Chen Y-Y, Huang H-H, et al Incidence of cardiovascular diseases in a nationwide HIV/AIDS patient cohort in Taiwan from 2000 to 2014. Epidemiol Infect2018; 146: 2066–71. https://doi.org/10.1017/S0950268818002339
- 36. Freiberg MS, Duncan MS, Alcorn C, et al HIV infection and the risk of World Health Organization-defined sudden cardiac death. J Am Heart Assoc2021; 10: e021268. https://doi.org/10.1161/JAHA.121.021268
- 37. Silverberg MJ, Leyden WA, Xu L, et al Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr2014; 65: 160–6. https://doi.org/10.1097/QAI.0000000000000009
- 38. Freiberg MS, Chang C-CH, Skanderson M, et al Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol2017; 2: 536–46. https://doi.org/10.1001/jamacardio.2017.0264
- 39. Feinstein MJ, Steverson AB, Ning H, et al Adjudicated heart failure in HIV-infected and uninfected men and women. J Am Heart Assoc2018; 7: e009985. https://doi.org/10.1161/JAHA.118.009985
- 40. So-Armah K, Benjamin LA, Bloomfield GS, et al HIV and cardiovascular disease. Lancet HIV2020; 7: e279–93. https://doi.org/10.1016/S2352-3018(20)30036-9
- 41. Tarr PE, Ledergerber B, Calmy A, et al Subclinical coronary artery disease in Swiss HIV-positive and HIV-negative persons. Eur Heart J2018; 39: 2147–54. https://doi.org/10.1093/eurheartj/ehy163
- 42. Heseltine T, Murray S, Ortega-Martorell S, et al Associations of hepatosteatosis with cardiovascular disease in HIV-positive and HIV-negative patients: the Liverpool HIV-Heart Project. J Acquir Immune Defic Syndr2021; 87: 1221–7. https://doi.org/10.1097/QAI.0000000000002721
- 43. Kazooba P, Kasamba I, Mayanja BN, et al Cardiometabolic risk among HIV-positive Ugandan adults: prevalence, predictors and effect of long-term antiretroviral therapy. Pan Afr Med J2017; 27: 40. https://doi.org/10.11604/pamj.2017.27.40.9840
- 44. Bailin SS, Gabriel CL, Wanjalla CN, et al Obesity and weight gain in persons with HIV. Curr HIV/AIDS Rep2020; 17: 138–50. https://doi.org/10.1007/s11904-020-00483-5
- 45. Hanna DB, Post WS, Deal JA, et al HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis2015; 61: 640–50. https://doi.org/10.1093/cid/civ325
- 46. Freiberg MS, Chang C-CH, Kuller LH, et al HIV infection and the risk of acute myocardial infarction. JAMA Intern Med2013; 173: 614–22. https://doi.org/10.1001/jamainternmed.2013.3728
- 47. Cervo A, Sebastiani G, Milic J, et al Dangerous liaisons: NAFLD and liver fibrosis increase cardiovascular risk in HIV. HIV Med2022; 23: 911–21. https://doi.org/10.1111/hiv.13274
- 48. Fedele F, Bruno N, Mancone M. Cardiovascular risk factors and HIV disease. AIDS Rev2011; 13: 119–29.
- 49. El-Sadr WM, Lundgren JD, Neaton JD, et al CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med2006; 355: 2283–96. https://doi.org/10.1056/NEJMoa062360
- 50. Kaplan RC, Kingsley LA, Gange SJ, et al Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS2008; 22: 1615–24. https://doi.org/10.1097/QAD.0b013e328300581d
- 51. Dorjee K, Choden T, Baxi SM, et al Risk of cardiovascular disease associated with exposure to abacavir among individuals with HIV: a systematic review and meta-analyses of results from 17 epidemiologic studies. Int J Antimicrob Agents2018; 52: 541–53. https://doi.org/10.1016/j.ijantimicag.2018.07.010
- 52. Worm SW, Sabin C, Weber R, et al Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis2010; 201: 318–30. https://doi.org/10.1086/649897
- 53. Jaschinski N, Greenberg L, Neesgaard B, et al Recent abacavir use and incident cardiovascular disease in contemporary-treated people with HIV. AIDS2023; 37: 467–75. https://doi.org/10.1097/QAD.0000000000003373
- 54. Nan C, Shaefer M, Urbaityte R, et al Abacavir use and risk for myocardial infarction and cardiovascular events: pooled analysis of data from clinical trials. Open Forum Infect Dis2018; 5: ofy086. https://doi.org/10.1093/ofid/ofy086
- 55. Ryom L, Lundgren JD, El-Sadr W, et al Cardiovascular disease and use of contemporary protease inhibitors: the D:A:D international prospective multicohort study. Lancet HIV2018; 5: e291–300. https://doi.org/10.1016/S2352-3018(18)30043-2
- 56. Hernandez-Romieu AC, Garg S, Rosenberg ES, et al Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care2017; 5: e000304. https://doi.org/10.1136/bmjdrc-2016-000304
- 57. Camps-Vilaró A, Pérez-Fernández S, Subirana I, et al Standardized comparison of cardiovascular risk factors prevalence in Spanish women and men living with HIV and in the general population. J Pers Med2021; 11: 1085. https://doi.org/10.3390/jpm11111085
- 58. Lopez-Alvarenga JC, Martinez DA, Diaz-Badillo A, et al Association of HIV-1 infection and antiretroviral therapy with type 2 diabetes in the Hispanic population of the Rio Grande Valley, Texas, USA. Front Med (Lausanne)2021; 8: 676979. https://doi.org/10.3389/fmed.2021.676979
- 59. Bratu A, McLinden T, Kooij K, et al Incidence of diabetes mellitus among people living with and without HIV in British Columbia, Canada between 2001 and 2013: a longitudinal population-based cohort study. BMJ Open2021; 11: e048744. https://doi.org/10.1136/bmjopen-2021-048744
- 60. Prioreschi A, Munthali RJ, Soepnel L, et al Incidence and prevalence of type 2 diabetes mellitus with HIV infection in Africa: a systematic review and meta-analysis. BMJ Open2017; 7: e013953. https://doi.org/10.1136/bmjopen-2016-013953
- 61. Samad F, Harris M, Puskas CM, et al Incidence of diabetes mellitus and factors associated with its development in HIV-positive patients over the age of 50. BMJ Open Diabetes Res Care2017; 5: e000457. https://doi.org/10.1136/bmjdrc-2017-000457
- 62. Njoroge A, Augusto O, Page ST, et al Increased risk of prediabetes among virally suppressed adults with HIV in Central Kenya detected using glycated haemoglobin and fasting blood glucose. Endocrinol Diabetes Metab2021; 4: e00292. https://doi.org/10.1002/edm2.292
- 63. Cassenote AJF, Grangeiro A, Escuder MM, et al Incidence and associated factors of type 2 diabetes mellitus onset in the Brazilian HIV/AIDS cohort study. Braz J Infect Dis2021; 25: 101608. https://doi.org/10.1016/j.bjid.2021.101608
- 64. Sarfo FS, Norman B, Nichols M, et al Prevalence and incidence of pre-diabetes and diabetes mellitus among people living with HIV in Ghana: evidence from the EVERLAST study. HIV Med2021; 22: 231–43. https://doi.org/10.1111/hiv.13007
- 65. Nansseu JR, Bigna JJ, Kaze AD, et al Incidence and risk factors for prediabetes and diabetes mellitus among HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Epidemiology2018; 29: 431–41. https://doi.org/10.1097/EDE.0000000000000815
- 66. Guimarães MMM, Greco DB, Moreira AN, et al Lipid accumulation product index in HIV-infected patients: a marker of cardiovascular risk. Braz J Infect Dis2018; 22: 171–6. https://doi.org/10.1016/j.bjid.2018.03.006
- 67. Colberg SR, Sigal RJ, Yardley JE, et al Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care2016; 39: 2065–79. https://doi.org/10.2337/dc16-1728
- 68. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med2017; 15: 131. https://doi.org/10.1186/s12916-017-0901-x
- 69. Hu FB, Manson JE, Stampfer MJ, et al Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med2001; 345: 790–7. https://doi.org/10.1056/NEJMoa010492
- 70. Noubissi EC, Katte J-C, Sobngwi E. Diabetes and HIV. Curr Diab Rep2018; 18: 125. https://doi.org/10.1007/s11892-018-1076-3
- 71. Eslam M, Newsome PN, Sarin SK, et al A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol2020; 73: 202–9. https://doi.org/10.1016/j.jhep.2020.03.039
- 72. Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology2020; 158: 1999–2014.e1. https://doi.org/10.1053/j.gastro.2019.11.312
- 73. Jongraksak T, Sobhonslidsuk A, Jatchavala J, et al Prevalence and predicting factors of metabolic-associated fatty liver disease diagnosed by transient elastography with controlled attenuation parameters in HIV-positive people. Int J STD AIDS2021; 32: 266–75. https://doi.org/10.1177/0956462420960997
- 74. Liu D, Shen Y, Zhang R, et al Prevalence and risk factors of metabolic associated fatty liver disease among people living with HIV in China. J Gastroenterol Hepatol2021; 36: 1670–8. https://doi.org/10.1111/jgh.15320
- 75. Kirkegaard-Klitbo DM, Fuchs A, Stender S, et al Prevalence and risk factors of moderate-to-severe hepatic steatosis in human immunodeficiency virus infection: the Copenhagen Co-morbidity Liver Study. J Infect Dis2020; 222: 1353–62. https://doi.org/10.1093/infdis/jiaa246
- 76. Perazzo H, Cardoso SW, Yanavich C, et al Predictive factors associated with liver fibrosis and steatosis by transient elastography in patients with HIV mono-infection under long-term combined antiretroviral therapy. J Int AIDS Soc2018; 21: e25201. https://doi.org/10.1002/jia2.25201
- 77. Bischoff J, Gu W, Schwarze-Zander C, et al Stratifying the risk of NAFLD in patients with HIV under combination antiretroviral therapy (cART). EClinicalMedicine2021; 40: 101116. https://doi.org/10.1016/j.eclinm.2021.101116
- 78. Pezzini MF, Cheinquer H, de Araujo A, et al Transient elastography in HIV infected patients with liver steatosis identifies a high-risk group for non-alcoholic steatohepatitis. Curr HIV Res2021; 19: 311–6. https://doi.org/10.2174/1570162X19666210225085002
- 79. Fernandez-Botran R, Plankey MW, Ware D, et al Changes in liver steatosis in HIV-positive women are associated with the BMI, but not with biomarkers. Cytokine2021; 144: 155573. https://doi.org/10.1016/j.cyto.2021.155573
- 80. Wei Q, Lin H, Ding Y, et al Liver fibrosis after antiretroviral therapy in a longitudinal cohort of sexually infected HIV patients in eastern China. Biosci Trends2017; 11: 274–81. https://doi.org/10.5582/bst.2017.01071
- 81. Saracino A, Cozzi-Lepri A, Shanyinde M, et al HIV-1 co-receptor tropism and liver fibrosis in HIV-infected patients. PLoS ONE2018; 13: e0190302. https://doi.org/10.1371/journal.pone.0190302
- 82. Battalora L, Buchacz K, Armon C, et al Low bone mineral density and risk of incident fracture in HIV-infected adults. Antivir Ther2016; 21: 45–54. https://doi.org/10.3851/IMP2979
- 83. Chang C-J, Chan Y-L, Pramukti I, et al People with HIV infection had lower bone mineral density and increased fracture risk: a meta-analysis. Arch Osteoporos2021; 16: 47. https://doi.org/10.1007/s11657-021-00903-y
- 84. Goh SSL, Lai PSM, Tan ATB, et al Reduced bone mineral density in human immunodeficiency virus-infected individuals: a meta-analysis of its prevalence and risk factors. Osteoporos Int2018; 29: 595–613. https://doi.org/10.1007/s00198-017-4305-8
- 85. Sharma A, Hoover DR, Shi Q, et al Human immunodeficiency virus (HIV) and menopause are independently associated with lower bone mineral density: results from the Women's Interagency HIV Study. Clin Infect Dis2022; 75: 65–72. https://doi.org/10.1093/cid/ciab874
- 86. Madanhire T, Goedecke JH, Ward KA, et al The impact of human immunodeficiency virus and menopause on bone mineral density: a longitudinal study of urban-dwelling South African women. J Bone Miner Res2023; 38: 619–30. https://doi.org/10.1002/jbmr.4765
- 87. Tinago W, Cotter AG, Sabin CA, et al Predictors of longitudinal change in bone mineral density in a cohort of HIV-positive and negative patients. AIDS2017; 31: 643–52. https://doi.org/10.1097/QAD.0000000000001372
- 88. Thomsen MT, Wiegandt YL, Gelpi M, et al Prevalence of and risk factors for low bone mineral density assessed by quantitative computed tomography in people living with HIV and uninfected controls. J Acquir Immune Defic Syndr2020; 83: 165–72. https://doi.org/10.1097/QAI.0000000000002245
- 89. Negredo E, Langohr K, Bonjoch A, et al High risk and probability of progression to osteoporosis at 10 years in HIV-infected individuals: the role of PIs. J Antimicrob Chemother2018; 73: 2452–9. https://doi.org/10.1093/jac/dky201
- 90. Guo F, Song X, Li Y, et al Longitudinal change in bone mineral density among Chinese individuals with HIV after initiation of antiretroviral therapy. Osteoporos Int2021; 32: 321–32. https://doi.org/10.1007/s00198-020-05584-w
- 91. Guan W, Pan W, Yu W, et al Long-term trabecular bone score and bone mineral density changes in Chinese antiretroviral-treated HIV-infected individuals. Arch Osteoporos2021; 16: 41. https://doi.org/10.1007/s11657-021-00890-0
- 92. Erlandson KM, Lake JE, Sim M, et al Bone mineral density declines twice as quickly among HIV-infected women compared with men. J Acquir Immune Defic Syndr2018; 77: 288–94. https://doi.org/10.1097/QAI.0000000000001591
- 93. McGinty T, Cotter AG, Sabin CA, et al Assessment of trabecular bone score, an index of bone microarchitecture, in HIV positive and HIV negative persons within the HIV UPBEAT cohort. PLoS ONE2019; 14: e0213440. https://doi.org/10.1371/journal.pone.0213440
- 94. Han WM, Wattanachanya L, Apornpong T, et al Bone mineral density changes among people living with HIV who have started with TDF-containing regimen: a five-year prospective study. PLoS ONE2020; 15: e0230368. https://doi.org/10.1371/journal.pone.0230368
- 95. Kalayjian RC, Albert JM, Cremers S, et al Women have enhanced bone loss associated with phosphaturia and CD4+ cell restoration during initial antiretroviral therapy. AIDS2018; 32: 2517–24. https://doi.org/10.1097/QAD.0000000000001995
- 96. McComsey GA, Tebas P, Shane E, et al Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis2010; 51: 937–46. https://doi.org/10.1086/656412
- 97. Walker Harris V, Brown TT. Bone loss in the HIV-infected patient: evidence, clinical implications, and treatment strategies. J Infect Dis2012; 205:S391–8. https://doi.org/10.1093/infdis/jis199
- 98. Pezzaioli LC, Porcelli T, Delbarba A, et al Impact of hypogonadism on bone mineral density and vertebral fractures in HIV-infected men. J Endocrinol Invest2022; 45: 433–43. https://doi.org/10.1007/s40618-021-01665-7
- 99. LeBoff MS, Greenspan SL, Insogna KL, et al The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int2022; 33: 2049–102. https://doi.org/10.1007/s00198-021-05900-y
- 100. Bedimo R, Maalouf NM, Lo Re V III. Hepatitis C virus coinfection as a risk factor for osteoporosis and fracture. Curr Opin HIV AIDS2016; 11: 285–93. https://doi.org/10.1097/COH.0000000000000259
- 101. Hoy JF, Grund B, Roediger M, et al Immediate initiation of antiretroviral therapy for HIV infection accelerates bone loss relative to deferring therapy: findings from the START bone mineral density substudy, a randomized trial. J Bone Miner Res2017; 32: 1945–55. https://doi.org/10.1002/jbmr.3183
- 102. Spinelli MA, Glidden DV, Anderson PL, et al Impact of estimated pre-exposure prophylaxis (PrEP) adherence patterns on bone mineral density in a large PrEP demonstration project. AIDS Res Hum Retroviruses2019; 35: 788–93. https://doi.org/10.1089/aid.2018.0297
- 103. Brown TT, Yuhas K, Mayer KH, et al Bone changes with candidate PrEP regimens containing tenofovir disoproxil fumarate and/or maraviroc and/or emtricitabine in US men and women: HPTN 069/ACTG A5305. J Antimicrob Chemother2022; 77: 500–6. https://doi.org/10.1093/jac/dkab400
- 104. Brown TT, McComsey GA, King MS, et al Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr2009; 51: 554–61. https://doi.org/10.1097/QAI.0b013e3181adce44
- 105. Duvivier C, Kolta S, Assoumou L, et al Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS2009; 23: 817–24. https://doi.org/10.1097/QAD.0b013e328328f789
- 106. Huhn GD, Wilkin A, Mussini C, et al Week 96 subgroup analyses of the phase 3, randomized AMBER and EMERALD trials evaluating the efficacy and safety of the once daily darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) single-tablet regimen in antiretroviral treatment (ART)-naïve and -experienced, virologically-suppressed adults living with HIV-1. HIV Res Clin Pract2020; 21: 151–67. https://doi.org/10.1080/25787489.2020.1844520
- 107. Bonfanti P, De Vito A, Ricci E, et al Bone safety of dolutegravir-containing regimens in people living with HIV: results from a real-world cohort. Infect Drug Resist2020; 13: 2291–300. https://doi.org/10.2147/IDR.S260449
- 108. Orkin C, DeJesus E, Sax PE, et al Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir-containing regimens for initial treatment of HIV-1 infection: week 144 results from two randomised, double-blind, multicentre, phase 3, non-inferiority trials. Lancet HIV2020; 7: e389–400. https://doi.org/10.1016/S2352-3018(20)30099-0
- 109. Bi X, Liu F, Zhang X, et al Vitamin D and calcium supplementation reverses tenofovir-caused bone mineral density loss in people taking ART or PrEP: a systematic review and meta-analysis. Front Nutr2022; 9: 749948. https://doi.org/10.3389/fnut.2022.749948
- 110. Overton ET, Chan ES, Brown TT, et al Vitamin D and calcium attenuate bone loss with antiretroviral therapy initiation: a randomized trial. Ann Intern Med2015; 162: 815–24. https://doi.org/10.7326/M14-1409
- 111. Baranek B, Wang S, Cheung AM, et al The effect of tenofovir disoproxil fumarate on bone mineral density: a systematic review and meta-analysis. Antivir Ther2020; 25: 21–32. https://doi.org/10.3851/IMP3346
- 112. Brown TT, Moser C, Currier JS, et al Changes in bone mineral density after initiation of antiretroviral treatment with tenofovir disoproxil fumarate/emtricitabine plus atazanavir/ritonavir, darunavir/ritonavir, or raltegravir. J Infect Dis2015; 212: 1241–9. https://doi.org/10.1093/infdis/jiv194
- 113. Chang J, Do D, Delgado H, et al A retrospective analysis of bone loss in tenofovir-emtricitabine therapy for HIV PrEP. Int J STD AIDS2022; 33: 1183–92. https://doi.org/10.1177/09564624221130129
- 114. Hill A, Hughes SL, Gotham D, et al Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety? J Virus Erad 2018; 4: 72–9. https://doi.org/10.1016/S2055-6640(20)30248-X
- 115. Kanda N, Okamoto K, Okumura H, et al Outcomes associated with treatment change from tenofovir disoproxil fumarate to tenofovir alafenamide in HIV-1-infected patients: a real-world study in Japan. HIV Med2021; 22: 457–66. https://doi.org/10.1111/hiv.13061
- 116. Hagins D, Orkin C, Daar ES, et al Switching to coformulated rilpivirine (RPV), emtricitabine (FTC) and tenofovir alafenamide from either RPV, FTC and tenofovir disoproxil fumarate (TDF) or efavirenz, FTC and TDF: 96-week results from two randomized clinical trials. HIV Med2018; 19: 724–33. https://doi.org/10.1111/hiv.12664
- 117. Maggiolo F, Rizzardini G, Raffi F, et al Bone mineral density in virologically suppressed people aged 60 years or older with HIV-1 switching from a regimen containing tenofovir disoproxil fumarate to an elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide single-tablet regimen: a multicentre, open-label, phase 3b, randomised trial. Lancet HIV2019; 6: e655–66. https://doi.org/10.1016/S2352-3018(19)30195-X
- 118. McComsey GA, Lupo S, Parks D, et al Switch from tenofovir disoproxil fumarate combination to dolutegravir with rilpivirine improves parameters of bone health. AIDS2018; 32: 477–85. https://doi.org/10.1097/QAD.0000000000001725
- 119. Ibrahim F, Samarawickrama A, Hamzah L, et al Bone mineral density, kidney function, weight gain and insulin resistance in women who switch from TDF/FTC/NNRTI to ABC/3TC/DTG. HIV Med2021; 22: 83–91. https://doi.org/10.1111/hiv.12961
- 120. Ciccullo A, Baldin G, Borghi V, et al Re: “No significant changes in weight and body fat mass in suppressed HIV infected patients switched to dual combination lamivudine plus dolutegravir or raltegravir” by Calza et al. AIDS Res Hum Retroviruses2021; 37: 333–4. https://doi.org/10.1089/aid.2020.0274
- 121. Marques EA, Mota J, Carvalho J. Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age (Dordr)2012; 34: 1493–515. https://doi.org/10.1007/s11357-011-9311-8
- 122. Kelley GA, Kelley KS, Kohrt WM. Exercise and bone mineral density in premenopausal women: a meta-analysis of randomized controlled trials. Int J Endocrinol2013; 2013: 741639. https://doi.org/10.1155/2013/741639
- 123. Oncken C, Prestwood K, Kleppinger A, et al Impact of smoking cessation on bone mineral density in postmenopausal women. J Womens Health (Larchmt)2006; 15: 1141–50. https://doi.org/10.1089/jwh.2006.15.1141
- 124. König D, Oesser S, Scharla S, et al Specific collagen peptides improve bone mineral density and bone markers in postmenopausal women—a randomized controlled study. Nutrients2018; 10: 97. https://doi.org/10.3390/nu10010097
- 125. Cruz-Jentoft AJ, Bahat G, Bauer J, et al Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing2019; 48: 16–31. https://doi.org/10.1093/ageing/afy169
- 126. Bhasin S, Travison TG, Manini TM, et al Sarcopenia definition: the position statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc2020; 68: 1410–8. https://doi.org/10.1111/jgs.16372
- 127. Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol2014; 2: 819–29. https://doi.org/10.1016/S2213-8587(14)70034-8
- 128. Gutierrez MDM, Mateo MG, Corbacho N, et al Drug-drug interactions when treating HIV-related metabolic disorders. Expert Opin Drug Metab Toxicol2019; 15: 787–802. https://doi.org/10.1080/17425255.2019.1667334
- 129. Courlet P, Livio F, Alves Saldanha S, et al Real-life management of drug-drug interactions between antiretrovirals and statins. J Antimicrob Chemother2020; 75: 1972–80. https://doi.org/10.1093/jac/dkaa099
- 130. Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab2015; 6: 19–28. https://doi.org/10.1177/2042018814559725
- 131. Nguyen QP, Wooten D, Duren K, et al GLP-1 receptor agonists promote weight loss among people with HIV. Open Forum Infect Dis2023; 10:S66–7. https://doi.org/10.1093/ofid/ofad500.109
- 132. Triant VA, Perez J, Regan S, et al Cardiovascular risk prediction functions underestimate risk in HIV infection. Circulation2018; 137: 2203–14. https://doi.org/10.1161/CIRCULATIONAHA.117.028975
- 133. Law MG, Friis-Møller N, El-Sadr WM, et al The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D study. HIV Med2006; 7: 218–30. https://doi.org/10.1111/j.1468-1293.2006.00362.x
- 134. Schouten J, Wit FW, Stolte IG, et al Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis2014; 59: 1787–97. https://doi.org/10.1093/cid/ciu701
- 135. Gosiker BJ, Lesko CR, Rich AJ, et al Cardiovascular disease risk among transgender women living with HIV in the United States. PLoS ONE2020; 15: e0236177. https://doi.org/10.1371/journal.pone.0236177
- 136. de Blok CJ, Wiepjes CM, van Velzen DM, et al Mortality trends over five decades in adult transgender people receiving hormone treatment: a report from the Amsterdam cohort of gender dysphoria. Lancet Diabetes Endocrinol2021; 9: 663–70. https://doi.org/10.1016/S2213-8587(21)00185-6
- 137. Toribio M, Fulda ES, Chu SM, et al Hot flashes and cardiovascular disease risk indices among women with HIV. Open Forum Infect Dis2021; 8: ofab011. https://doi.org/10.1093/ofid/ofab011
- 138. Kaplan RC, Hanna DB, Kizer JR. Recent insights into cardiovascular disease (CVD) risk among HIV-infected adults. Curr HIV/AIDS Rep2016; 13: 44–52. https://doi.org/10.1007/s11904-016-0301-4
- 139. Raposeiras-Roubín S, Abu-Assi E, Iñiguez-Romo A. Tobacco, illicit drugs use and risk of cardiovascular disease in patients living with HIV. Curr Opin HIV AIDS2017; 12: 523–7. https://doi.org/10.1097/COH.0000000000000407
- 140. Grinspoon SK, Fitch KV, Zanni MV, et al Pitavastatin to prevent cardiovascular disease in HIV infection. N Engl J Med2023; 389: 687–99. https://doi.org/10.1056/NEJMoa2304146
- 141. Erlandson KM, Plankey MW, Springer G, et al Fall frequency and associated factors among men and women with or at risk for HIV infection. HIV Med2016; 17: 740–8. https://doi.org/10.1111/hiv.12378
- 142. Oblak L, van der Zaag J, Higgins-Chen AT, et al A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Res Rev2021; 69: 101348. https://doi.org/10.1016/j.arr.2021.101348
- 143. Psomas CK, Hoover DR, Shi Q, et al Polypharmacy is associated with falls in women with and without HIV. J Acquir Immune Defic Syndr2022; 90: 351–9. https://doi.org/10.1097/QAI.0000000000002955
- 144. Kim TW, Walley AY, Ventura AS, et al Polypharmacy and risk of falls and fractures for patients with HIV infection and substance dependence. AIDS Care2018; 30: 150–9. https://doi.org/10.1080/09540121.2017.1384532
- 145. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv.
- 146. World Health Organization. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. https://www.who.int/publications/i/item/9789240031593.
- 147. Guaraldi G, Milic J, Mussini C. Aging with HIV. Curr HIV/AIDS Rep2019; 16: 475–81. https://doi.org/10.1007/s11904-019-00464-3