Introduction
Cerebral small vessel disease (CSVD) is associated with cognitive decline and is considered a risk factor for dementia.– The main brain MRI features of CSVD are white matter hyperintensities and lacunar infarcts of presumed vascular origin.– Brain autopsy studies showed the presence of cerebral small infarcts that can range from 50 µm to 15 mm in size and which are also considered a feature of CSVD.– In autopsy studies, these small infarcts can occur in up to 43% of non-demented older individuals in cortical and subcortical areas of the brain. Despite this high prevalence, these small infarcts are largely undetected on conventional 1.5T MRI due to their small size. Recently, it was shown that small infarcts in the cerebral cortex can be detected in vivo on 7T MRI. Although the presence and imaging characteristics of small infarcts in the cortical gray matter on high field MRI have been studied,– small infarcts in the subcortical gray matter on high field MRI have not been studied. To determine the total CSVD burden more accurately, it is necessary to assess not only cortical, but also subcortical gray matter in greater detail. The caudate nucleus has advantages over other subcortical gray matter structures when assessing small infarcts. The caudate nucleus has a paraventricular location and is better demarcated from surrounding tissues compared to the rest of the subcortical gray matter, thus allowing for a more accurate determination of the frequency of small infarcts in this subcortical structure. The aim of the present study was first, to establish imaging criteria for the detection of small infarcts in the caudate nucleus on 7T MRI; second, to determine the intra- and inter-rater agreement for detection of these small infarcts on 7T MRI; third, to estimate the frequency and explore possible determinants of these small infarcts in patients with a history of symptomatic atherosclerotic disease; and fourth, to assess differences in detection rate of these small infarcts between 7T MRI and 1.5T MRI.
Material and methods
Study populations
We used cross-sectional data from the SMART study and the SMART-MR study., The Second Manifestations of ARTerial disease (SMART) study is a prospective cohort study at the University Medical Center Utrecht designed to establish the prevalence of concomitant arterial diseases and risk factors for atherosclerosis in a high-risk population. The SMART-MR study is a sub-study of the SMART study, with the aim to investigate risk factors and consequences of brain changes on MRI in patients with symptomatic atherosclerotic disease. The SMART-MR study is an ongoing prospective cohort study in 1309 middle-aged and older adult patients newly referred to the University Medical Center Utrecht for treatment of symptomatic atherosclerotic disease (manifest coronary artery disease, cerebrovascular disease, peripheral arterial disease or abdominal aortic aneurysm) enrolled between May 2001 and December 2005 for baseline measurements, and of whom 754 patients had follow-up measurements four years later between January 2006 and May 2009. During a one-day visit to our medical center, a physical examination, ultrasonography of the carotid arteries, blood and urine samplings, neuropsychological assessment, and a 1.5T brain MRI scan were performed. Questionnaires were used for assessing demographics, risk factors and medical history, medication use, and functioning. Since November 2013, all patients alive are invited for a second follow-up, including a 7T brain MRI. For objectives 1, 3 and 4, we included 90 patients who had second follow-up measurements between December 2013 and May 2016 and who had a 7T MRI scan.
For objective 2, we used a random selection of 23 patients with asymptomatic (e.g. hypertension) or symptomatic (e.g. coronary artery disease) atherosclerotic disease from the SMART study who had a 7T MRI of the brain between September 2012 and September 2013. There was no overlap between the group of 23 patients used for objective 2 and the group of 90 patients used for objectives 1, 3 and 4.
To assess the frequency of small infarcts in the caudate nucleus in primary care patients not selected on disease status, we included 48 participants with a 7T brain MRI from the PREDICT-MR study. In this study, adult patients aged 18 years or older were included from the waiting room of the general practitioner, irrespective of their reasons for consulting their general practitioner. Participants were considered eligible for the PREDICT-MR study if they were not demented or severely ill. A 1.5T and 7T brain MRI was performed on the same day between June 2010 and January 2012 as part of follow-up measurements.
All studies were approved by the medical ethics committee of the University Medical Center Utrecht according to the guidelines of the Declaration of Helsinki of 1975 and written informed consent was obtained from all participants.
Magnetic resonance imaging protocol
Conventional MR imaging of the brain was performed on a 1.5T whole-body system (Gyroscan ACS-NT, Philips Medical Systems, Best, The Netherlands) using a standardized scan protocol. Transversal T1-weighted [repetition time (TR) = 235 ms; echo time (TE) = 2 ms], T2-weighted [TR = 2200 ms; TE = 11 ms], fluid-attenuated inversion recovery (FLAIR) [TR = 6000 ms; TE = 100 ms; inversion time (TI) = 2000 ms] and T1-weighted inversion recovery images [TR = 2900 ms; TE = 22 ms; TI = 410 ms] were acquired with a voxel size of 1.0 × 1.0 × 4.0 mm3 and contiguous slices. Infarcts on 1.5T MRI scans were rated on all MRI sequences (T1, T2 and FLAIR) by a rater with five years of experience in brain MRI (MHTZ). Ratings were performed blinded to patient characteristics.
High-field imaging of the brain was performed on a whole-body 7T MR system (Philips Healthcare, Cleveland, OH, USA) with a volume transmit and 32-channel receive head coil (Nova Medical, Wilmington, MA, USA). The standardized scan protocol consisted of volumetric (3D) T1-weighted [voxel size = 0.66 ×0.66 × 0.50 mm3; TR = 4.8 ms; TE = 2.2 ms; flip angle 8°], 3D T2-weighted turbo-spin echo [voxel size =0.35 × 0.35 × 0.35 mm3, TR = 3158 ms, TE = 301 ms] and 3D magnetization prepared FLAIR images [voxel size = 0.49 × 0.49 × 0.49 mm3, TR = 8000 ms, TE = 300 ms].,,
Cardiovascular risk factors
Smoking habits and alcohol intake were assessed with questionnaires, and were categorized as never, former or current. Height and weight were measured, and the body mass index (BMI) was calculated (kg/m2). Systolic blood pressure (SBP; mmHg) and diastolic blood pressure (DBP; mmHg) were measured three times with a sphygmomanometer, and the average of these measures was calculated. Hypertension was defined as a mean SBP of ≥ 160 mmHg, or a mean DBP of ≥ 95 mmHg, or self-reported use of antihypertensive drugs, or a known history of hypertension at inclusion. An overnight fasting venous blood sample was taken to determine glucose and lipid levels. Diabetes mellitus was defined as use of glucose-lowering agents, or a known history of diabetes mellitus, or a fasting plasma glucose level of > 11.1 mmol/l. Hyperlipidemia was defined as a total cholesterol of > 5.0 mmol/l, or a low-density lipoprotein cholesterol of > 3.2 mmol/l, or use of lipid-lowering drugs, or a known history of hyperlipidemia. Ultrasonography was performed with a 10 MHz linear-array transducer (ATL Ultramark 9) by an ultrasound technician. Mean carotid intima-media thickness (in mm) was calculated for the left and right common carotid arteries based on six far-wall measurements.
MRI analysis
All ratings were performed blinded to patient characteristics. For the first objective, to establish imaging criteria for the detection of small infarcts in the caudate nucleus on 7T MRI, the 7T MRI scans of patients with symptomatic atherosclerotic disease (SMART-MR study; n = 90) were examined by one rater (RG) for possible lesions in the caudate nucleus. Subsequently, a meeting was held with two raters with 30 and 15 years of experience in brain MRI imaging (TW and JH; senior neuroradiologists) and a rater with 9 years of experience in brain MRI imaging (JdB) to define and characterize lesions.
For the second objective, intra- and inter-rater agreement for the detection of small infarct on 7T MRI was determined. The 7T MRI scans of patients with atherosclerotic disease (SMART study; n = 23) were rated twice by one rater (TW) and rated once by two raters (JdB and JH) using all 7T MRI sequences (T1, T2 and FLAIR) in three orthogonal viewing directions (sagittal, coronal, and transversal). Intra-observer agreement was calculated for presence (Cohen’s kappa), number (intra-class correlation coefficient (ICC)) and overlap in individual locations (Dice similarity coefficient) of small infarcts in the caudate nucleus. Inter-observer agreement between the three raters was calculated for presence (Fleiss’ kappa), number (ICC) and overlap in individual locations (Dice similarity coefficient).
For the third objective, the scans of patients with symptomatic atherosclerotic disease (SMART-MR study; n = 90) and of primary care patients not selected on disease status (PREDICT-MR study; n = 48) were randomly mixed and rated for presence of small infarcts in the caudate nucleus by one rater (TW) blinded to patient status. Uncertain lesions were discussed during a consensus meeting (TW, JH and JdB) to reach agreement. The side (left, right), location (head, body or tail of the caudate nucleus) and maximal diameter of all small infarcts were determined on the FLAIR sequence in three orthogonal directions (sagittal, coronal or transversal). Baseline characteristics were compared between the patients with versus without small infarcts in the caudate nucleus by Chi square tests or t-tests. A Chi square test was performed to assess differences in frequency of small infarcts between patients with atherosclerotic disease (n = 90) and the primary care patients not selected on disease status (n = 48).
For the fourth objective, presence and individual locations of small infarcts identified on 7T MRI were compared to those on 1.5T MRI in patients with symptomatic atherosclerotic disease (SMART-MR study; n = 90).
Results
7T MRI imaging criteria of small infarcts in the caudate nucleus
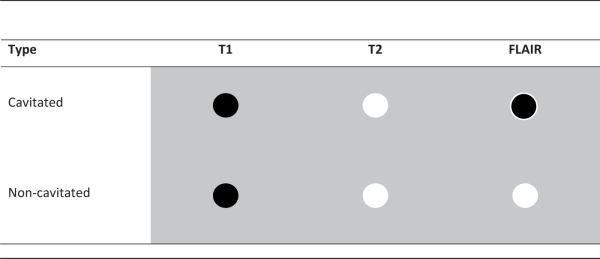
Based on our assessment of infarct types in the caudate nucleus on 7T MRI, we identified two distinct morphologies. First, cavitated small infarcts were identified as lesions that were hypointense on T1-weighted images, hyperintense on T2-weighted images and hypointense with a hyperintense rim on FLAIR images (Table 1). Second, non-cavitated small infarcts were identified as hypointense on T1-weighted images and hyperintense on T2-weighted and FLAIR images (Table 1). Tissue loss with a surrounding hyperintense rim on FLAIR images was characterized as a cavitated small infarct. Lesions with a maximum diameter of 15 mm were considered small infarcts in the caudate nucleus, while we did not define a minimum size criterion for small infarcts. Examples of cavitated and non-cavitated small infarcts in the caudate nucleus can be seen in Figures 1 and 2.

Figure 1
Two cavitated small infarcts (arrow) in the body of the left caudate nucleus in a 67-year-old female shown on sagittal FLAIR (a), T1-weighted (b) and T2-weighted images (c) of the 7T MRI scan. These lesions are hypointense with a hyperintense rim on the FLAIR image, hypointense on the T1-weighted image and hyperintense on the T2-weighted image. Scale bar indicates 10 mm.

Figure 2
A non-cavitated small infarct (arrow) in the body of the right caudate nucleus in a 69-year-old male shown on sagittal FLAIR (a), T1-weighted (b) and T2-weighted images (c) of the 7T MRI scan. This lesion is hyperintense on the FLAIR image, hypointense on the T1-weighted image and hyperintense on the T2-weighted image. Note the presence of a cavitated small infarct (*) in the head of the caudate nucleus. Scale bar indicates 10 mm.
Intra- and inter-rater agreement of small infarcts in the caudate nucleus
In the MRI scans of the 23 patients (mean age 61 ± 11 years; 65% male) used to measure intra- and inter-rater agreement, the first rater identified a total number of 13 cavitated small infarcts in five patients (22%) during the first rating. During the second rating, the first rater identified a total number of 12 cavitated small infarcts in the same five patients (22%). The first rater identified no non-cavitated infarcts (Supplementary Table 1). The second rater identified a total number of 12 small infarcts in 4 patients (17%), of which 10 were rated as cavitated and 2 as non-cavitated. The third rater identified a total number of 14 small infarcts in 5 patients (22%), of which 12 were rated as cavitated and 2 as non-cavitated (Supplementary Table 1). The intra-rater agreement for the first rater was excellent for presence (Cohen’s kappa: 1.00), number (ICC: 0.99 (95% CI 0.98 to 1.00)) and individual locations (Dice similarity coefficient: 0.96) of small infarcts in the caudate nucleus. The inter-rater agreement for all three raters was very good to excellent for presence (Fleiss’ kappa: 0.91), number (ICC: 0.99 (95% CI 0.98 to 1.00)) and individual locations (Dice similarity coefficient between rater 1 and 2/rater 1 and 3/rater 2 and 3, respectively: 0.88/0.89/0.85) of small infarcts in the caudate nucleus.
Patients with versus without small infarcts in the caudate nucleus
The baseline characteristics for the 90 patients with symptomatic atherosclerotic disease (SMART-MR study; mean age 68 ± 8 years; 80% male) are shown in Table 2. A total number of 21 small infarcts were identified in the caudate nucleus (20 cavitated; 1 non-cavitated) in 12 patients (13%). A maximum of five small infarcts was identified in one patient. The mean size of small infarcts in the caudate nucleus was 5.2 mm (range: 2.1 to 8.6 mm). Most small infarcts were located in the caudate head (11 infarcts), followed by the body (six infarcts) and tail (four infarcts) (Table 3). In two patients (2%), a cavitated small infarct was identified that consisted of tissue loss with a surrounding hyperintense rim on the FLAIR image (Figure 3). Three patients (3%) showed a large infarct in the flow territory of the middle cerebral artery, which included infarcted tissue in a large part of the caudate nucleus in one hemisphere.

Figure 3
An area of tissue loss with a hyperintense rim on FLAIR (arrow) in the body of the left caudate nucleus in a 80-year-old male shown on sagittal FLAIR (a), T1-weighted (b) and T2-weighted images (c) of the 7T MRI scan. We defined this type of infarct as a cavitated small infarct. Scale bar indicates 10 mm.
In the 48 primary care patients not selected on disease status (PREDICT-MR study; mean age 60 ± 10 years; 38% male), we identified one cavitated small infarct in the head of the caudate nucleus in one patient (2%). More small infarcts were detected in the caudate nucleus in the 90 patients with symptomatic atherosclerotic disease compared to the 48 primary care patients not selected on disease status (p = 0.031).
In the 90 patients with symptomatic atherosclerotic disease, patients with small infarcts (n = 12) in the caudate nucleus were older (mean difference of 6.9 years; 95% CI 2.1 to 11.6) than patients without small infarcts (n = 78). Furthermore, patients with small infarcts in the caudate nucleus showed a higher number of cerebral infarcts in the entire brain on 1.5T MRI (58% vs. 24%; p = 0.017), specifically of lacunar infarcts (58% vs. 13%; p < 0.001), compared to patients without small infarcts. No differences in sex, vascular disease location or cardiovascular risk factors were found between the patients with and without small infarcts in the caudate nucleus (Table 4).
Detection rate of small infarcts in the caudate nucleus on 7T MRI versus 1.5T MRI
In the 90 patients with symptomatic atherosclerotic disease, the median duration between the 1.5T and 7T MRI scans was 0 months (10th–90th percentile: 0–28 months). The majority of patients received both MRI scans on the same day (50 patients; 56%). A total number of 21 small infarcts were identified on 7T MRI compared to 7 small infarcts on 1.5T MRI scans. On 7T MRI scans (n = 12; 13%), twice the number of patients had small infarcts compared to 1.5T MRI scans (n = 6; 7%). A similar difference between detection of small infarcts on 7T MRI and 1.5T MRI scans was seen in patients who did not receive both MRI scans on the same day. All small infarcts detected on 1.5T MRI scans were also rated on the 7T MRI scans (Supplementary Table 2). An example of a small infarct in the caudate nucleus that was rated on both 1.5T and 7T MRI and one that was only rated on 7T MRI can be seen in Supplementary Figures 1 and 2, respectively.
Discussion
We established imaging criteria for the detection of small infarcts in the caudate nucleus on 7T MRI that differentiate between cavitated and non-cavitated small infarcts. The intra- and inter-rater agreement for detection of these small infarcts was very good to excellent. We observed that small infarcts in the caudate nucleus were a relatively common finding on 7T MRI in patients with symptomatic atherosclerotic disease. Furthermore, they were associated with older age and presence of other cerebral infarcts on 1.5T MRI. More small infarcts were detected on 7T MRI compared to 1.5T MRI.
To our knowledge, this is the first study that used 7T MRI to evaluate the deep gray matter for the presence of small infarcts, both in terms of characterizing lesions and assessing intra- and inter-rater agreement for detection. We showed that on 7T MRI, the MRI signal intensity characteristics of small infarcts in the caudate nucleus are similar to those described in the STRIVE criteria. The STRIVE criteria, which were originally formulated for use on 1.5T or 3T MRI, characterize small infarcts as lesions between 3 mm and 15 mm in diameter. Contrary to the STRIVE criteria, we did not define a minimum size criterion for small infarcts in the caudate nucleus, because smaller infarcts can be detected on 7T MRI compared to 1.5T or 3T MRI. This increased detection can be explained by the enhanced resolution of 7T MRI and the higher image quality due to a higher signal-to-noise and contrast-to-noise ratio. Hence, we believe that the STRIVE criteria should be extended by a lower minimum size criterion for small infarcts on 7T MRI images.
The prevalence of small infarcts in the caudate nucleus (13%) in patients with symptomatic atherosclerotic disease in our subset of the SMART-MR study (n = 90; mean age 68 ± 8 years) is higher than reported in studies using low field MRI. Only few studies reported small infarcts in the caudate nucleus separately. In a study including patients with CSVD (n = 633; mean age 74 ± 5 years), the prevalence of small infarcts in the caudate nucleus on 1.5T MRI was 7.7%. Although our findings cannot be directly compared, we will provide prevalences of small infarcts in the basal ganglia and brain as a frame of reference. In a study in community dwelling older individuals (n = 477; mean age 63 ± 2 years), small infarcts in the basal ganglia on 1.5T MRI were observed in 4.6%. In other studies in community dwelling individuals, the combined prevalence of small infarcts in the entire brain on 1.5T MRI was 15.4% (range: 5.8 to 23.0%; mean age range: 49 to 72 years).– The higher prevalence of small infarcts in the caudate nucleus found in our study may be explained by the higher detection rate on 7T MRI, which is caused by a combination of an increased signal-to-noise ratio, an increased contrast-to-noise ratio and a higher resolution compared to conventional 1.5T MRI scans.
In previous studies using 7T or 3T MRI to detect small infarcts in the cortical gray matter, lesions smaller than 5 mm were sometimes defined as microinfarcts.,– If we apply this size criterion to our study, 9 of 21 small infarcts (43%) are smaller than 5 mm and could thus be defined as microinfarcts. However, we would like to emphasize that the etiological significance of the term “microinfarct” in the context of the caudate nucleus remains questionable and its use is not in concordance with current consensus reporting standards. This lack of consistency does not only apply to microinfarcts, but also to other manifestations of CSVD. Definitions and terminology for imaging features of CSVD vary widely in the literature, as was highlighted in the Position Paper for the STRIVE criteria. Our use of the consensus term small infarct is in concordance with the STRIVE reporting standards, in which small infarcts are defined as cavitated lesions (e.g. lacunes of presumed vascular origin) or in some cases non-cavitated lesions without a fluid cavity on FLAIR images. Use of standardized neuroimaging criteria for CSVD will facilitate across-study comparisons of findings, thereby possibly accelerating the translation of new findings into clinical practice.,,
The strengths of our study are the use of high quality 7T MRI data that enabled us to accurately detect and characterize small infarcts in the caudate nucleus. Our study made it possible to detect small infarcts that would have remained undetected on conventional 1.5T MRI scans. Also, compared to other 7T MRI studies, we had a large cohort of patients. A limitation of our study may be the lack of histopathological validation of the observed small infarcts. However, we applied strict MRI imaging criteria aimed at identifying lesions with a high probability of being small infarcts. This approach could also have resulted in an underrating of small infarcts in the caudate nucleus. For example, we found one potential lesion that did not conform to our imaging criteria. This lesion was hypointense with a hyperintense rim on FLAIR and hyperintense on T2; however, on T1, this lesion appeared isointense to the surrounding tissue. It is possible that this lesion was a small infarct. Infarcts in the caudate nucleus of around 1 mm in diameter are at the detection limit of 7T MRI, thus the true burden of small infarcts might be underestimated. Another limitation might be that although the sample size of our study is among the largest for 7T MRI studies, a larger sample is needed to examine determinants of small infarcts in the caudate nucleus in more detail. Lastly, a limitation may be that the intra- and inter-rater agreement was performed by three raters with extensive experience in neuroradiology. Performing ratings by less experienced raters could result in a lower intra- and inter-rater agreement.
The increased detection rate of small infarcts at 7T MRI is most likely not limited to the caudate nucleus. We started with the caudate nucleus due to its paraventricular location. This paraventricular location allowed for a better demarcation from surrounding tissues compared to other structures of the subcortical gray matter, thus enabling a more accurate determination of the frequency of small infarcts in the caudate nucleus. Expanding the research of small infarcts to other structures of the subcortical gray matter will most likely lead to other challenges. For example, compared to the caudate nucleus, the lentiform nucleus and thalamus are less well-demarcated from surrounding tissues on MRI scans. This can lead to challenges in the localization of small infarcts in these structures in some cases.
In conclusion, we established reproducible imaging criteria for the detection of small infarcts in the caudate nucleus on 7T MRI and showed that 7T MRI allows for a higher detection rate than conventional 1.5T MRI. These imaging criteria can be used in future studies to provide new insights into the pathophysiology of CSVD.
Acknowledgements
We gratefully acknowledge the contribution of the SMART research personnel: R van Petersen and BGF. Dinther and also the participants of the SMART Study Group: A. Algra MD, PhD; Y van der Graaf, MD, PhD; D.E. Grobbee, MD, PhD; G.E.H.M. Rutten, MD, PhD, Julius Center for Health Sciences and Primary care; FLJ Visseren, MD, PhD, Department of Internal Medicine; GJ de Borst, MD, PhD, Department of Vascular Surgery; LJ Kappelle, MD, PhD, Department of Neurology; T Leiner, MD, PhD, Department of Radiology; PA Doevendans, MD, PhD, Department of Cardiology.
References
- 1. Pantoni L, Poggesi A, Inzitari D. Cognitive decline and dementia related to cerebrovascular diseases: some evidence and concepts. Cerebrovasc Dis 2009; 27(Suppl 1): 191–196.
- 2. Makin SD, Turpin S, Dennis MS, et al Cognitive impairment after lacunar stroke: systematic review and meta-analysis of incidence, prevalence and comparison with other stroke subtypes. J Neurol Neurosurg Psychiatry 2013; 84: 893–900.
- 3. Ostergaard L, Engedal TS, Moreton F, et al Cerebral small vessel disease: capillary pathways to stroke and cognitive decline. J Cereb Blood Flow Metab 2016; 36: 302–325.
- 4. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013; 12: 483–497.
- 5. Gouw AA, Seewann A, van der Flier WM, et al Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 2011; 82: 126–135.
- 6. De Guio F, Jouvent E, Biessels GJ, et al Reproducibility and variability of quantitative magnetic resonance imaging markers in cerebral small vessel disease. J Cereb Blood Flow Metab 2016; 36: 1319–1337.
- 7. Brundel M, de Bresser J, van Dillen JJ, et al Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab 2012; 32: 425–436.
- 8. Kalaria RN, Kenny RA, Ballard CG, et al Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci 2004; 226: 75–80.
- 9. Smith EE, Schneider JA, Wardlaw JM, et al Cerebral microinfarcts: the invisible lesions. Lancet Neurol 2012; 11: 272–282.
- 10. van Veluw SJ, Zwanenburg JJ, Engelen-Lee J, et al In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI. J Cereb Blood Flow Metab 2013; 33: 322–329.
- 11. van Veluw SJ, Zwanenburg JJ, Rozemuller AJ, et al The spectrum of MR detectable cortical microinfarcts: a classification study with 7-tesla postmortem MRI and histopathology. J Cereb Blood Flow Metab 2015; 35: 676–683.
- 12. Brundel M, Reijmer YD, van Veluw SJ, et al Cerebral microvascular lesions on high-resolution 7-Tesla MRI in patients with type 2 diabetes. Diabetes 2014; 63: 3523–3529.
- 13. De Reuck J, Deramecourt V, Auger F, et al Post-mortem 7.0-tesla magnetic resonance study of cortical microinfarcts in neurodegenerative diseases and vascular dementia with neuropathological correlates. J Neurol Sci 2014; 346: 85–89.
- 14. De Reuck JL, Deramecourt V, Auger F, et al The significance of cortical cerebellar microbleeds and microinfarcts in neurodegenerative and cerebrovascular diseases. A post-mortem 7.0-tesla magnetic resonance study with neuropathological correlates. Cerebrovasc Dis 2015; 39: 138–143.
- 15. de Rotte AA, Koning W, den Hartog AG, et al 7.0 T MRI detection of cerebral microinfarcts in patients with a symptomatic high-grade carotid artery stenosis. J Cereb Blood Flow Metab 2014; 34: 1715–1719.
- 16. Pellizzaro Venti M, Paciaroni M, Caso V. Caudate infarcts and hemorrhages. Front Neurol Neurosci 2012; 30: 137–140.
- 17. Simons PC, Algra A, van de Laak MF, et al Second manifestations of ARTerial disease (SMART) study: rationale and design. Eur J Epidemiol 1999; 15: 773–781.
- 18. Geerlings MI, Appelman AP, Vincken KL, et al Brain volumes and cerebrovascular lesions on MRI in patients with atherosclerotic disease. The SMART-MR study. Atherosclerosis 2010; 210: 130–136.
- 19. Wisse LE, Biessels GJ, Stegenga BT, et al Major depressive episodes over the course of 7 years and hippocampal subfield volumes at 7 tesla MRI: the PREDICT-MR study. J Affect Disord 2015; 175: 1–7.
- 20. Visser F, Zwanenburg JJ, Hoogduin JM, et al High-resolution magnetization-prepared 3D-FLAIR imaging at 7.0 Tesla. Magn Reson Med 2010; 64: 194–202.
- 21. Kuijf HJ, van Veluw SJ, Viergever MA, et al How to assess the reliability of cerebral microbleed rating? Front Aging Neurosci 2013; 5: 57.
- 22. Wardlaw JM, Smith EE, Biessels GJ, et al Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838.
- 23. Benisty S, Gouw AA, Porcher R, et al Location of lacunar infarcts correlates with cognition in a sample of non-disabled subjects with age-related white-matter changes: the LADIS study. J Neurol Neurosurg Psychiatry 2009; 80: 478–483.
- 24. Chen X, Wen W, Anstey KJ, et al Prevalence, incidence, and risk factors of lacunar infarcts in a community sample. Neurology 2009; 73: 266–272.
- 25. Das RR, Seshadri S, Beiser AS, et al Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke 2008; 39: 2929–2935.
- 26. Kohara K, Fujisawa M, Ando F, et al MTHFR gene polymorphism as a risk factor for silent brain infarcts and white matter lesions in the Japanese general population: The NILS-LSA Study. Stroke 2003; 34: 1130–1135.
- 27. Lee SC, Park SJ, Ki HK, et al Prevalence and risk factors of silent cerebral infarction in apparently normal adults. Hypertension 2000; 36: 73–77.
- 28. Longstreth WT Jr, Bernick C, Manolio TA, et al Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol 1998; 55: 1217–1225.
- 29. Schmidt R, Schmidt H, Pichler M, et al C-reactive protein, carotid atherosclerosis, and cerebral small-vessel disease: results of the Austrian Stroke Prevention Study. Stroke 2006; 37: 2910–2916.
- 30. van Dijk EJ, Prins ND, Vermeer SE, et al C-reactive protein and cerebral small-vessel disease: the Rotterdam Scan Study. Circulation 2005; 112: 900–905.
- 31. Madai VI, von Samson-Himmelstjerna FC, Bauer M, et al Ultrahigh-field MRI in human ischemic stroke – a 7 tesla study. PLoS One 2012; 7: e37631.
- 32. van Veluw SJ, Hilal S, Kuijf HJ, et al Cortical microinfarcts on 3T MRI: clinical correlates in memory-clinic patients. Alzheimers Dement 2015; 11: 1500–1509.
- 33. Hilal S, Sikking E, Shaik MA, et al Cortical cerebral microinfarcts on 3T MRI: a novel marker of cerebrovascular disease. Neurology 2016; 87: 1583–1590.
- 34. Wang Z, van Veluw SJ, Wong A, et al Risk factors and cognitive relevance of cortical cerebral microinfarcts in patients with ischemic stroke or transient ischemic attack. Stroke 2016; 47: 2450–2455.
- 35. van Dalen JW, Scuric EE, van Veluw SJ, et al Cortical microinfarcts detected in vivo on 3 Tesla MRI: clinical and radiological correlates. Stroke 2015; 46: 255–257.
- 36. Dichgans M, Wardlaw J, Smith E, et al METACOHORTS for the study of vascular disease and its contribution to cognitive decline and neurodegeneration: an initiative of the joint programme for neurodegenerative disease research. Alzheimers Dement 2016; 12: 1235–1249.
- 37. Rosenberg GA, Wallin A, Wardlaw JM, et al Consensus statement for diagnosis of subcortical small vessel disease. J Cereb Blood Flow Metab 2016; 36: 6–25.