Introduction
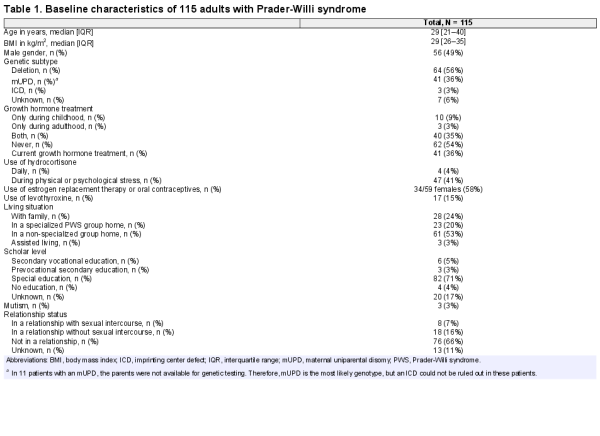
Prader-Willi syndrome (PWS) is a rare genetic, neuroendocrine condition caused by the absence of a normal paternal contribution to the 15q11-13 region. It is most commonly caused by a paternal deletion (65–75%) or a maternal uniparental disomy 15 ([mUPD], 20–30%). In the minority of cases, PWS is caused by an imprinting center defect (ICD, 1–3%) or a paternal chromosomal translocation (0.1%) (, ). The syndrome is characterized by hypotonia, behavioral challenges, typical dysmorphic features, and hypothalamic dysfunction resulting in hyperphagia, pituitary hormone deficiencies, abnormal temperature regulation, and inadequate pain registration ().
Annual mortality in adults with PWS is high (3%) (, ) compared with 1.3% annual mortality in non-PWS adults with an intellectual disability (). More than half of these deaths are caused by cardiopulmonary pathology (, ) and another 7% of deaths are directly related to obesity (). Seventy-eight percent of deaths in patients with PWS are unexpected ().
Multiple factors contribute to the increased risk of cardiopulmonary pathology in patients with PWS. Based on our clinical experience with more than a hundred PWS adults, we hypothesize that there is a complex interaction between obesity and behavioral, endocrine, and cardiovascular (CV) risk factors that contribute to the high prevalence of cardiopulmonary disease in patients with PWS, as shown in Fig. 1.
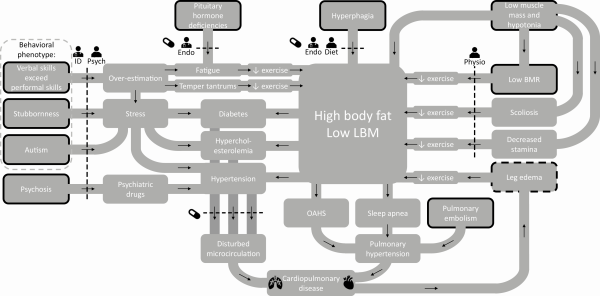
Figure 1
Factors contributing to cardiopulmonary disease in patients with PWS. Abbreviations: BMR, basal metabolic rate; diet ,dietitian; endo, endocrinologist; ID, physician for people with intellectual disabilities; LBM, lean body mass; OAHS, obesity associated hypoventilation syndrome; physio, physiotherapist; PWS, Prader-Willi syndrome; psych, psychologist. Legend: black arrows indicate a cause-and-effect relationship; dotted lines indicate an intervention; the stands for an intervention with medication; black borders indicate that the factor is inherent to the syndrome; dotted black border indicates that the factor is inherent to the syndrome, but can be aggravated by cardiopulmonary disease ().
Obesity in patients with PWS is caused by hyperphagia (leading to a high energy intake) combined with a low energy expenditure (, ). This low energy expenditure is caused by low muscle mass, which is part of the syndrome. Untreated pituitary hormone deficiencies like hypogonadism, hypothyroidism, and growth hormone deficiency can affect muscle mass and function, causing a further decrease in basal metabolic rate (, ).
The total energy expenditure in adults with PWS is 20% lower than in age-matched obese adults (). This difference in energy expenditure should be compensated by either a strict diet or by exercising for at least 1 hour a day (). However, exercise tolerance may be low, due to hypotonia, pituitary hormone deficiencies, and (severe) vitamin D deficiency (, ). Moreover, the typical behavioral phenotype and musculoskeletal problems like scoliosis, hypotonia, and leg edema impair physical activity in adults with PWS. This results in a vicious circle of muscle weakness, exercise intolerance, and a further decrease in physical activity. The subsequent sedentary lifestyle can induce CV risk factors like hypertension, hypercholesterolemia, and type 2 diabetes mellitus (DM2) (). Other CV risk factors often present in PWS are obesity hypoventilation syndrome and sleep apnea, which can be central sleep apnea, obstructive sleep apnea, or both. Central sleep apnea, obstructive sleep apnea, and obesity hypoventilation syndrome can lead to pulmonary hypertension (, ), DM2 (), and a further increase in obesity () and CV risk (). Lastly, the cognitive phenotype of PWS (often higher verbal comprehension skills compared with performal/reasoning skills, which can easily lead to overestimation) and autism-related behavioral challenges could induce stress. Stress can induce hypertension, another important CV risk factor (). Moreover, psychosis is prevalent in patients with PWS, often requiring psychiatric drugs. As many psychiatric drugs have CV side effects, this can lead to a further increase in CV risk ().
The complex interplay between somatic and psychological factors requires a syndrome-specific approach to health problems. However, as PWS is a rare disorder (), most physicians are unfamiliar with the syndrome and its associated comorbidities. Furthermore, the PWS-specific behavioral phenotype (high pain threshold and the inability to express complaints) often leads to underdiagnosis and undertreatment. Combined doctors’ and patients’ delay can lead to medical complications and hospital admission. Timely recognition of comorbidities can reduce medical complications and associated personal and financial burdens ().
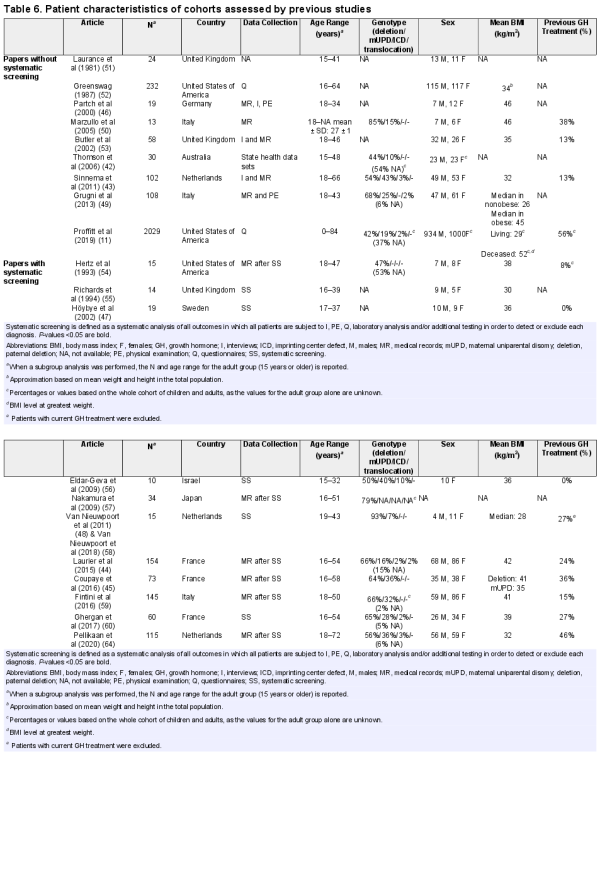
Previous authors have reported health problems in adults with PWS (, ). However, most of them did not perform a systematic screening and only reported health problems that had already been diagnosed. As underdiagnosis is a serious problem in this patient population, the prevalences reported in these studies are most likely underestimated. Data from systematic health screenings in adults with PWS are scarce (, , , , ) and little is known about the relation between patient characteristics (living situation, presence or absence of obesity, genotype, and demographic factors) and health problems. As a consequence, there is no consensus about periodical screening.
In our reference center, we routinely perform a systematic health screening in all adults with PWS in order to detect comorbidities at an early stage. In this article, we report the prevalence of the physical health problems detected by our screening. Based on their associations with the aforementioned patient characteristics, we provide practical advice for medical screening.
Methods
This study was approved by the Medical Ethics Committee of the Erasmus University Medical Center. In this retrospective study, we reviewed the medical files of all adults who visited the multidisciplinary outpatient clinic of the PWS reference center in the Erasmus University Medical Center, Rotterdam, Netherlands, between January 2015 and April 2020 and who underwent a routine systematic health screening. All patients that visited our outpatient clinic were already diagnosed with PWS, often years before visiting our outpatient clinic and/or during childhood. Before the launch of our multidisciplinary outpatient clinic in 2015, many Dutch adults with PWS were treated by their general practitioner or physician for people with intellectual disabilities (ID physician).
Our systematic screening consists of a structured interview, a complete physical examination, a medical questionnaire, a review of the medical records, biochemical measurements and, if indicated and feasible, additional tests such as dual energy X-ray absorptiometry (DEXA), polygraphy (PG), and polysomnography (PSG). We report the hidden health problems that were present but undetected and/or untreated until the moment of screening. Conditions that developed during later follow-up visits were not taken into account. Forty-two patients in the cohort that we describe were also mentioned in a previous study by Sinnema et al (), who gave an overview of 102 adults with PWS and the health problems that had already been diagnosed (without systematic screening).
Genetic diagnosis
We performed genetic testing or collected previous genetic test results from other Dutch academic hospitals to confirm the PWS diagnosis and to determine the genetic subtype.
Medical questionnaire
As part of regular patient care, primary caregivers filled out a medical questionnaire before visiting the outpatient clinic. This questionnaire included questions on the patient’s medical history, medication, family history, symptoms of disease, physical complaints, behavioral challenges, and social aspects such as school, relationships, and living situation. The symptoms of disease, physical complaints, and behavioral challenges are rated on a 5-point Likert scale (1 = rarely or never, 2 = not often and/or not severe, 3 = quite often and/or quite severe, 4 = often and/or severe, 5 = very often and/or very severe). A score of 3 or higher was considered clinically relevant and was further explored during the visit. Mutism is defined as the absence of speech.
Biochemical analysis
During the visit, blood samples were taken for general medical screening, including the evaluation of fat metabolism (low density lipoprotein [LDL]-cholesterol), glucose metabolism (nonfasting glucose, hemoglobin A1c), thyroid function (free T4), gonadal function (random luteinizing hormone [LH], follicle-stimulating hormone, estradiol or testosterone, sex hormone binding globulin), liver enzymes (aspartate transaminase, alanine transaminase, alkaline phosphatase, gamma glutamyl transpeptidase, total bilirubin, lactate dehydrogenase), kidney function (urea, creatinine, estimated glomerular filtration rate [eGFR]), the hematopoietic system (hemoglobin, hematocrit, mean corpuscular volume, leukocytes, thrombocytes and, in case of microcytic anemia, ferritin), and vitamin D status (25-hydroxyvitamin D). The eGFR is estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.
Cutoff levels.
A hypercholesterolemia diagnosis was confirmed if the patient had a nonfasting LDL-cholesterol above 4.0 mmol/L. Type 2 diabetes mellitus was defined as a repeated fasting glucose above 6.7 mmol/L (or nonfasting glucose above 11.0 nmol/L). Hemoglobin A1c was used to assess long-term glycemic control. As hypothyroidism in PWS can be both primary and central (), hypothyroidism was defined as a free T4 below 11 pmol/L, regardless of thyroid stimulating hormone. Hypogonadism in males was defined as a morning testosterone level below 10.0 nmol/L or a random testosterone level below 10.0 nmol/L combined with clear clinical features of hypogonadism (sparse body hair, micropenis, and the absence of spontaneous morning erections). Hypogonadism in females was defined as absent, scarce, or irregular menses. The diagnosis of hypogonadism was based on both laboratory values and clinical parameters because of the effect of adipose tissue aromatase activity on estradiol and testosterone levels (), and the fact that hypogonadism in PWS can be both primary and central (). Due to intellectual disability in most patients, gynecological evaluation was not routinely performed. When females used oral contraceptives or estrogen replacement therapy before screening, we asked for the presence of the menstrual cycle before the start of estrogen replacement therapy.
Severe vitamin D deficiency was defined as a 25-hydroxyvitamin D level below 20 nmol/L and a mild vitamin D deficiency was defined as a 25-hydroxyvitamin D level below 50 nmol/L. When patients used cholesterol-lowering medications, oral antidiabetics, insulin, levothyroxine, or testosterone replacement therapy before the start of the screening, we requested pretreatment laboratory values to verify the diagnosis.
Additional tests
We screened for hypertension by measuring blood pressure. If the patient’s blood pressure was above 140/90 mmHg, the measurement was repeated, and if it was still elevated, a 30-minute blood pressure measurement was performed. If the patient already used antihypertensive drugs, we requested pretreatment blood pressure values.
If risk factors for osteoporosis were present (untreated hypogonadism, family history of osteoporosis, previous fractures, untreated vitamin D deficiency, and/or corticosteroid treatment), we performed a DEXA scan to screen for osteoporosis or osteopenia. Osteoporosis was defined as a T-score (comparison of a person’s bone density with that of a healthy 30-year-old of the same sex) below -2.5, osteopenia was defined as a T-score between -1.0 and -2.5.
If there was a clinical suspicion of scoliosis (based on a gibbus deformity during physical examination), we performed an X-ray of the spine if (1) the patient was not previously diagnosed with scoliosis, (2) the patient suffered from back pain, or (3) the caregivers reported new or progressive postural abnormalities. Radiologically confirmed scoliosis was defined as a Cobb angle of 10° or more, as measured on a spinal X-ray.
The indication for sleep studies was based on the presence of clinical signs of sleep apnea: severe snoring, witnessed apneas, daytime sleepiness, morning headaches, hypertension, or waking up with shortness of breath, headaches, or panic. If indicated and feasible, we performed PG (ie, the continuous recording of nasal airflow, thoracic and abdominal movements, heart rate, and oxygen saturation during 1 night) or a PSG (ie, PG measurements and electroencephalography, electro-oculography, and electromyography). Also, before the start of growth hormone (GH) treatment, we performed a PSG to exclude sleep apnea, as untreated sleep apnea is an absolute contraindication for GH treatment.
Data analysis
Statistical analysis was performed using R version 3.6.3. Descriptive statistics for continuous variables are reported as median and interquartile range (IQR). For dichotomous variables, we display the number of people and the percentage of total people, n (%). We used a chi-squared test to compare the prevalence of health problems between different groups based on patient characteristics: genotype, living situation, and gender. To investigate the relationship between body mass index (BMI), age, patient characteristics, and the prevalence of health problems, we used the Wilcoxon rank sum test. For the relationship between BMI, age, and living situation, we used the Kruskal-Wallis test. A chi-squared test for trend was used to compare the number of undiagnosed health problems between subgroups. To investigate the relationship between age and BMI, the Kendall rank correlation test was used. To investigate the effect of BMI and age on health problems and the number of undiagnosed health problems corrected for age and BMI respectively, logistic and ordinal regression models were used and a likelihood ratio test was performed.
Literature review
We reviewed the literature for studies that report physical health problems in patients with PWS. Inclusion criteria were original research articles and observational studies that reported the prevalence of physical health problems in a cohort of patients with PWS of 15 years of age or older. Exclusion criteria were clinical trials, basic or translational research, case reports, case series that included less than 10 adults with PWS, articles that were not available online, articles that were not available in English, and mixed pediatric–adult articles that did not report separate prevalences for patients with PWS of 15 years of age or older. The full search strategy used is available upon request.
Results
We included 115 (56 male and 59 female) patients. Median age was 29 years (IQR 21–40) and median BMI 29 kg/m2 (IQR 26–35). Baseline characteristics are shown in Table 1. The exact age at diagnosis was known for 72 patients, of which 59 patients were diagnosed during childhood. Of 115 patients, 42 underwent transition after years of medical supervision at the pediatric multidisciplinary outpatient clinic. All patients referred by the pediatrician had a personal care plan. Of the remaining 73 patients, 17 patients had been followed by an endocrinologist elsewhere during the year before the screening. A total of 46 patients had been followed by an ID physician and 14 had never visited an adult endocrinologist or ID physician before.
We refer to the repository () for the following supplementary data: baseline characteristics and health problems by living situation and genotype; health problems by BMI, age, and gender; information about lifestyle, behavior, and physical complaints; details of biochemical analysis (liver panel, kidney function, hematopoiesis, and electrolyte values); and data about sleep apnea, bone mineral density, and vitamin D deficiency.
Health problems detected by screening
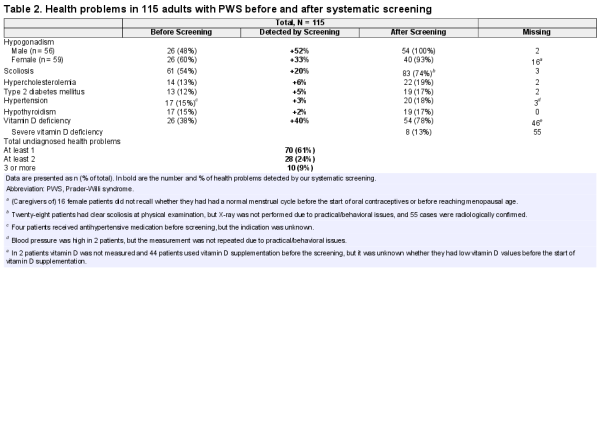
The results of our systematic health screening are shown in Table 2 and Fig. 2. We found undetected health problems in 61% of adults with PWS. One-fourth had more than 1 undetected simultaneous health problem. The most common undetected health problem was hypogonadism, which had gone unnoticed in 52% of males and 33% of females. Other undiagnosed health problems were scoliosis (20%), hypercholesterolemia (6%), DM2 (5%), hypertension (3%), and hypothyroidism (2%). Forty-five patients underwent DEXA scans as part of medical screening. This revealed 3 new cases of osteoporosis and 8 cases of osteopenia, on top of the 9 patients already known with osteoporosis and the 22 patients with osteopenia. Two males and 1 female (all older than 30 years of age during the screening) had osteoporosis despite previous treatment for hypogonadism. Both males had received testosterone replacement therapy for more than 15 years before screening. For the female, the exact duration of estrogen replacement therapy was unknown. Nine patients were known to have sleep apnea before the screening. Nineteen patients underwent PG or PSG, of which 11 patients were diagnosed with sleep apnea.
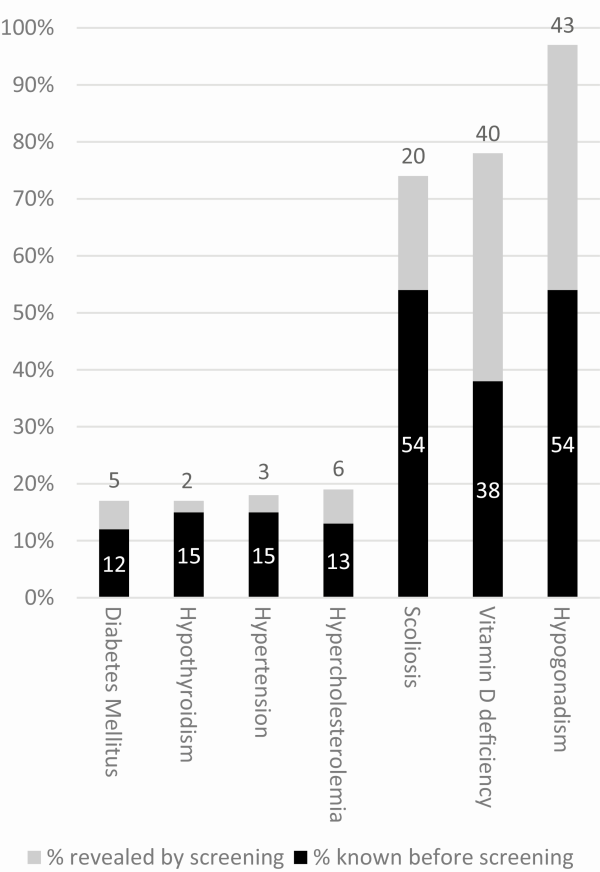
Figure 2
Health problems detected by systematic health screening in 115 adults with PWS. Abbreviation: PWS, Prader-Willi syndrome. Legend: black bars indicate the percentage of health problems already diagnosed before the screening; gray bars indicate the percentage of health problems that were revealed by screening.
Comparison of health problems between groups
Living situation.
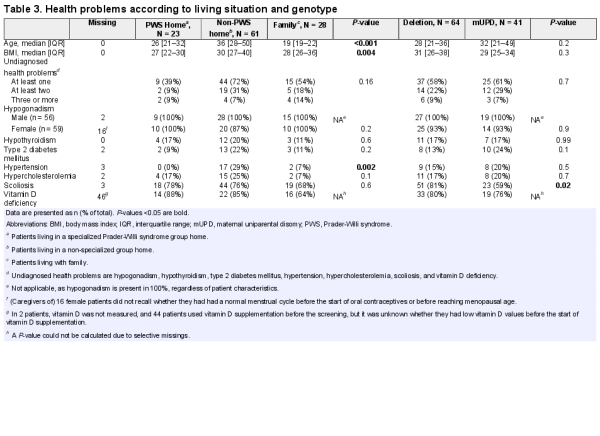
Twenty-three patients lived in a specialized PWS group home (PWS home), 61 in a nonspecialized group home (non-PWS home), 28 with family, and 3 in an assisted living facility. Patients living in non-PWS homes were significantly older (median age 36 years, IQR 28–50) than those living in PWS homes (median age 26 years, IQR 21–32) or with family (median age 19 years, IQR 19–22). Body mass index and prevalence of hypertension were significantly higher in patients living in non-PWS homes (see Table 3).
Patients in PWS homes exercised more than those living with family or in non-PWS homes. Patients in PWS homes all exercised at least 30 minutes a day versus 75% and 70% of those living with family or in non-PWS homes, respectively. A dietitian was involved in the care of 87% of patients living in PWS homes, 74% of those living in a non-PWS home, and 29% of those living with family.
Genotype.
When comparing health problems between the 2 largest genotypic subgroups (64 patients with a deletion and 41 with mUPD), scoliosis was more frequent in patients with a deletion than in patients with an mUPD (81% vs 59%, P = 0.02). Other variables were not remarkably different between the genotypes (see Table 3).
Body mass index.
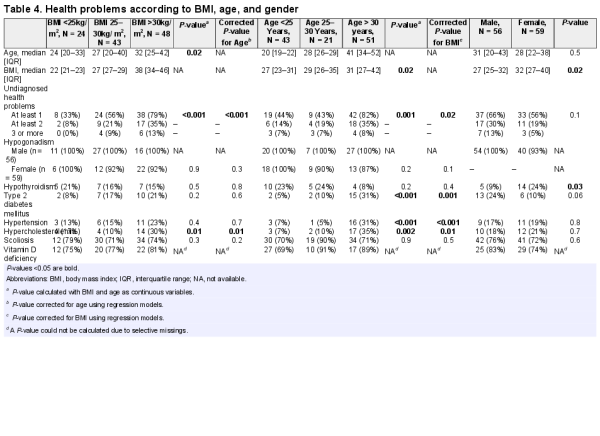
Body mass index increased with age (P = 0.02). Patients with a higher BMI had a higher total number of undiagnosed health problems (P < 0.001) and more hypercholesterolemia (P = 0.01) (see Table 4). This remained significant after correction for age.
Age.
Older patients had a higher prevalence of DM2 (P < 0.001), hypertension (P < 0.001), and hypercholesterolemia (P < 0.002), and a higher total number of undiagnosed health problems (P = 0.001) (see Table 4). This remained significant after correction for BMI.
Gender.
Body mass index was significantly higher in females than in males (P = 0.02). Hypothyroidism was more prevalent in females than in males (24% vs 9%, P = 0.03) (see Table 4).
Age and BMI.
Thirteen patients had BMI < 25kg/m2 and age < 25 years. None of these patients had DM2, 1 patient had hypertension, and 2 had hypercholesterolemia (of which 1 case was undiagnosed before screening).
Fatigue and daytime sleepiness
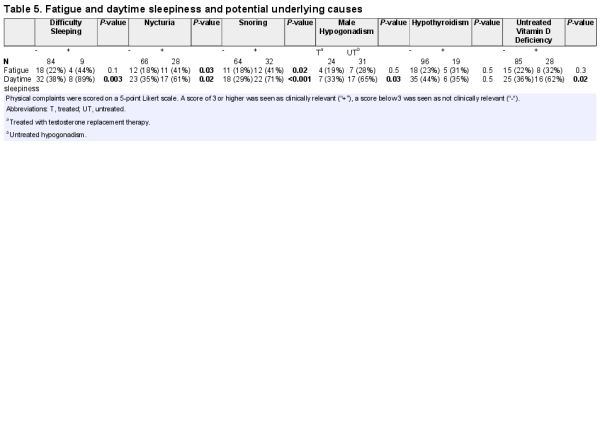
Fatigue and daytime sleepiness were common. One-third of the patients (40/115) had clinically relevant daytime sleepiness (score of 3 or higher on a 5-point Likert scale). All of these 40 patients had either untreated vitamin D deficiency, untreated male hypogonadism (Table 5), or another treatable cause such as sleep apnea, narcolepsia, nycturia, or use of drugs that can cause sleepiness (antiepileptic drugs, antipsychotics, benzodiazepines, tricyclic antidepressants, or antihistamines). Daytime sleepiness was present in 62% of the patients with untreated vitamin D deficiency versus 36% of the patients with normal vitamin D levels (P = 0.02). It was also related to the severity of the deficieny: daytime sleepiness was present in 80% of patients with untreated severe vitamin D defiency, 57% of patients with untreated mild vitamin D defiency, and 36% of patients with normal vitamin D levels.
Biochemical analysis
Liver panel was normal in most patients. However, 19 patients had alkaline phosphatase levels above the upper limit of normal. The vast majority of them had potential underlying causes such as vitamin D deficiency (63%) and/or obesity (58%). Normocytic anemia was common in males, but not in females. There were no cases of micro- or macrocytic anemia. Of the 17 males with anemia, 13 (76%) had untreated hypogonadism. Creatinine levels were generally low: 35 males (63%) and 28 females (47%) had creatinine levels below the lower limit of normal, and this was independent of BMI and age. Of all males, 95% had creatinine levels between 46 and 93µmol/L and eGFR levels between 93 and 149ml/min/1.73m2. Of all females, 95% had creatinine levels between 37 and 76 µmol/L and eGFR levels between 98 and 140 ml/min/1.73m2.
Literature review
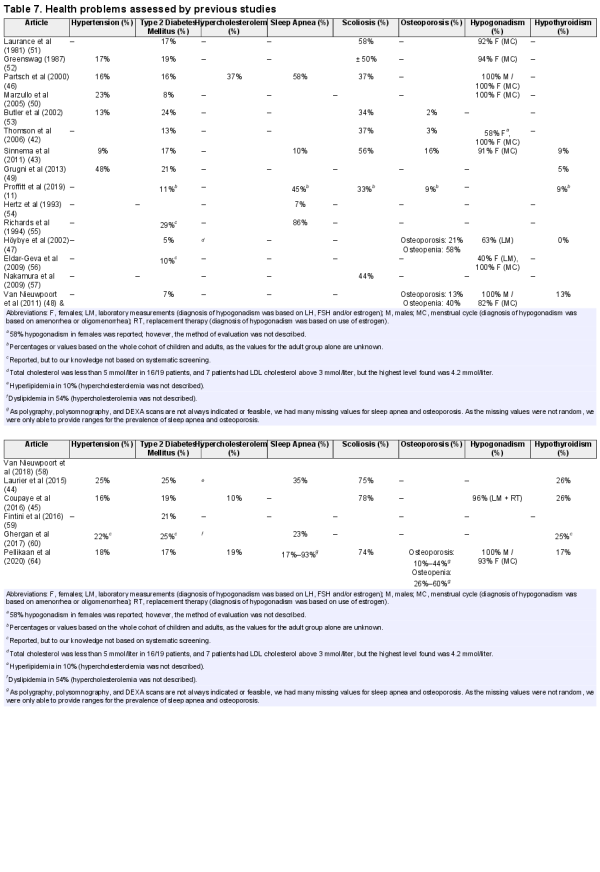
We found 21 publications reporting 1 or more of the following health problems in PWS: hypogonadism, hypothyroidism, DM2, hypertension, hypercholesterolemia, scoliosis, vitamin D deficiency, sleep apnea, or osteoporosis/osteopenia. Outcomes of these studies are summarized in Tables 6 and 7. None of the papers reported the prevalence of vitamin D deficiency in PWS.
Algorithm for diagnostics and treatment
Based on our analysis of patients data and the literature review, we defined diagnostic and therapeutic recommendations, presented in the algorithm in Fig. 3.
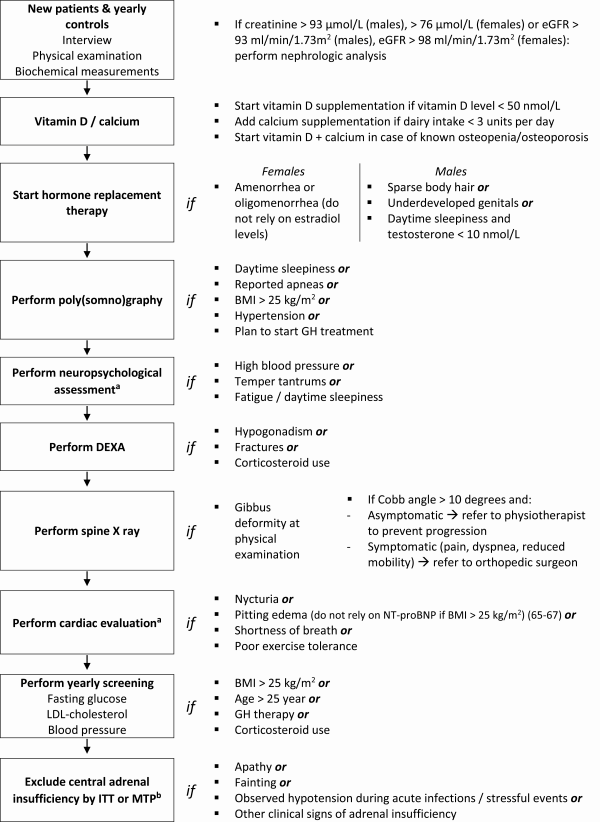
Figure 3
Algorithm for diagnostics and treatment in adults with PWS. Abbreviations: BMI, body mass index; DEXA, dual energy X-ray absorptiometry; FSH, follicle-stimulating hormone; FT4, free thyroxin; HbA1c, hemoglobin A1c; ITT, insulin tolerance test; LDL, low density lipoprotein; LH, luteinizing hormone; MTP, metyrapone test; PWS, Prader-Willi syndrome; SHBG, sex hormone binding globulin. aRecommendation based on expert opinion and literature review (). bBased on previously published data ().
Discussion
We found a large number of undetected health problems among adults with PWS during our systematic health screening. To our knowledge, we are the first to translate this into an evidence-based algorithm for screening and treatment of adults with PWS. We hypothesize that the high prevalence of undiagnosed health problems is the result of most physicians’ unfamiliarity with the syndrome, in combination with the complex PWS-specific behavioral phenotype.
Previous studies
Previous studies have reported prevalences of health problems in adults with PWS. However, most studies (, , , , ) did not perform a systematic screening. Of the 4 studies that performed a systematic health screening (, , , , ), only 2 (Laurier et al () and Coupaye et al () included a substantial number of (over 20) patients. Six studies (, , ) performed a systematic screening, but focused on only 1 health problem of interest. Compared to the systematic screening described by Laurier et al () and Coupaye et al (), we found a lower prevalence of DM2, scoliosis, and hypothyroidism. The difference in the prevalence of DM2 could be partly explained by the large difference in BMI, which was much lower in our cohort than in the French cohorts (Table 7). Moreover, the patients in our cohort had more often been treated with GH during childhood. Although GH treatment may have a short-term negative effect on glucose homeostasis due to increased insulin resistance, GH treatment also improves body composition and exercise tolerance, which has positive effects on glucose metabolism in the long term ().
Prader-Willi syndrome homes and non-PWS homes
Patients in specialized PWS homes had a lower BMI and a lower frequency of hypertension than patients living in non-PWS homes. This could probably be largely explained by the age difference between the groups. Another contributing factor could be that patients in a specialized PWS home are subject to strict supervision from trained personnel. In PWS homes, food is kept out of sight and food storages are locked. According to caregivers, this greatly reduces the stress and conflicts caused by food-seeking behavior. We hypothesize it might even prevent stress-related hypertension. The fact that all patients living in PWS homes received portion-controlled meals (as determined by a dietitian) and often exercised under supervision probably explained part of the difference in the BMI between patients living in PWS homes and those living in non-PWS homes.
Fatigue and daytime sleepiness
Fatigue and daytime sleepiness were very common problems among adults with PWS. According to caregivers, these complaints often prevented them from taking part in day trips and physical activities. Daytime sleepiness is usually attributed to a lack of hypothalamic arousal and is regarded as a problem that is inherent to the syndrome. However, when we looked in more detail, all patients with clinically relevant fatigue or daytime sleepiness had treatable underlying problems such as sleep apnea, narcolepsia, nycturia, vitamin D deficiency, untreated male hypogonadism, or use of drugs that can cause sleepiness. Although we could not perform a randomized controlled trial to assess whether treating these underlying problems resolved the complaints, our clinical experience is that the majority of the patients reported less fatigue after treatment of the underlying cause. Also, caregivers reported that these patients were more actively participating in daily activities. This indicates that daytime sleepiness is not necessarily just “part of the syndrome”, but could be the symptom of an underlying, treatable problem. Treating the underlying cause is important to reduce daytime sleepiness and increase physical activity.
Vitamin D deficiency and hypogonadism
Both vitamin D deficiency and hypogonadism are frequently present in adults with PWS. Low levels of vitamin D and testosterone are often attributed to obesity, as vitamin D is fat-soluble and testosterone production can be diminished by increased estradiol levels due to adipose tissue aromatase activity. However, lean male patients also had hypogonadism and vitamin D defiency. Although there is no consensus on the clinical effects of vitamin D (, ), we found a clear relation between (the severity of) vitamin D deficiency and daytime sleepiness. Although the cause of daytime sleepiness in this complex patient population is likely to be multifactorial, we believe that prescribing vitamin D may be beneficial for all PWS adults with vitamin D deficiency. The high prevalence of osteoporosis and osteopenia in adults with PWS combined with the fact that vitamin D knows little side effects () are additional arguments for treatment. Therefore, we recommend prescribing vitamin D supplementation in all adults with PWS with a vitamin D level below 50 ng/mL.
Creatinine levels
Creatinine levels were low in the majority of patients, regardless of sex and BMI. This indicates that normal creatinine levels in patients with PWS are lower than in healthy controls, which is explained by their low muscle mass (). Therefore, in PWS patients, presence of high-normal creatinine levels might actually indicate impaired renal function. We recommend to adjust the reference values with –24% for males and –18% for females. In our hospital, the PWS-specific reference range for creatinine is 46 to 93 µmol/L (compared with 65–115 µmol/L for non-PWS adult males) and 37 to 76 µmol/L for females (compared with 55–90 µmol/L for non-PWS adult females). For the same reasons, we propose using PWS-specific reference values for eGFR of >98 ml/min/1.73m2 for PWS adult males and >93 ml/min/1.73m2 for PWS adult females.
Adrenal insufficiency
This is rare in adults with PWS (). However, in cases of clinical signs of hypocortisolism, we recommend assessing the hypothalamic-pituitary-adrenal axis using the metyrapone test or, in the absence of contraindications, the insulin tolerance test (see Fig. 3).
Strengths and limitations
Like every study, our study has strengths and limitations. The strengths of our study are the large sample size (considering the fact that PWS is a rare syndrome), its focus on adults, and the fact that we investigated health problems in relation to living situation, BMI, genotype, and demographic factors, thus making our study a powerful source of new information. Limitations may include selection bias (due to selective referral to our specialized facility) and survival bias. Moreover, we have many missing values for osteoporosis and sleep apnea. These additional tests were not always performed because they were either not indicated or impossible to perform due to behavioral issues. Therefore, these results should be interpreted with caution.
Conclusion
We found undetected health problems in 61% of adults with PWS. On top of this, one-third of the patients had clinically relevant fatigue or daytime sleepiness which, according to caregivers, prevented them from taking part in physical activities. Although daytime sleepiness is usually considered just “part of the syndrome,” all of these patients turned out to have treatable causes such as sleep apnea, narcolepsia, nycturia, vitamin D deficiency, untreated male hypogonadism, or use of drugs that can cause sleepiness. Therefore, fatigue and daytime sleepiness should be considered not just “part of the syndrome,” but the symptom of an underlying health problem. We recommend exploring and treating these underlying causes in order to optimize physical activity and prevent obesity-related cardiopulmonary problems. We provide an algorithm for diagnostics and treatment, taking into account PWS-specific pitfalls like falsely low creatinine levels and false-normal cardiac markers. Use of the algorithm will optimize the mental and physical health of adults with PWS. This will improve exercise tolerance and reduce the personal and financial burden of cardiopulmonary complications in this vulnerable patient group.
Acknowledgments
We thank the patients with PWS, their families, and their caregivers who contributed to this study.
Author Contributions: K.P. collected all data, wrote the first draft of the manuscript, was involved in all revisions of the manuscript, and performed the statistical analysis. A.J.v.d.L. and L.d.G. were responsible for the conception and design of the study. All authors were involved in data interpretation, revision of the manuscript, and final approval of the manuscript.
ALAT: alanine transaminase
ALP: alkaline phosphatase
ASAT: aspartate transaminase
BMI: body mass index
CV: cardiovascular
DEXA: dual energy x-ray absorptiometry
DM2: type 2 diabetes mellitus
eGFR: estimated glomerular filtration rate
FSH: follicle-stimulating hormone
FT4: free thyroxin
GGT: gamma glutamyl transpeptidase
GH: growth hormone
ICD: imprinting center defect
IQR: interquartile range
LDH: lactate dehydrogenase
LDL: low-density lipoprotein
LH: luteinizing hormone
mUPD: maternal uniparental disomy
PG: polygraphy
PSG: polysomnography
PWS: Prader-Willi syndrome
SHBG: sex hormone binding globulin
References
- 1. Cheon CK. Genetics of Prader-Willi syndrome and Prader-Will-Like syndrome. Ann Pediatr Endocrinol Metab.2016;21(3):126–135.
- 2. Cassidy SB, Driscoll DJ. Prader-Willi syndrome. Eur J Hum Genet.2009;17(1):3–13.
- 3. Angulo MA, Butler MG, Cataletto ME. Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest.2015;38(12):1249–1263.
- 4. Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M; speakers contributors at the Second Expert Meeting of the Comprehensive Care of Patients with PWS. Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab.2008;93(11):4183–4197.
- 5. Cassidy SB. Prader-Willi syndrome. J Med Genet.1997;34(11):917–923.
- 6. Holm VA, Cassidy SB, Butler MG, et al Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics.1993;91(2):398–402.
- 7. Whittington JE, Holland AJ, Webb T, Butler J, Clarke D, Boer H. Population prevalence and estimated birth incidence and mortality rate for people with Prader-Willi syndrome in one UK Health Region. J Med Genet.2001;38(11):792–798.
- 8. Butler MG, Manzardo AM, Heinemann J, Loker C, Loker J. Causes of death in Prader-Willi syndrome: Prader-Willi Syndrome Association (USA) 40-year mortality survey. Genet Med.2017;19(6):635–642.
- 9. Hosking FJ, Carey IM, Shah SM, et al Mortality among adults with intellectual disability in England: comparisons with the general population. Am J Public Health.2016;106(8):1483–1490.
- 10. Pacoricona Alfaro DL, Lemoine P, Ehlinger V, et al Causes of death in Prader-Willi syndrome: lessons from 11 years’ experience of a national reference center. Orphanet J Rare Dis.2019;14(1):238.
- 11. Proffitt J, Osann K, McManus B, et al Contributing factors of mortality in Prader-Willi syndrome. Am J Med Genet A.2019;179(2):196–205.
- 12. Schoeller DA, Levitsky LL, Bandini LG, Dietz WW, Walczak A. Energy expenditure and body composition in Prader-Willi syndrome. Metabolism.1988;37(2):115–120.
- 13. Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med.2012;14(1):10–26.
- 14. Cobo G, Gallar P, Di Gioia C, et al Hypogonadism associated with muscle atrophy, physical inactivity and ESA hyporesponsiveness in men undergoing haemodialysis. Nefrologia.2017;37(1):54–60.
- 15. Speakman JR, Selman C. Physical activity and resting metabolic rate. Proc Nutr Soc.2003;62(3):621–634.
- 16. Zheng X, Cheng Q, Long J, et al Prevalence of low lean mass in patients with adult growth hormone deficiency with or without low-dose growth hormone therapy. Clin Endocrinol (Oxf).2019;90(6):834–841.
- 17. Kraemer WJ, Ratamess NA, Hymer WC, Nindl BC, Fragala MS. Growth hormone(s), testosterone, insulin-like growth factors, and cortisol: roles and integration for cellular development and growth with exercise. Front Endocrinol (Lausanne).2020;11:33.
- 18. Tzankoff SP, Norris AH. Effect of muscle mass decrease on age-related BMR changes. J Appl Physiol Respir Environ Exerc Physiol.1977;43(6):1001–1006.
- 19. Bekx MT, Carrel AL, Shriver TC, Li Z, Allen DB. Decreased energy expenditure is caused by abnormal body composition in infants with Prader-Willi Syndrome. J Pediatr.2003;143(3):372–376.
- 20. Yavuz S, Salgado Nunez Del Prado S, Celi FS. Thyroid hormone action and energy expenditure. J Endocr Soc.2019;3(7):1345–1356.
- 21. Salvatore D, Simonides WS, Dentice M, Zavacki AM, Larsen PR. Thyroid hormones and skeletal muscle–new insights and potential implications. Nat Rev Endocrinol.2014;10(4):206–214.
- 22. Butler MG, Theodoro MF, Bittel DC, Donnelly JE. Energy expenditure and physical activity in Prader-Willi syndrome: comparison with obese subjects. Am J Med Genet A.2007;143A(5):449–459.
- 23. United States. Department of Health and Human Services., United States. Department of Agriculture., United States. Dietary Guidelines Advisory Committee. Dietary Guidelines for Americans. 6th ed. Washington, D.C.: G.P.O; 2005.
- 24. Roy S, Sherman A, Monari-Sparks MJ, Schweiker O, Hunter K. Correction of low vitamin D improves fatigue: effect of correction of low vitamin D in fatigue study (EViDiF Study). N Am J Med Sci.2014;6(8):396–402.
- 25. Nowak A, Boesch L, Andres E, et al Effect of vitamin D3 on self-perceived fatigue: a double-blind randomized placebo-controlled trial. Medicine (Baltimore).2016;95(52):e5353.
- 26. Beckmann Y, Türe S, Duman SU. Vitamin D deficiency and its association with fatigue and quality of life in multiple sclerosis patients. Epma J.2020;11(1):65–72.
- 27. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet.2017;390(10101):1550–1562.
- 28. Heidelbaugh JJ. Endocrinology update: testicular hypogonadism. FP Essent.2016;451:31–41.
- 29. Galesanu C, Mocanu V. Vitamin D deficiency and the clinical consequences. Rev Med Chir Soc Med Nat Iasi.2015;119(2):310–318.
- 30. Young DR, Hivert MF, Alhassan S, et al; Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; and Stroke Council. Sedentary behavior and cardiovascular morbidity and mortality: a science advisory from the American Heart Association. Circulation.2016;134(13):e262–e279.
- 31. Kholdani C, Fares WH, Mohsenin V. Pulmonary hypertension in obstructive sleep apnea: Is it clinically significant? A critical analysis of the association and pathophysiology. Pulm Circ.2015;5(2):220–227.
- 32. Almeneessier AS, Nashwan SZ, Al-Shamiri MQ, Pandi-Perumal SR, BaHammam AS. The prevalence of pulmonary hypertension in patients with obesity hypoventilation syndrome: a prospective observational study. J Thorac Dis.2017;9(3):779–788.
- 33. Lacedonia D, Carpagnano GE, Patricelli G, et al Prevalence of comorbidities in patients with obstructive sleep apnea syndrome, overlap syndrome and obesity hypoventilation syndrome. Clin Respir J.2018;12(5):1905–1911.
- 34. Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest.2010;137(3):711–719.
- 35. Floras JS. Sleep apnea and cardiovascular disease: an enigmatic risk factor. Circ Res.2018;122(12):1741–1764.
- 36. Athayde RAB, Oliveira Filho JRB, Lorenzi Filho G, Genta PR. Obesity hypoventilation syndrome: a current review. J Bras Pneumol.2018;44(6):510–518.
- 37. Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S; INCOSACT Initiative (International Collaboration of Sleep Apnea Cardiovascular Trialists). Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation.2017;136(19):1840–1850.
- 38. Castro-Añón O, Pérez de Llano LA, De la Fuente Sánchez S, et al Obesity-hypoventilation syndrome: increased risk of death over sleep apnea syndrome. Plos One.2015;10(2):e0117808.
- 39. Boone JL. Stress and hypertension. Prim Care.1991;18(3):623–649.
- 40. Piña IL, Di Palo KE, Ventura HO. Psychopharmacology and cardiovascular disease. J Am Coll Cardiol.2018;71(20):2346–2359.
- 41. Chevreul K, Berg Brigham K, Clément MC, Poitou C, Tauber M; Members of the BURQOL-RD Research Network listed in the Online Appendix. Economic burden and health-related quality of life associated with Prader-Willi syndrome in France. J Intellect Disabil Res.2016;60(9):879–890.
- 42. Thomson AK, Glasson EJ, Bittles AH. A long-term population-based clinical and morbidity review of Prader-Willi syndrome in Western Australia. J Intellect Disabil Res.2006;50(Pt 1):69–78.
- 43. Sinnema M, Maaskant MA, van Schrojenstein Lantman-de Valk HM, et al Physical health problems in adults with Prader-Willi syndrome. Am J Med Genet A.2011;155A(9):2112–2124.
- 44. Laurier V, Lapeyrade A, Copet P, et al Medical, psychological and social features in a large cohort of adults with Prader-Willi syndrome: experience from a dedicated centre in France. J Intellect Disabil Res.2015;59(5):411–421.
- 45. Coupaye M, Tauber M, Cuisset L, et al Effect of genotype and previous GH treatment on adiposity in adults with Prader-Willi syndrome. J Clin Endocrinol Metab.2016;101(12):4895–4903.
- 46. Partsch CJ, Lämmer C, Gillessen-Kaesbach G, Pankau R. Adult patients with Prader-Willi syndrome: clinical characteristics, life circumstances and growth hormone secretion. Growth Horm IGF Res.2000;10 Suppl B:S81–S85.
- 47. Höybye C, Hilding A, Jacobsson H, Thorén M. Metabolic profile and body composition in adults with Prader-Willi syndrome and severe obesity. J Clin Endocrinol Metab.2002;87(8):3590–3597.
- 48. van Nieuwpoort IC, Sinnema M, Castelijns JA, Twisk JW, Curfs LM, Drent ML. The GH/IGF-I axis and pituitary function and size in adults with Prader-Willi syndrome. Horm Res Paediatr.2011;75(6):403–411.
- 49. Grugni G, Crinò A, Bedogni G, et al Metabolic syndrome in adult patients with Prader-Willi syndrome. Nutr Metab Cardiovasc Dis.2013;23(11):1134–1140.
- 50. Marzullo P, Marcassa C, Campini R, et al The impact of growth hormone/insulin-like growth factor-I axis and nocturnal breathing disorders on cardiovascular features of adult patients with Prader-Willi syndrome. J Clin Endocrinol Metab.2005;90(10):5639–5646.
- 51. Laurance BM, Brito A, Wilkinson J. Prader-Willi syndrome after age 15 years. Arch Dis Child.1981;56(3):181–186.
- 52. Greenswag LR. Adults with Prader-Willi syndrome: a survey of 232 cases. Dev Med Child Neurol.1987;29(2):145–152.
- 53. Butler JV, Whittington JE, Holland AJ, Boer H, Clarke D, Webb T. Prevalence of, and risk factors for, physical ill-health in people with Prader-Willi syndrome: a population-based study. Dev Med Child Neurol.2002;44(4):248–255.
- 54. Hertz G, Cataletto M, Feinsilver SH, Angulo M. Sleep and breathing patterns in patients with Prader Willi syndrome (PWS): effects of age and gender. Sleep.1993;16(4):366–371.
- 55. Richards A, Quaghebeur G, Clift S, Holland A, Dahlitz M, Parkes D. The upper airway and sleep apnoea in the Prader-Willi syndrome. Clin Otolaryngol Allied Sci.1994;19(3):193–197.
- 56. Eldar-Geva T, Hirsch HJ, Rabinowitz R, Benarroch F, Rubinstein O, Gross-Tsur V. Primary ovarian dysfunction contributes to the hypogonadism in women with Prader-Willi Syndrome. Horm Res.2009;72(3):153–159.
- 57. Nakamura Y, Nagai T, Iida T, Ozeki S, Nohara Y. Epidemiological aspects of scoliosis in a cohort of Japanese patients with Prader-Willi syndrome. Spine J.2009;9(10):809–816.
- 58. van Nieuwpoort IC, Twisk JWR, Curfs LMG, Lips P, Drent ML. Body composition, adipokines, bone mineral density and bone remodeling markers in relation to IGF-1 levels in adults with Prader-Willi syndrome. Int J Pediatr Endocrinol.2018;2018(1).
- 59. Fintini D, Grugni G, Bocchini S, et al; Genetic Obesity Study Group of the Italian Society of Pediatric Endocrinology and Diabetology (ISPED). Disorders of glucose metabolism in Prader-Willi syndrome: results of a multicenter Italian cohort study. Nutr Metab Cardiovasc Dis.2016;26(9):842–847.
- 60. Ghergan A, Coupaye M, Leu-Semenescu S, et al Prevalence and phenotype of sleep disorders in 60 adults with prader–Willi syndrome. Sleep2017; 40(12).
- 61. Iughetti L, Vivi G, Balsamo A, et al Thyroid function in patients with Prader-Willi syndrome: an Italian multicenter study of 339 patients. J Pediatr Endocrinol Metab.2019;32(2):159–165.
- 62. Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab.2012;23(2):83–89.
- 63. Hirsch HJ, Eldar-Geva T, Bennaroch F, Pollak Y, Gross-Tsur V. Sexual dichotomy of gonadal function in Prader-Willi syndrome from early infancy through the fourth decade. Hum Reprod.2015;30(11):2587–2596.
- 64. Pellikaan K, Rosenberg AGW, Kattentidt-Mouravieva AA, et al Supplementary data for: Missed Diagnoses and Health Problems in Adults With Prader-Willi Syndrome: Recommendations for Screening and Treatment RePub Erasmus University Reposittory 2020. Deposited July 9, 2020. hdl.handle.net/1765/128375.
- 65. Buckley LF, Canada JM, Del Buono MG, et al Low NT-proBNP levels in overweight and obese patients do not rule out a diagnosis of heart failure with preserved ejection fraction. ESC Heart Fail.2018;5(2):372–378.
- 66. Clerico A, Giannoni A, Vittorini S, Emdin M. The paradox of low BNP levels in obesity. Heart Fail Rev.2012;17(1):81–96.
- 67. Krauser DG, Lloyd-Jones DM, Chae CU, et al Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am Heart J.2005;149(4):744–750.
- 68. Rosenberg AGW, Pellikaan K, Poitou C, et al Central adrenal insufficiency is rare in adults with Prader-Willi syndrome. J Clin Endocrinol Metab2020; 105(7):e2563–e2571.
- 69. Vogt KS, Emerick JE. Growth hormone therapy in adults with Prader-Willi syndrome. Diseases.2015;3(2):56–67.
- 70. Hong S, Chang Y, Jung HS, Yun KE, Shin H, Ryu S. Relative muscle mass and the risk of incident type 2 diabetes: A cohort study. Plos One.2017;12(11):e0188650.
- 71. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol.2016;94(2):146–150.
- 72. LeFevre ML, LeFevre NM. Vitamin D screening and supplementation in community-dwelling adults: common questions and answers. Am Fam Physician.2018;97(4):254–260.
- 73. Reid IR. Vitamin D effect on bone mineral density and fractures. Endocrinol Metab Clin North Am.2017;46(4):935–945.
- 74. Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet.2014;383(9912):146–155.
- 75. Malihi Z, Wu Z, Mm Lawes C, Scragg R. Noncalcemic adverse effects and withdrawals in randomized controlled trials of long-term vitamin D2 or D3 supplementation: a systematic review and meta-analysis. Nutr Rev.2017;75(12):1007–1034.