Iodine is a micronutrient essential for thyroid hormone production, for which the recommended intake for US adults is 150 µg daily. Iodine is present in contrast medium that is commonly required for radiologic studies in health care settings; a single dose of iodine contrast administered for computed tomography (CT) scans, coronary angiography, and other radiologic procedures may contain up to 13 500 μg of free iodine and 15 to 60 g of bound iodine, amounts that equate to several hundred times the recommended daily requirement for iodine ().
Iodine-induced hyperthyroidism is a well-recognized clinical entity that results from the Jöd-Basedow phenomenon, in which an iodine load provides a large supply of iodine required for thyroid hormone synthesis, resulting in increased circulating thyroid hormone levels (). Although most individuals are able to adapt to acute iodine exposure, certain susceptible subgroups (eg, those with thyroid nodules or a history of iodine deficiency) are at higher risk for iodine-induced hyperthyroidism, thought to be due to impaired thyroid regulation in such individuals ().
The risks of hyperthyroidism may be particularly concerning among older individuals, in whom its associated adverse health effects may worsen underlying health conditions. Atrial fibrillation may be seen in up to 60% of individuals with hyperthyroidism, depending on age, sex, and underlying cardiovascular disease (). Given the known risk of hyperthyroidism following iodine exposure and the long-term adverse health outcomes of atrial fibrillation, including embolic events, heart failure, and death (), it is imperative to elucidate the relationships between iodine-induced hyperthyroidism and incident risk of atrial fibrillation/flutter in alignment with a 2019 white paper (reflecting an expert panel convened by the U.S. National Heart, Lung, and Blood Institute) calling for additional research on the inter-relationships between thyroid and cardiovascular diseases (). However, robust evidence is lacking on this topic to date.
To address this knowledge gap, we conducted a cohort study of the U.S. Veterans Health Administration to investigate the associations between iodine-induced hyperthyroidism and the incident risks of atrial fibrillation/flutter. Given the older age and high prevalence of comorbidities in U.S. Veterans (), including cardiovascular disease burden (), this population may be particularly vulnerable to the adverse effects of hyperthyroidism. A better understanding of these relationships would help clinicians consider the importance of monitoring for hyperthyroidism following iodinated contrast use in select population subgroups.
Materials and Methods
Study Design and Population
The study population was extracted from the Veterans Affairs (VA) Corporate Data Warehouse database, the national repository of clinical and administrative records from all U.S. Veterans Health Administration patients in both hospitalized and ambulatory settings, from March 10, 1988, to October 20, 2021. The eligible sample included adults aged ≥18 years with a follow-up serum thyrotropin (TSH) measurement within 60 days after iodine contrast administration, and up to 1 year of a normal baseline serum TSH concentration.
We then excluded subjects with a history of hypothyroidism, hyperthyroidism, atrial fibrillation/flutter, heart failure, thyroid surgery, thyroid cancer, radioactive iodine treatment, use of thyroid hormone or antithyroid medications, and use of other medications that may alter serum thyroid function (Table 1A ()). To ascertain the use of non-VA medications and electronic medical records that may not be present in VA Corporate Data Warehouse (), exclusion criteria were also extracted and cross-referenced against the VA Observation Medical Partnership Database (). The process of study sample selection is shown in Fig. 1. The study was approved by the VA Greater Los Angeles Healthcare System Institutional Review Board.
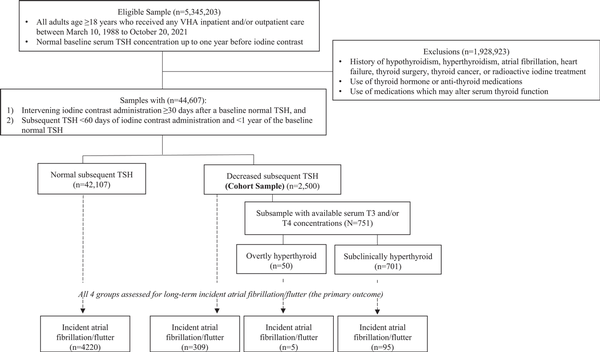
Figure 1
Flow chart of study sample selection.
Ascertainment of Iodine-Induced Hyperthyroidism and Atrial Fibrillation/Flutter
Our exposure was defined by a follow-up serum TSH measurement less than the lower limit of each VA site-specific reference range within 60 days after iodine contrast administration, and restricted to only those TSH values available up to 1 year after a normal baseline serum TSH level. Hyperthyroid individuals with available serum triiodothyronine (T3) and/or thyroxine (T4) concentrations were further categorized into overt hyperthyroidism (decreased TSH and elevated T3 and/or T4 levels) and subclinical hyperthyroidism (decreased TSH and normal T3 and/or T4 levels).
Iodine contrast administration was determined using ICD-9 and ICD-10 codes of radiologic procedures that employed iodinated contrast media (Table 1B ()). The outcome was atrial fibrillation/flutter identified using ICD-9 and ICD-10 codes (427.3X and I48.X, respectively, where X denotes any number).
Covariates
Covariates included age, sex, race/ethnicity (Hispanic, non-Hispanic White, non-Hispanic Black, and non-Hispanic other races), body mass index, and history of several comorbidities. Body mass index was recorded as the most proximal measurement within 1 year of the TSH measurement. History of several comorbidities was extracted based on ICD-10 codes: coronary heart disease (I21, I22, I23, I24, I25), dyslipidemia (E78), diabetes (E08, E09, E11, E13), and hypertension (I10, I11, I12, I13, I15, I16).
Statistical Analyses
Summary statistics of the study cohort were reported as mean ± SD for numerical characteristics and frequency (%) for categorical characteristics. Inverse propensity score–weighted cumulative incidence curves for incident atrial fibrillation were generated according to thyroid status, with weight adjusting for age, sex, race/ethnicity, body mass index, and history of coronary heart disease, dyslipidemia, diabetes, and hypertension. We then employed Cox proportional hazards regression models to estimate the adjusted hazard ratio (HR) with 95% CI of the association between iodine-induced hyperthyroidism and incident atrial fibrillation/flutter, adjusting for age, sex, race/ethnicity, body mass index, and history of coronary heart disease, dyslipidemia, diabetes, and hypertension. The date of the follow-up TSH result was designated as time 0 relative to incident atrial fibrillation/flutter.
To evaluate the heterogeneity in the association by demographic characteristics, we also stratified the analyses by sex (male, female), age (<65 years, ≥ 65 years), and race/ethnicity (Hispanic, non-Hispanic White, non-Hispanic Black, and non-Hispanic others). Statistical significance was defined as 2-sided α < .05.
We also conducted the following 3 sensitivity analyses. First, we reanalyzed the data using 2 shorter thresholds of the duration (ie, up to 12 and up to 24 months), in addition to our main analysis performed without time restriction, of the date of atrial fibrillation/flutter during long-term follow-up. Second, we assessed the strength of the association according to the type of radiologic study requiring iodinated contrast (ie, iodinated CT scans, angiograms, venograms, cystograms, hysterosalpingograms, urograms, and other iodinated radiologic procedures). Third, we calculated the E value to quantify the minimum strength of the association between an unmeasured confounder and both the exposure (iodine-induced hyperthyroidism) and the outcome (atrial fibrillation/flutter), conditional on the measured covariates, to determine whether or not this could explain the observed overall exposure–outcome association (). All analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
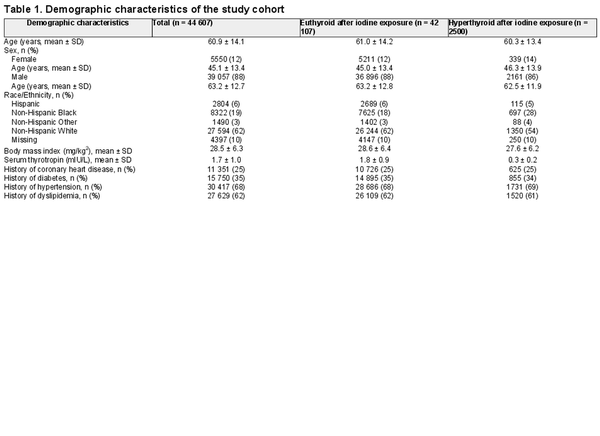
Among 44 607 Veterans defined as our cohort sample (mean ± SD age, 60.9 ± 14.1 years; 88% men), there were 2500 (5.6%) individuals found to be hyperthyroid (mean ± SD TSH, 0.30 ± 0.18 mIU/L) within 60 days after iodine contrast exposure and a previously normal baseline serum TSH (Fig. 1). Hyperthyroid individuals were more likely to be female and non-Hispanic Black than those who were euthyroid (Table 1).
Hyperthyroidism Following Iodine Exposure and Incident Atrial Fibrillation
Over a median follow-up period of 3.7 years (IQR 1.9-7.4), atrial fibrillation/flutter was observed in 4629 (10.4%) individuals, composed of 409 individuals who had developed hyperthyroidism following iodine exposure and 4220 who had remained euthyroid following iodine exposure (Fig. 1). In Cox regression models adjusting for sociodemographic and cardiovascular risk factors, hyperthyroidism after iodine exposure in the setting of a previously normal baseline TSH was associated with an increased risk of atrial fibrillation (HR 1.19, 95% CI 1.06-1.33; Fig. 2); subanalyses of those with available peripheral thyroid hormone levels (serum T3 and/or T4) were unable to be performed due to the small sample sizes of those subgroups.
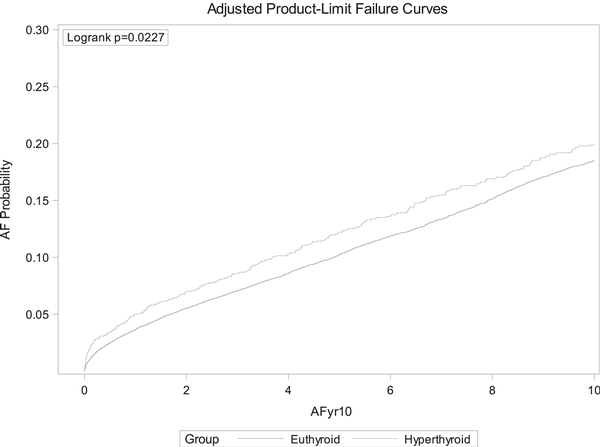
Figure 2
Cumulative incidence of atrial fibrillation according to thyroid function (euthyroid vs hyperthyroid) following iodine exposure.
Stratified Analyses by Age, Sex, and Race/Ethnicity
In stratified analyses, we found no evidence of heterogeneity in the association between hyperthyroidism following an iodine load and atrial fibrillation/flutter by age (Fig. 3). The association was stronger among females than males (females, HR 1.81, 95% CI 1.12-2.92; males, HR 1.15, 95% CI 1.03-1.30; P for interaction between hyperthyroidism and sex = .04). Across race/ethnicity, the association was the strongest among Hispanic (HR 1.60, 95% CI 0.96-2.66) followed by non-Hispanic Black (HR 1.23, 95% CI 0.89-1.69) and non-Hispanic White (HR 1.07, 95% CI 0.93-1.23), although the estimates did not have enough statistical power to detect heterogeneity due to limited sample size.
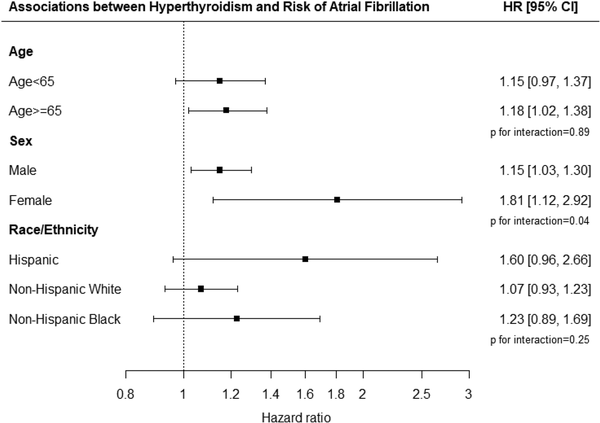
Figure 3
Associations between hyperthyroidism and risk of atrial fibrillation by age, sex, and race/ethnicity.
Sensitivity Analyses
The risks remained significant in the sensitivity analysis restricting the detection of incident atrial fibrillation/flutter to shorter durations; HR was 1.44 (1.19-1.75, P = .0002) for incident atrial fibrillation/flutter <12 months, and HR was 1.35 (1.15-1.59, P = .0003) for incident atrial fibrillation/flutter <24 months. The sensitivity analysis examining incident atrial fibrillation/flutter by type of radiologic study requiring iodinated contrast showed similar results. Iodine-induced hyperthyroidism was associated with a greater risk of atrial fibrillation/flutter compared with those who remained euthyroid after iodine administration, both in those who had received iodine contrast for a CT scan (iodine-induced hyperthyroidism, 281/2252 [12.5%]) vs iodine-induced euthyroidism, 3786/36 705 [10.3%]; HR 1.15, 95% CI 1.02-1.3, P = .0239) and in those who had received iodine contrast for all other types of radiologic imaging (iodine-induced hyperthyroidism, 28/248 [11.3%] vs iodine-induced euthyroidism, 434/5401 [8.0%]; HR 1.76, 95% CI 1.24-2.49, P = .0017). Finally, examination of the E value showed that an unmeasured confounder would need to be associated with both hyperthyroidism and incident atrial fibrillation/flutter with an HR >1.67 to explain away the observed overall association conditional on measured covariates.
Discussion
In this cohort study of the largest integrated health care system in the United States, hyperthyroidism observed within 60 days of an acute iodine load was associated with a significantly increased risk of incident atrial fibrillation/flutter over a median follow-up of 3.7 years. The association was stronger among females than males. The sex-specific differences seen are notable, as women are more likely to die from atrial fibrillation-related stroke than men (), which may be related to different thresholds of membrane potential and action potential duration between the sexes (). Although sex-specific data focused on cardiovascular disease have been increasing steadily, this understanding of sex as a biologic variable has not been routinely translated into clinical practice ().
The findings shed further understanding on the complex relationship between thyroid dysfunction and cardiac arrhythmias (). As thyroid hormone receptors are present in the myocardium and vascular tissue, mechanistic data show a reduction of circulating endothelial progenitor cells and impaired endothelial function in hyperthyroidism, through both the genomic and nongenomic effects of T3 (). In human studies, both short- and long-term cardiovascular risks of hyperthyroidism have been described (); furthermore, hyperthyroidism-related atrial fibrillation is thought to confer higher risks of ischemic stroke and systemic embolism than atrial fibrillation resulting from nonthyroidal causes (). In longitudinal epidemiologic studies, abnormally low serum TSH concentration (such as in inadequately treated Graves disease or in hypothyroidism that is over-replaced with thyroid hormone) is associated with both cardiovascular morbidity and mortality (, ). A recent meta-analysis of 13 studies that included 649 293 participants showed that both subclinical and overt hyperthyroidism was associated with a risk of incident atrial fibrillation over a mean follow-up period of 7.1 years (range 1.0-13.2) ().
Our previous longitudinal analysis of the U.S. Veterans Health Administration system reported that there is only a small increased risk of thyroid dysfunction following iodine contrast administration, with males in particular having a higher risk (1.4-fold) than unexposed individuals (), findings which support only targeted screening of thyroid dysfunction after iodine exposure. In accordance with those findings, the European Thyroid Association recommends an individualized approach toward whether or not to ascertain iodine-induced thyroid dysfunction after iodine administration on the basis of the patient's age, clinical symptoms, the presence of any pre-existing thyroid diseases, coexisting morbidities, and iodine intake (, ). In the United States, there are currently no clinical recommendations for the generalized screening or monitoring of at-risk patients receiving iodinated contrast radiologic scans, with the exception of infants aged <3 years in whom the developing thyroid gland remains immature. In March 2022, the U.S. Food and Drug Administration issued guidance to screen for thyroid dysfunction within 3 weeks of intravascular iodinated contrast medium administration in this particularly vulnerable subgroup (, ). The present study suggests that in the select individuals who are found to have iodine-induced hyperthyroidism, thyroidal dysfunction poses a long-term risk of atrial fibrillation/flutter, and such patients should be considered for increased monitoring. At present, the mechanism for a long-lasting effect is unknown but may be related to alternations in cardiovascular hemodynamics following thyroid dysfunction. Rigorous future data examining parameters evaluating changes in cardiac rhythm and/or structure in such individuals are needed.
Strengths of this study include its large sample size, use of a highly detailed demographic, medical/surgical, laboratory, radiologic, and pharmacy database, and the use of the largest integrated health care system in the United States (considered an overall iodine-sufficient population), thereby allowing for the long-term capture of incident atrial fibrillation/flutter. In addition, analysis was restricted to the observation of individuals with iodine-induced thyroid dysfunction following a baseline normal serum TSH concentration within the past year. These data are the first to link the understanding of the thyroid dysfunction risks following iodine exposure and the arrhythmia-related risks of hyperthyroidism.
Our study has some limitations. First, as with any database analysis, potential misclassifications due to miscoding of inclusion and exclusion criteria, iodinated contrast use, and incident atrial fibrillation/flutter are possible, and the outcome of these cardiac arrhythmias was not further ascertained by electrocardiograms. Second, thyroid dysfunction was denoted by an abnormal serum TSH that may not always reflect hyperthyroidism (ie, nonthyroidal acute illness), although all included subjects were required to have a baseline normal serum TSH prior to iodine exposure. Also, as routine thyroid function (ie, TSH) monitoring following iodine contrast administration is not standardly obtained in the United States, we acknowledge that ascertainment bias of iodine-induced hyperthyroidism is possible, although this would not alter the associations observed between this exposure and incident atrial fibrillation/flutter. Third, we cannot rule out the possibility of unmeasured confounding between iodine-induced hyperthyroidism and atrial fibrillation, including smoking status, that we felt reflected substantial missing and/or potentially inaccurate capture in this dataset. However, the E value in our sensitivity analysis (1.67) indicated that it is unlikely for such unmeasured confounders to fully explain away the observed overall association between iodine-induced hyperthyroidism and atrial fibrillation. It should be noted that caution is needed when extrapolating our results to external populations, given that VA data are composed primarily of older, non-Hispanic, White men. Fourth, known risk factors for iodine-induced thyroid dysfunction such as thyroid nodules and iodine deficiency were unable to be captured. Since thyroid nodules and goiter are known to be significantly underdiagnosed compared with autopsy data (), they were not excluded and/or adjusted for in our study. In addition, as the United States is considered generally iodine sufficient () and iodine status cannot be reliably ascertained in a given individual (), iodine status could not be determined at the individual patient level for this existing electric database study. Finally, thyroid hormone action and cardiac arrhythmias are related by the complex interplay of many factors (), including genetically predicted thyroid hormone levels even within the normal range (, ), the positive association between serum free thyroxine levels (but not TSH) and atrial fibrillation from individual participant data in a study of 30 085 subjects (), and a possible bidirectional effect (), such that the reasons for increased atrial fibrillation/flutter risk seen in younger individuals in our study require further investigation. In addition, our study's finding that females have a higher risk of atrial fibrillation/flutter following iodine-induced hyperthyroidism than males may be related to the higher prevalence of thyroid autoimmunity in females (), as well as differences in genetically determined thyroid hormone traits () and risks of atrial fibrillation between the sexes (). Further study of the associations in a more sex-diverse study population spanning a larger age range are needed.
In summary, among US adults, hyperthyroidism following iodine exposure was associated with a significantly increased risk of incident atrial fibrillation/flutter over a median follow-up of 3.7 years. Given the high prevalence of asymptomatic atrial fibrillation/flutter in elderly people, and increased risk of stroke and systemic embolism in patients with atrial fibrillation/flutter, the European, Canadian, and Australian guidelines currently advocate for the universal screening of atrial fibrillation in those age ≥65 years (). Regarding the population analyzed in the current study, we believe the present data support the need for further research to refine the clinical significance of this issue. Although the results demonstrate an increased risk of incident atrial fibrillation/flutter following iodine-induced thyroid dysfunction, particularly among females, the cost–benefit analysis of such long-term monitoring and identification of underlying risk factors requires further investigation using sex-specific analytic approaches.
Abbreviations
CT: computed tomography
T3: triiodothyronine
T4: thyroxine
TSH: thyrotropin
References
- 1. Lee SY, Rhee CM, Leung AM, Braverman LE, Brent GA, Pearce EN. A review: radiographic iodinated contrast media-induced thyroid dysfunction. J Clin Endocrinol Metab. 2015;100(2):376–383.
- 2. Stanbury JB, Ermans AE, Bourdoux P, et al Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid. 1998;8(1):83–100.
- 3. Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol. 2014;10(3):136-142.
- 4. Shimizu T, Koide S, Noh JY, Sugino K, Ito K, Nakazawa H. Hyperthyroidism and the management of atrial fibrillation. Thyroid. 2002;12(6):489–493.
- 5. Martin FI, Deam DR. Hyperthyroidism in elderly hospitalised patients. Clinical features and treatment outcomes. Med J Aust. 1996;164(4):200–203.
- 6. Frost L, Vestergaard P, Mosekilde L. Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Arch Intern Med. 2004;164(15):1675–1678.
- 7. January CT, Wann LS, Calkins H, et alWriting Group Members. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16(8):e66–e93.
- 8. Cappola AR, Desai AS, Medici M, et al Thyroid and cardiovascular disease research agenda for enhancing knowledge, prevention, and treatment. Thyroid. 2019;29(6):760–777.
- 9. Bloomfield H, Linsky A, Bolduc J, et al Deprescribing for Older Veterans: A Systematic Review. U.S. Department of Veterans Affairs; 2019.
- 10. Al Rifai M, Blumenthal RS, Stone NJ, et al Department of Veterans Affairs (VA) and U.S. Department of Defense (DoD) guidelines for management of dyslipidemia and cardiovascular disease risk reduction: putting evidence in context. Prog Cardiovasc Dis. 2021;68:2–6.
- 11. Inoue K, Guo R, Lee ML, et al. Iodine-induced hyperthyroidism and long-term risks of incident atrial fibrillation and flutter: Supplemental Table 1. Available at: https://data.mendeley.com/datasets/b7gcc2db3y/1
- 12. Lynch K. The VA OMOP Common Data Model: The Basics. May 9, 2019. Accessed March 13, 2023.https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=3632.
- 13. Viernes B, Lynch KE, South B, Coronado G, DuVall SL. Characterizing VA users with the OMOP common data model. Stud Health Technol Inform. 2019;264:1614–1615.
- 14. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274.
- 15. Prabakaran S, Gongora Nieto MC, Lundberg G. Atrial fibrillation in women: risks and management. J Womens Health (Larchmt). 2018;27(1):107–114.
- 16. Ko D, Rahman F, Schnabel RB, Yin X, Benjamin EJ, Christophersen IE. Atrial fibrillation in women: epidemiology, pathophysiology, presentation, and prognosis. Nat Rev Cardiol. 2016;13(6):321–332.
- 17. Garcia M, Mulvagh SL, Merz CNB, Buring JE, Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res. 2016;118(8):1273–1293.
- 18. Bekiaridou A, Kartas A, Moysidis DV, et al The bidirectional relationship of thyroid disease and atrial fibrillation: established knowledge and future considerations. Rev Endocr Metab Disord. 2022;23(3):621–630.
- 19. Phowira J, Bakhashab S, Doddaballapur A, Weaver JU. Subclinical thyrotoxicosis and cardiovascular risk: assessment of circulating endothelial progenitor cells, proangiogenic cells, and endothelial function. Front Endocrinol (Lausanne). 2022;13:894093.
- 20. Razvi S, Jabbar A, Pingitore A, et al Thyroid hormones and cardiovascular function and diseases. J Am Coll Cardiol. 2018;71(16):1781–1796.
- 21. Bielecka-Dabrowa A, Mikhailidis DP, Rysz J, Banach M. The mechanisms of atrial fibrillation in hyperthyroidism. Thyroid Res. 2009;2(1):4.
- 22. Gencer B, Cappola AR, Rodondi N, Collet TH. Challenges in the management of atrial fibrillation with subclinical hyperthyroidism. Front Endocrinol (Lausanne). 2021;12:795492.
- 23. Kim K, Yang PS, Jang E, et al Increased risk of ischemic stroke and systemic embolism in hyperthyroidism-related atrial fibrillation: a nationwide cohort study. Am Heart J. 2021;242:123–131.
- 24. Okosieme OE, Taylor PN, Evans C, et al Primary therapy of Graves' disease and cardiovascular morbidity and mortality: a linked-record cohort study. Lancet Diabetes Endocrinol. 2019;7(4):278–287.
- 25. Evron JM, Hummel SL, Reyes-Gastelum D, Haymart MR, Banerjee M, Papaleontiou M. Association of thyroid hormone treatment intensity with cardiovascular mortality among US Veterans. JAMA Netw Open. 2022;5(5):e2211863.
- 26. Huang M, Yang S, Ge G, Zhi H, Wang L. Effects of thyroid dysfunction and the thyroid-stimulating hormone levels on the risk of atrial fibrillation: a systematic review and dose-response meta-analysis from cohort studies. Endocr Pract. 2022;28(8):822–831.
- 27. Inoue K, Guo R, Lee ML, et al Iodinated contrast administration and risks of thyroid dysfunction: a retrospective cohort analysis of the U.S. Veterans health administration system. Thyroid. 2023;33(2):230–238.
- 28. van der Molen AJ, Thomsen HS, Morcos SKContrast Media Safety CommitteeEuropean Society of Urogenital Radiology. Effect of iodinated contrast media on thyroid function in adults. Eur Radiol. 2004;14(5):902-907.
- 29. Bednarczuk T, Brix TH, Schima W, Zettinig G, Kahaly GJ. 2021 European Thyroid Association guidelines for the management of iodine-based contrast media-induced thyroid dysfunction. Eur Thyroid J. 2021;10(4):269–284.
- 30. Callahan MJ, Iyer RS, Wassner AJ. Is thyroid monitoring warranted in infants and young children after intravascular administration of iodine-based contrast media?AJR Am J Roentgenol. 2022;220(1):144–145.
- 31. U.S. Food and Drug Administration. FDA recommends thyroid monitoring in babies and young children who receive injections of iodine-containing contrast media for medical imaging. March 30, 2022. Accessed March 13, 2023.https://www.fda.gov/drugs/drug-safety-and-availability/fda-recommends-thyroid-monitoring-babies-and-young-children-who-receive-injections-iodine-containing
- 32. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553–559.
- 33. Iodine Global Network. Accessed April 3, 2023https://www.ign.org
- 34. Leung AM. What's the best measure of population iodine status? It's Not a simple answer. Am J Clin Nutr. 2019;110(4):797–798.
- 35. Ellervik C, Roselli C, Christophersen IE, et al Assessment of the relationship between genetic determinants of thyroid function and atrial fibrillation: a Mendelian randomization study. JAMA Cardiol. 2019;4(2):144–152.
- 36. Gammage MD, Parle JV, Holder RL, et al Association between serum free thyroxine concentration and atrial fibrillation. Arch Intern Med. 2007;167(9):928–934.
- 37. Baumgartner C, da Costa BR, Collet TH, et al Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation. Circulation. 2017;136(22):2100–2116.
- 38. Hollowell JG, Staehling NW, Flanders WD, et al Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499.
- 39. McLachlan SM, Hamidi S, Aliesky H, Williams RW, Rapoport B. Sex, genetics, and the control of thyroxine and thyrotropin in mice. Thyroid. 2014;24(7):1080–1087.
- 40. Cheng EY, Kong MH. Gender differences of thromboembolic events in atrial fibrillation. Am J Cardiol. 2016;117(6):1021–1027.
- 41. Hindricks G, Potpara T, Dagres N, et al 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC), developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498.
- 42. Brieger D, Amerena J, Attia J, et alNHFA CSANZ Atrial Fibrillation Guideline Working Group National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Heart Lung Circ. 2018;27(10):1209–1266.
- 43. Andrade JG, Aguilar M, Atzema C, et al The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36(12):1847–1948.