Since receiving its first Food and Drug Administration approval in 2011, tumor immunotherapy has emerged as 1 of the most sought-after oncology treatments available today. Immune checkpoints are small molecules expressed by immune cells, playing a critical role in maintaining immune homeostasis. However, tumor cells can suppress the body's immune response by overexpressing inhibitory checkpoint molecules, enabling them to evade immune surveillance and promote the growth of tumor cells (, ). This phenomenon has underscored the need for novel immunotherapeutic strategies to enhance the body's natural immune response against cancer. Immune checkpoint inhibitors (ICIs) are a class of drugs that have shown impressive clinical benefits in the treatment of advanced malignancies (). They work by blocking immunosuppressive molecules and reactivating the function of effector T cells, which can then specifically target and kill tumor cells. Currently, there are 3 main categories of ICIs in clinical use: monoclonal antibodies targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed death receptor 1 (PD-1), and programmed death ligand-1 (PD-L1) (). Although ICIs have the potential to modulate the immune response and kill tumors, they can also overactivate the immune cells, leading to autoimmune damage in the body, also known as immune-related adverse events (irAEs) (). The endocrine system is frequently affected by immune-related disorders, such as thyroid dysfunction, pituitary inflammation, parathyroid dysfunction, primary adrenal insufficiency, and insulin-deficient diabetes (). Multiple studies have demonstrated that immune-related thyroid dysfunction (ITD) is a common endocrine immune disorder, often occurring within weeks to months after beginning drug administration. In the early stages, most clinical symptoms associated with ICIs are relatively insidious and nonspecific; however, if left unrecognized or unaddressed, they can impede the treatment of patients with ICIs and may even be life-threatening in severe cases (, ). Additionally, subgroup analysis of CheckMate141 revealed a higher incidence of immune-related endocrine adverse reactions in the Asian population than the global average (). Nevertheless, these studies primarily compared the incidence of thyroid dysfunction with different drugs, with only a few reports on the influencing factors and clinical characteristics.
Most of the available data on ICI-associated ITD come from pharmaceutical clinical studies, where the inclusion of participants is strictly screened, so there is a gap between their data and the real world. Therefore, we describe the clinical characteristics of ICI-related thyroid dysfunction and explore the risk factors through a case-control study.
Materials and Methods
Study Design and Participants
We report a retrospective analysis of patients with malignancies who were hospitalized at the Second Affiliated Hospital of Nanchang University for ICIs from January 2019 to December 2021. All subjects underwent thyroid function tests (TFTs) before starting immunotherapy and thyroid function was rechecked every 2 to 3 weeks while receiving ICIs.
Inclusion criteria were (1) having a clinically or pathologically histologically confirmed malignancy, (2) receiving at least 2 cycles of ICIs on a 2-week or 3-week regimen, (3) complete clinical history, (4) age ≥ 18 years, and (5) having assessable baseline information before receiving ICIs.
Exclusion criteria were (1) serious organic diseases of the heart, liver, and kidney and autoimmune deficiency diseases; (2) abnormal levels of baseline TFTs; (3) incomplete clinical data; (4) having thyroid disease, thyroid surgery; (5) confirmation that thyroid function is abnormal because of a toxic nodular goiter; (6) the malignant tumor is thyroid cancer or thyroid metastasis; and (7) having received radiotherapy in the thyroid region.
Definitions
The diagnostic criteria for immune-related thyroid dysfunction refer to the 2020 edition of the National Comprehensive Cancer Network guidelines for the management of immune-related toxicity (). Clinical thyrotoxicosis is characterized by decreased serum TSH and elevated free thyroxine (FT4) and free triiodothyronine (FT3) concentrations; subclinical thyrotoxicosis is characterized by decreased serum TSH and normal FT3/FT4 ratio. Clinical hypothyroidism is characterized by elevated serum TSH and reduced FT4/FT3 ratio; subclinical hypothyroidism is characterized by elevated serum TSH and normal FT3/FT4 ratio.
Thyroid dysfunction was assessed in patients treated with ICIs with reference to the Common Terminology Criteria for Adverse Events grading issued by the National Cancer Institute (). The ITD was classified into grades 1 to 5 (1 = asymptomatic, requiring only clinical or diagnostic observation; 2 = symptomatic, with no restriction of daily activities; 3 = severe symptoms, with restriction of daily activities, requiring hospitalization; 4 = life-threatening, requiring urgent intervention; 5 = lethal).
Assay Characteristics
Serum levels of FT3, FT4, TSH, antithyroid microsomal antibody (TMAb), and thyroglobulin antibody (TgAb) were measured by immunochemiluminescence. The normal reference range for FT3 is 2.3 to 4.2 pg/mL and the normal reference range for FT4 is 0.89 to 1.8 ng/dL. TMAb and TgAb titers with values >32 IU/mL and >15 IU/mL are considered positive, respectively.
Procedures and Outcomes
The primary outcomes are the prevalence, timing of occurrence, and factors influencing the development of ITD in a cohort of patients with malignancies treated with ICIs. In this paper, we describe the clinical features, autoantibody levels, and impact on outcome of patients with ITD.
The time to onset of ITD is defined as the time from initiation of treatment with ICIs to the first documented laboratory evaluation and clinical signs consistent with immune-related thyroid dysfunction documented during the course of the disease.
Statistical Analysis
The statistical analysis was conducted using R software (version 4.2.1; https://www.r-project.org) and SPSS 25.0. Continuous variables were summarized as median and interquartile range or frequencies and percentages reported for categorical variables. Baseline characteristics of patients with ITD are compared with those of patients without ITD in Table 1 using the Mann-Whitney U test for continuous outcome variables and outcome variables for categorical outcome
variables. Associations between demographic factors and ITD were assessed using multivariable logistic regression that included all factors with P < .05 from a univariate analysis. Results of multivariable logistic regression are presented as odds ratio (OR) and associated 95% CI. Survival differences between patients with ITD and patients without ITD were compared using Kaplan-Meier log-rank analysis. The effect of ITD on overall survival (OS) was estimated using Cox regression adjusted for age, sex, and ICI type. P < .05 was considered a statistically significant difference.
Results
Comparison of General Information
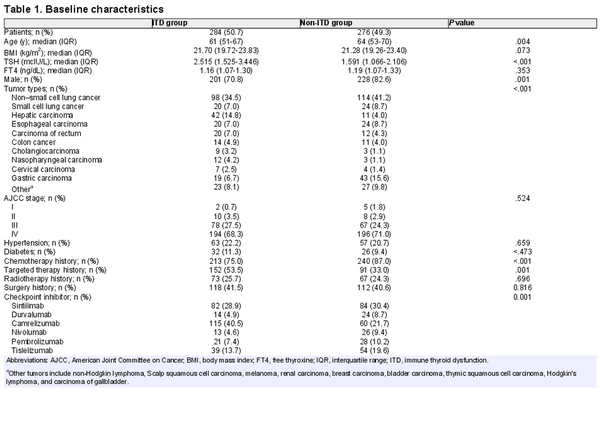
A total of 560 patients met the inclusion criteria and 284 developed ITD, with an incidence of 50.7%. As can be seen by Table 1, the median age of patients starting treatment with ICIs was 59.82 (24-95) years among all included patients, 76.6% of whom were male (n = 429). The primary tumor types included were predominantly non–small cell carcinoma of the lung (n = 212), gastric cancer (n = 62), hepatocellular carcinoma (n = 53), and small cell carcinoma of the lung (n = 44). Stage III (n = 145) and IV (n = 390) tumor stages were predominant at the time of treatment with ICIs. The majority of patients had received treatment such as radiotherapy, chemotherapy, or targeted drugs before treatment with ICIs. The included ICIs were dominated by sintilimab (n = 166), tislelizumab (n = 93), and camrelizumab (n = 175). The remaining baseline characteristics included medical history (whether combined with hypertension and/or diabetes), smoking status, and alcohol consumption status.
As seen in Table 1, the age of patients in the ITD group was lower than that in the non-ITD group (P = .004; Table 1), the incidence of ITD was significantly higher in female patients than in males (P = .001; Table 1), and the baseline TSH was significantly higher in the ITD group than in the control group (P < .001; Table 1). In terms of primary tumor, there was a statistically significant difference in the incidence of ITD among different cancer types in the 2 groups (P < .001; Table 1). In terms of previous treatment history, the incidence of ITD was significantly higher in patients who had received chemotherapy and targeted therapy than in those who had not (P < .001 for chemotherapy, P < .001 for targeted therapy; Table 1). The differences in ITD incidence among the 6 different ICIs were statistically significant (P < .001; Table 1).
Clinical Features of ITD due to ICIs and Time of Occurrence
Among ITD patients, 62 (21.8%) had clinical hypothyroidism and 117 (41.2%) had subclinical hypothyroidism, 87 (30.6%) had clinical thyrotoxicosis, and 18 (6.4%) had subclinical thyrotoxicosis. There were 272 patients (95.8%) with grade 1 or 2 thyroid dysfunction, who showed only minor adverse effects such as discomfort and fatigue. Twelve patients (4.2%) experienced grade 3 adverse effects, with TSH increased above 100 mcIU/L and were hospitalized after discontinuation of immunotherapy, including 9 who became euthyroid and immediately resumed immunotherapy after receiving L-T4. Three patients did not continue immunotherapy until the end of the follow-up period (121 days in 1 case, 78 days in 1 case, and 67 days in 1 case). Eighty-seven patients with ICIs did not receive treatment for thyroid dysfunction and only 1 patient with clinical thyrotoxicosis received antithyroid medication. Fifty-five of 179 patients with hypothyroidism and subclinical hypothyroidism received L-T4 replacement therapy, all at a starting dose of 50 µg every day. No patient developed cortisol deficiency.
The median time to ITD in patients receiving ICIs medication was 73 (34.5-149) days, with the shortest being only 9 days and the longest 983 days. The median time to ITD was 83.5 days (33-168 days) in the patients who developed hypothyroidism and 64 days (37-107 days) in those who developed thyrotoxicosis with a statistically significant difference between the 2 (P = .027; Supplementary Fig. 1 ) ().
Comparison of the Occurrence of ITD due to Different ICIs
In this study, 6 ICI drugs were used to compare the differences in ITD incidence among different types of drugs. As seen in Table 1 and Supplementary Table S1(), there was a statistically significant difference in the incidence of ITD among the 6 drugs (P < .001), with the highest incidence of camrelizumab (65.7%) and the lowest incidence of nivolumab (33.3%). The median time difference in the occurrence of ITD by drug was also large for camrelizumab (64 days), sintilimab (84 days), pembrolizumab (116.5 days), durvalumab (82 days), tislelizumab (69 days), and nivolumab (34.5 days).
Analysis of Factors Associated With the Occurrence of ITD due to ICIs
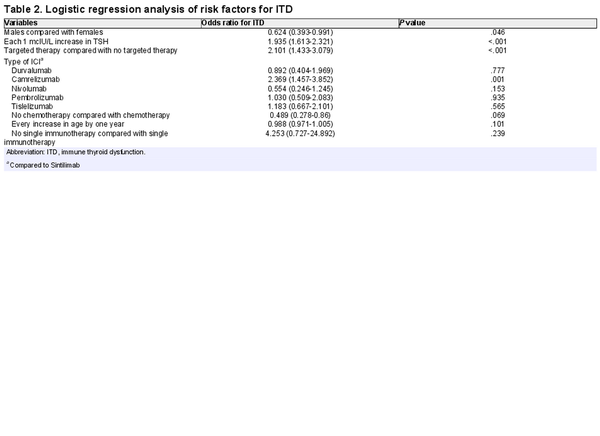
Previous studies have shown that the occurrence of ITD due to ICIs is associated with sex, age, baseline TSH, history of previous treatment, and type of ICI drug. We analyzed the presence or absence of correlation between ITD and selected variables in case and control groups by χ2 test and independent samples t-test. We included sex, age, baseline TSH, history of chemotherapy, history of targeted therapy, combination therapy, and type of ICIs in a binary logistic regression analysis. The results showed that being female, baseline TSH, history of targeted therapy, and type of ICIs used were associated with the occurrence of ITD (P < .05; Table 2).
Evolution of Patients With Immune-related Thyroiditis
Of the 105 patients initially diagnosed with thyrotoxic ITD, 46 (43.8%) developed hypothyroidism over the course of the disease, which resembled thyroiditis with transient thyrotoxicosis. Baseline characteristics of these 46 patients are described in Supplementary Table S2 (). The median age at initiation of treatment with ICIs was 55 years (from 30 to 75 years). The most common primary tumor types were non–small cell carcinoma of the lung (39.1%), esophageal cancer (13%), and colon cancer (13%). Thirty-three cases (71.8%) were treated with camrelizumab, the predominant ICI type. The median baseline TSH for those treated with ICIs was 2.479 mcIU/L (1.455-3.430 mcIU/L).
All 46 patients had normal TFTs before starting treatment with ICIs. The median time to onset of thyrotoxicosis was 61.5 days (range, 13-495 days). The median time to onset of hypothyroidism was 130.5 days (from 19 to 604 days). The median duration of the thyrotoxic phase was 72 days (from 4 to 286 days). Of the 46 patients, 32 (69.7%) started L-T4 replacement therapy after the onset of hypothyroidism. Six (12.1%) patients recovered from transient hypothyroidism without L-T4 replacement. Eight (18.2%) patients were not given L-T4 replacement therapy after the onset of hypothyroidism and had not returned to normal thyroid function until the end of the follow-up time. One of the 46 patients started methimazole therapy 34 days after the development of thyrotoxicosis when the FT4 was 3.4 ng/dL and TSH 0.004 mcIU/L, hypothyroidism occurred after 69 days, so methimazole was discontinued. L-T4 replacement was given to this patient afterwards, but thyroid function had not returned to normal until the end of the follow-up time (225 days). Thirty-two patients on L-T4 replacement therapy from the start of treatment with ICIs were still on L-T4 at a median follow-up time of 442 days (range, 193-858 days). All 32 patients were given a starting dose of 25 µg/d or 50 µg/d of L-T4. The thyroiditis time profile is shown in Fig. 1.
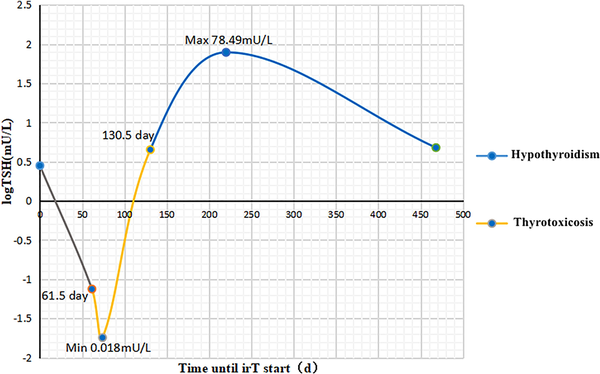
Figure 1
Timeline of thyroiditis. The median time to thyrotoxicosis was 61.5 days (range, 13-495 days). The median time to hypothyroidism was 130.5 days (from 19 to 604 days). The median thyrotoxicosis phase was 72 days (from 4 to 286 days).
Baseline TGAb and TMAb Antibody Levels
Baseline thyroid-associated antibody (TGAb and TMAb) levels were measured in 121 patients. Of these, 32 (39.1%) were positive for baseline antibodies, including 29 (33.3%) with abnormal TGAb, 22 (26.1%) with abnormal TMAb, and 19 (20.3%) with concurrent abnormalities. The baseline characteristics of all patients are presented in Supplementary Table S3().
The incidence of ITD was 93.8% (N = 30/32) in patients positive for thyroid-associated antibodies at baseline compared with 23.6% (N = 21/89) in patients negative for thyroid-associated antibodies (P < .001). The incidence of ITD was 96.6% (N = 28/29) in patients with positive baseline TgAb and 25.0% (N = 23/92) in patients with negative baseline TgAb (P < .001). The incidence of ITD was 90.9% (N = 20/22) in patients with positive baseline TMAb and 31.3% (N = 31/99) in patients with negative baseline TMAb (P < .001).
In univariate analysis, sex, TSH level, combination chemotherapy, presence of TgAb and TMAb were significantly associated with ITD (Supplementary Table S4) (). When multivariate analysis was performed using a model including age, sex, TSH level, combination chemotherapy, combination radiotherapy, combination targeted therapy, thyroid peroxidase antibody presence, and TgAb presence, ITD was significantly associated with women (OR, 0.22; 95% CI, 0.066-0.729; P = .013) and TgAb presence (OR, 67.393; 95% CI, 5.637-805.656; P < .001).
The Relationship Between the Occurrence and Prognosis of ITD
The median follow-up time was 11.0 months for the 560 patients included. The median survival time was 267 days in the ITD-free group and 378 days in the ITD group, and the OS rate was significantly higher for patients in the ITD group than for those in the ITD-free group (P < .001) (Supplementary Fig. 2) (). After adjusting for age, sex, and primary tumor type, the OS rate was significantly lower in patients without ITD than in those with ITD (hazard ratio [HR], 0.523; 95% CI, 0.599-0.97; P < .001) (Supplementary Table S5)(). In subgroup analysis, hypothyroid ITD (HR, 0.526; 95% CI, 0.404-0.685; P < .001), thyrotoxic ITD (HR, 0.695; 95% CI, 0.469-1.03; P = .005), and immune-related thyroiditis (HR, 0.330; 95% CI, 0.193-0.563; P < .001) prognoses were significantly improved (Supplementary Table S5 (); Fig. 2).
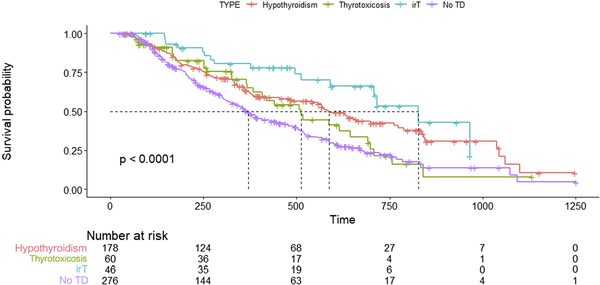
Figure 2
Survival curves for each thyroid dysfunction group after ICIs treatment using a Cox proportional-hazards model.
Discussion
As an autoimmune phenomenon, irAE can affect any organ system with inflammatory manifestations. Endocrine adverse events are one of the most common toxic reactions to ICIs, and the endocrine organ most susceptible to ICIs is the thyroid (, ).
In large phase III clinical trials targeting CTLA-4, PD-1, and PD-L1, the reported prevalence of thyroid dysfunction varied from 10% to 20% (, ). In a 2017 meta-analysis (), a total of 472 (6.3%) cases of immune-related hypothyroidism were observed in 7551 patients in 38 studies. In addition, 194 cases (2.9%) of thyrotoxicosis were observed. Another meta-analysis() (101 studies with 19 922 patients included) showed that PD-1/PD-L1 inhibitors alone had a higher incidence of ITD, especially hypothyroidism (8.0% for nivolumab, 8.5% for pembrolizumab, and 4.7% for durvalumab). The current study found that the incidence of thyrotoxicosis caused by ICIs is significantly lower than that of hypothyroidism (, ), mainly because thyrotoxicosis occurs before hypothyroidism in ITD caused by ICIs and can eventually evolve into hypothyroidism, and therefore cases of thyrotoxicosis may be missed in some clinical studies. In this study, the overall incidence of ITD caused by ICIs drugs in patients with advanced malignancies was 50.71%, including the incidence of thyrotoxicosis-type ITD about 18.75% and hypothyroidism-type ITD about 31.96%, which was significantly higher than the incidence of ITD reported in drug clinical trials, suggesting that the incidence of ITD caused by ICIs in the clinical sphere may be underestimated.
The European Society for Medical Oncology guidelines () and American Society of Clinical Oncology/National Comprehensive Cancer Network guidelines (, ) recommend follow-up thyroid function every 4 to 6 weeks for patients receiving immunotherapy, making it possible that most thyroid function abnormalities induced by ICIs are diagnosed at an early stage, and patients are generally asymptomatic or have mild symptoms, rarely inducing life-threatening thyroid storm. Two studies (, ) demonstrated that among patients who developed immune thyroid dysfunction during the administration of ICIs, there was a low incidence of grade 2 or higher related adverse reactions, few required L-T4 replacement therapy or the application of glucocorticoids, and usually there was no need to suspend immunotherapy. Patients in the current study underwent thyroid function tests on an average of every 3 weeks. Among patients who developed ITD, the incidence of grade 1 or 2 thyroid dysfunction was 95.8%; the incidence of grade 3 thyroid dysfunction was 4.2%, with no grade 4 or higher thyroid-related adverse effects. Therefore, it can be presumed that thyroid-related adverse events occurring in patients treated with ICIs in clinical practice are not severe and are manageable with regular monitoring of thyroid function. For serious life-altering thyroid adverse events, National Comprehensive Cancer Network guidelines recommend suspending ICIs therapy until symptoms resolve ().
In our study, women and higher baseline TSH were risk factors for immune-related ITD. The predilection of ITD toward women may be similar to the female preponderance of autoimmune thyroid disease observed in the general population(). A retrospective study of 68 patients by Pollack et al () found that a pretreatment TSH > 2.19 mcIU/mL was associated with an increased risk of overt thyroid dysfunction (OR, 3.46; 95% CI, 1.2-9.8). The majority of patients who received targeted therapy in this study were treated with tyrosine kinase inhibitors (TKIs), which function by inhibiting the activity of tyrosine kinases, blocking their signaling pathways, and impeding the proliferation and development of tumor cells (). Our study findings suggest that targeted drug therapy may increase the risk of immune-related ITD. Previous research has demonstrated that patients receiving ICI treatment after TKI use are more likely to experience thyroid dysfunction (). One possible mechanism for this phenomenon is that TKIs have immune-stimulating properties, which act on cancer by inhibiting the expression of CTLA4 and PD1 on CD4+ and CD8+ T cells. However, TKIs may also affect healthy tissues, such as the thyroid, leading to complications (). For high-risk patients, clinical symptoms should be closely followed, and thyroid function should be dynamically reviewed to permit early detection and treatment of thyroid dysfunction associated with ICIs, thereby avoiding serious adverse events.
The median time to onset of thyroid dysfunction, most of which is hypothyroidism, is 6 weeks after ICI initiation (, ), although this may occur essentially any time on therapy. The median time to ITD in patients treated with ICIs in this study was 73 days, with the shortest being only 9 days. Therefore, routine monitoring and evaluation of thyroid function can be recommended during the first 2 to 3 months of treatment with ICIs when the risk of immune-related endocrine toxic reactions is high.
Notably, some patients present with a course of immune-associated thyroiditis that manifests as an asymptomatic transient thyrotoxic phase that subsequently progresses to hypothyroidism. Based on prior clinical studies () and the rarity of Graves’ disease following the use of ICIs (, ), we can hypothesize that, in most cases, thyrotoxicosis and hypothyroidism following the use of these ICI drugs should be different manifestations of the same pathological entity: a destructive thyroiditis mediated by cytotoxic T cells against the thyroid gland. Studies indicate that the incidence of TD after administration of ICIs may be as high as 30% to 40% (). One study confirmed that 80% of patients with thyrotoxicosis progress to hypothyroidism, with a median time for this transformation process of 4 to 7 weeks(). The patients in the present study were followed for a prolonged time after development of hypothyroidism, and all patients who were started on levothyroxine remained on thyroid hormone replacement with normal TFTs at their last follow-up. These results suggest that hypothyroidism may require lifelong treatment, which is consistent with the results of other relevant studies (, , ). Most patients had a history of painless thyroiditis and were asymptomatic. Thirteen patients in our study had palpitations and tremors during the thyrotoxic phase but were able to continue treatment with ICIs after treatment with β-blockers. The American Society of Clinical Oncology guidelines () recommend that patients on ICIs who develop thyrotoxicosis should be closely monitored for thyroid function; if necessary, suspension of ICIs therapy should be considered until symptoms resolve and then evaluated for reactivation of immunotherapy.
In our study, the highest incidence of ITD is due to camrelizumab (58.3%). Previous studies have shown a higher incidence of ITD with the combination of CTLA-4 inhibitors and PD-1/PD-L1 inhibitors than with PD-1/PD-L1 inhibitors alone (). The incidence of ITD resulting from PD-1/PD-L1 inhibitor monotherapy versus combined CTLA-4 inhibitor therapy was not analyzed because CTLA-4 inhibitors have not yet entered the clinic at our institution.
Studies suggest that ITD is associated with antitumor effects and can be used as a predictor of clinical response to therapy (). Thuillier et al () found that the group of 40 patients with thyroid irAE had longer OS (29.8 months vs 8.1 months; P < .001) and longer progression-free survival (PFS) (8.7 months vs 1.7 months; P < .001) compared with the group of patients without ITD. A recent meta-analysis () showed that the occurrence of ITD during treatment with ICIs was associated with improved OS and PFS (OS: HR, 0.52; 95% CI, 0.43-0.62; P < .001; PFS: HR, 0.58; CI, 0.50-0.67; P < .001). In this study, median survival was used as an indicator for statistical analysis, and the results showed that the median survival time of patients with ITD was significantly higher than that of patients without ITD (P < .001). It may be explained by the fact that when immune-related adverse reactions occur after treatment with ICIs, it indicates that the body's immune system has been effectively activated, allowing for better antitumor efficacy and possibly better patient prognosis ().A recent study in a larger cohort of more than 1200 patients demonstrated that overt thyrotoxicosis irAEs were associated with increased immune toxicity in other organ systems as well as improved cancer survival and is hypothesized to be a surrogate marker of patients who demonstrate more robust immune activation following ICI treatment. However, the existence of a specific mechanistic link between the 2 is currently unclear and needs to be confirmed by data from larger studies.
The pathogenesis of ICI-associated ITD is unclear and is currently found to be associated with T cell-mediated thyrotoxicosis, CD14+ monocyte HLA-DR molecular expression, and other factors(, ), and to destruction of thyroid tissue by thyroid autoantibodies. Among 209 patients treated with PD-1 inhibitors, those positive for antithyroid antibodies at baseline had a significantly higher incidence of ITD than those who were negative (34.1% vs 2.4%) (). Because thyrotoxicosis associated with ICIs can spontaneously resolve or convert to hypothyroidism, routine antithyroid antibody testing has not been considered helpful or recommended for cancer patients treated with ICIs (). However, assessment of thyroid autoantibodies may be considered as a prognostic biomarker if the development of a thyroid autoimmune response predicts a better outcome of cancer immunotherapy. A recent study reported that thyroid antibody production was associated with significantly longer survival in cancer patients treated with PD-1 inhibitors (). Data from the current study support the recommendation that patients be evaluated for baseline thyroid-related antibodies before initiating pharmacological treatment with ICIs. However, some experts also believe that thyroid autoantibodies do not correlate with ICIs-induced ITD (, ). Therefore, whether thyroid autoantibodies are involved in the occurrence and development of ITD associated with ICIs also needs to be further explored in large data samples.
In conclusion, this study offers a comprehensive description of the clinical features of ICI-associated thyroid dysfunction. Through an exploration of the impact of ICIs on thyroid function, our goal is to increase awareness among endocrinologists and oncologists regarding these conditions. By doing so, we hope to mitigate the adverse effects of ICIs on cancer patients, improving their quality of life.
Supplementary Material
The web address of the article's supplementary file is in reference ().
Abbreviations
CTLA: cytotoxic T-lymphocyte-associated protein
FT3: free triiodothyronine
FT4: free thyroxine
HR: hazard ratio
ICI: immune checkpoint inhibitor
irAE: immune-related adverse event
ITD: immune thyroid dysfunction
OR: odds ratio
OS: overall survival
PD-1: programmed cell death protein 1
PD-L1: programmed cell death 1 ligand 1
PFS: progression-free survival
TFT: thyroid function test
TKI: tyrosine kinase inhibitor
TGAb: anti-thyroglobulin antibody
TMAb: thyroid microsomal antibody
References
- 1. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736.
- 2. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264.
- 3. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16(1):223–249.
- 4. Marin-Acevedo JA, Kimbrough EO, Lou Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. 2021;14(1):45.
- 5. Haanen J, Carbonnel F, Robert C, et al Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv119–iv142.
- 6. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):173–182.
- 7. Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13(4):195–207.
- 8. Kiyota N, Hasegawa Y, Takahashi S, et al A randomized, open-label, phase III clinical trial of nivolumab vs. therapy of investigator's choice in recurrent squamous cell carcinoma of the head and neck: a subanalysis of Asian patients versus the global population in checkmate 141. Oral Oncol. 2017;73:138–146.
- 9. Thompson JA, Schneider BJ, Brahmer J, et al NCCN Guidelines insights: management of immunotherapy-related toxicities, version 1.2020: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18(3):230–241.
- 10. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. National Institutes of Health, National Cancer Institute, 2017.
- 11. Qin Zhang, Data from: Clinical characters and influence factors of immune checkpoint inhibitor related thyroid dysfunction. http://doi.org/10.6084/m9.figshare.22587019.
- 12. Arnaud-Coffin P, Maillet D, Gan HK, et al A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer. 2019;145(3):639–648.
- 13. Larkin J, Chiarion-Sileni V, Gonzalez R, et al Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546.
- 14. De Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res. 2019;51(3):145–156.
- 15. Wright JJ, Powers AC, Johnson DB. Endocrine toxicities of immune checkpoint inhibitors. Nat Rev Endocrinol. 2021;17(7):389–399.
- 16. Brahmer JR, Lacchetti C, Schneider BJ, et al Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–1768.
- 17. Thompson JA. New NCCN guidelines: recognition and management of immunotherapy-related toxicity. J Natl Compr Canc Netw. 2018;16(5S):594–596.
- 18. Barroso-Sousa R, Ott PA, Hodi FS, Kaiser UB, Tolaney SM, Min L. Endocrine dysfunction induced by immune checkpoint inhibitors: practical recommendations for diagnosis and clinical management. Cancer. 2018;124(6):1111–1121.
- 19. Muir CA, Menzies AM, Clifton-Bligh R, Tsang VH. Thyroid toxicity following immune checkpoint inhibitor treatment in advanced cancer. Thyroid. 2020;30(10):1458–1469.
- 20. Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. N Engl J Med. 1996;335(2):99–107.
- 21. Pollack RM, Kagan M, Lotem M, Dresner-Pollak R. Baseline TSH level is associated with risk of anti–PD-1–induced thyroid dysfunction. Endocr Pract. 2019;25(8):824–829.
- 22. Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315(3):971–979.
- 23. Soni S, Rastogi A, Prasad KT, Behera D, Singh N. Thyroid dysfunction in non-small cell lung cancer patients treated with epidermal growth factor receptor and anaplastic lymphoma kinase inhibitors: results of a prospective cohort. Lung Cancer (Amsterdam, Netherlands). 2021;151:16–19.
- 24. Jannin A, Penel N, Ladsous M, Vantyghem MC, Do Cao C. Tyrosine kinase inhibitors and immune checkpoint inhibitors-induced thyroid disorders. Crit Rev Oncol Hematol. 2019;141:23–35.
- 25. Tan MH, Iyengar R, Mizokami-Stout K, et al Spectrum of immune checkpoint inhibitors-induced endocrinopathies in cancer patients: a scoping review of case reports. Clin Diabetes Endocrinol. 2019;5(1):1.
- 26. Lee H, Hodi FS, Giobbie-Hurder A, et al Characterization of thyroid disorders in patients receiving immune checkpoint inhibition TherapyImmune checkpoint blockade and thyroid disorders. Cancer Immunol Res. 2017;5(12):1133–1140.
- 27. Iyer PC, Cabanillas ME, Waguespack SG, et al Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. 2018;28(10):1243–1251.
- 28. Brancatella A, Viola N, Brogioni S, et al Graves’ disease induced by immune checkpoint inhibitors: a case report and review of the literature. Eur Thyroid J. 2019;8(4):192–195.
- 29. Peiffert M, Cugnet-Anceau C, Dalle S, et al Graves’ disease during immune checkpoint inhibitor therapy (a case series and literature review). Cancers (Basel). 2021;13(8):1944.
- 30. Martins F, Sofiya L, Sykiotis GP, et al Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–580.
- 31. Delivanis DA, Gustafson MP, Bornschlegl S, et al Pembrolizumab-induced thyroiditis: comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab. 2017;102(8):2770–2780.
- 32. Osorio J, Ni A, Chaft J, et al Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28(3):583–589.
- 33. Inaba H, Ariyasu H, Okuhira H, et al Endocrine dysfunctions during treatment of immune-checkpoint inhibitors. Trend Immunother. 2020;4(1):18–26.
- 34. Cheung Y-MM, Wang W, McGregor B, Hamnvik O-PR. Associations between immune-related thyroid dysfunction and efficacy of immune checkpoint inhibitors: a systematic review and meta-analysis. Cancer Immunol Immunother. 2022;71(8):1795–1812.
- 35. Thuillier P, Joly C, Alavi Z, et al Thyroid dysfunction induced by immune checkpoint inhibitors is associated with a better progression-free survival and overall survival in non-small cell lung cancer: an original cohort study. Cancer Immunol Immunother. 2021;70(7):2023–2033.
- 36. Angell TE, Min L, Wieczorek TJ, Hodi FS. Unique cytologic features of thyroiditis caused by immune checkpoint inhibitor therapy for malignant melanoma. Genes dis. 2018;5(1):46–48.
- 37. Okada N, Iwama S, Okuji T, et al Anti-thyroid antibodies and thyroid echo pattern at baseline as risk factors for thyroid dysfunction induced by anti-programmed cell death-1 antibodies: a prospective study. Br J Cancer. 2020;122(6):771–777.
- 38. Chang L-S, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. 2019;40(1):17–65.
- 39. Basak EA, van der Meer JW, Hurkmans DP, et al Overt thyroid dysfunction and anti-thyroid antibodies predict response to anti-PD-1 immunotherapy in cancer patients. Thyroid. 2020;30(7):966–973.
- 40. Kotwal A, Gustafson MP, Bornschlegl S, et al Immune checkpoint inhibitor-induced thyroiditis is associated with increased intrathyroidal T lymphocyte subpopulations. Thyroid. 2020;30(10):1440–1450.