Case Presentations
Case 1
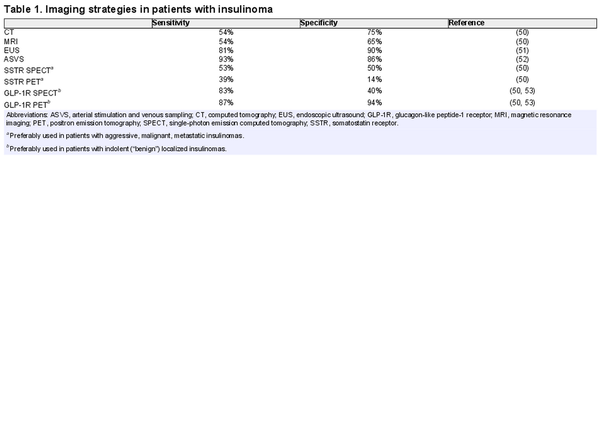
A 45-year-old man was referred for the evaluation of hypoglycemic symptoms. For approximately 1 year, he had been experiencing episodes of tremors, blurriness of vision, loss of attention, and headaches. There was no excessive perspiration, hunger, or episodes of loss of consciousness. He had fewer symptoms when he ate frequent meals. His weight had gradually increased over a period of 2 years (0.5-0.9 kg/year) and at first admission was 110 kg. His primary care provider had documented a “very low blood glucose” concentration during an episode. Additional investigations included a fasting test () with the following results: after 16 hours, he developed hypoglycemic symptoms and his blood glucose was 36 mg/dL (2.0 mmol/L), insulin = 42 μIU/mL (253 pmol/L), proinsulin = 43 pmol/L, and C-peptide = 1.35 nmol/L (4.05 ng/mL). The fast was stopped and the patient was allowed to eat food. His hypoglycemic symptoms subsequently rapidly disappeared. Urine toxicology was negative for sulfonylurea derivatives. An 3-phase abdominal computed tomography (CT) scan did not demonstrate a pancreatic lesion and there was no evidence for liver or lymph node metastases. A 68Ga-DOTATATE positron emission tomography (PET)-CT showed physiological uptake without demonstration of uptake of the radioligand in the pancreatic lesion. A 68Ga-Exendin 4 PET/CT, however, showed positive uptake in a 13 × 10-mm lesion in the pancreatic body without pathological uptake in other parts of the body (Fig. 1). An endoscopic ultrasound (EUS) showed a 14 × 11-mm hypoechoic lesion in the pancreatic body without a close relation to the pancreatic duct. The patient subsequently underwent a robot-assisted laparoscopic enucleation of the insulinoma. Histology revealed a 12 × 10-mm grade 1, ENETS/AJCC T1 pancreatic neuroendocrine tumor (NET) that stained positive for insulin and negative for the somatostatin receptor subtype 2 (SSTR2). The patient is still free of NET disease 10 years after surgery.
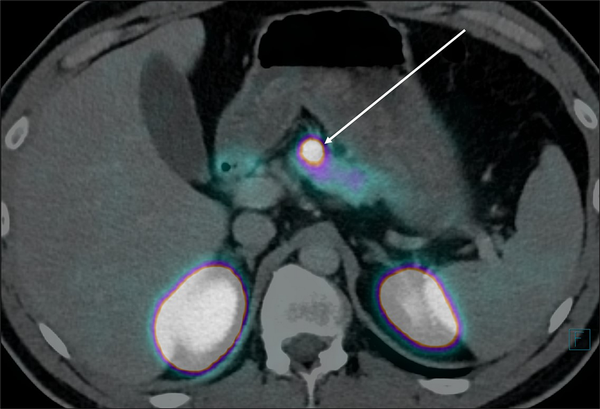
Figure 1
Axial 68Ga-exendin-PET/CT fused image of a 45-year-old man with documented endogenous hyperinsulinemic hypoglycemia (case 1) showing a 13 × 10-mm lesion (arrow) positive for the GLP-1 receptor and renal elimination of the tracer with uptake in the kidneys.
Case 2
A 50-year-old woman was referred for the evaluation of hypoglycemic symptoms. For less than 1 year, she had been experiencing episodes of tremors, blurriness of vision, loss of attention, and headaches. She had fewer symptoms when she ate frequent meals. Her weight was stable at 163 lb (74 kg). She was admitted to the emergency department following loss of consciousness. Her blood glucose level was 1.4 mmol/L. She was treated with IV glucose 20% and rapidly recovered. Additional investigations included a fasting test () with the following results: after 6 hours, she developed hypoglycemic symptoms and her blood glucose was 21.6 mg/dL (1.2 mmol/L), insulin = 132 μIU/mL (794 pmol/L), proinsulin > 200 pmol/L, and C-peptide = 1.69 nmol/L (5.07 ng/mL). The fast was stopped at that time. Her hypoglycemic symptoms rapidly disappeared after food intake. Urine toxicology was negative for sulfonylurea derivatives. An abdominal ultrasound demonstrated a pancreatic tumor and extensive liver metastases. A 68Ga-DOTATATE PET/CT scan demonstrated a 14 × 15-mm pancreatic head tumor and extensive liver and lymph node metastases that all showed positive PET uptake (Fig. 2). Pathology of a liver biopsy showed a grade 2, SSTR2-positive NET with positive immunohistochemistry for insulin. Initial treatment consisted of frequent meals, diazoxide 100 mg/day and lanreotide-Autogel 120 mg/4 weeks. Three months later, she experienced recurrent hypoglycemic episodes and radiological tumor progression and was started on peptide radionuclide receptor therapy (PRRT) with 177Lu-DOTATATE, in 4 cycles at 8-week intervals with a cumulative dose of 29.8 GBq. PRRT resulted in tumor control and normalization of blood glucose levels for 3 years. Subsequently, she developed tumor progression and a recurrence of hypoglycemic events and was retreated with 2 cycles of PRRT using 177Lu-DOTATATE, resulting in a cumulative dose of 44.5 GBq. Thereafter, she was treated with everolimus 10 mg/day. This resulted in euglycemia. Four years and 1 month after initial diagnosis, radiological and hormonal progressive disease occurred in combination with progressive liver failure, which led to her death.
Figure 2
Left: coronal CT image of a 50-year-old woman with documented endogenous hyperinsulinemic hypoglycemia (case 2) showing a 14 × 15-mm pancreatic lesion (box) and a gallbladder stone (arrow). Right: coronal 68Ga-DOTATATE PET/CT fused images of a 50-year-old woman with documented endogenous hyperinsulinemic hypoglycemia (case 2) in the same plane as Fig. 2A showing a 14 × 15-mm PET-positive pancreatic lesion (box) and extensive 68Ga-DOTATATE PET-positive liver metastases.
Diagnosis and Presentation
The Whipple triad, named after the American surgeon Allen O. Whipple (1881-1963), is the diagnostic hallmark establishing the existence of a hypoglycemic disorder and has the following 3 features: (1) symptoms, signs, or both consistent with hypoglycemia; (2) low plasma glucose measured at the time of the symptoms and signs; and (3) relief of symptoms and signs when the glucose is raised to a normal level (, ). In addition to the familiar hypoglycemic symptoms such as sweating, palpitations, nervousness, and feeling of hunger, neuroglycopenic symptoms such as confusion, visual disturbances, or seizures may also occur. The latter may cause patients to be misdiagnosed with a psychiatric or neurological disorder. Symptoms occur mainly in the fasting state, but up to 20% of patients also described postprandial symptoms (, ). By definition, in endogenous hyperinsulinism, the insulin secretion does not decrease to very low levels when plasma glucose concentrations decrease to hypoglycemic levels (, ). Thus, in patients with insulinoma, plasma insulin, C-peptide, and proinsulin concentrations are inappropriately high in the setting of low fasting plasma glucose concentrations (, ). According to the most recent clinical practice guidelines, critical diagnostic findings are present when the confirmed fasting plasma glucose concentrations are preferably below 45 mg/dL (2.5 mmol/L) or below 55 mg/dL (3.0 mmol/L) (=hypoglycemia—depending on guidelines and cutoffs—the optimal cutoff level for blood glucose is currently still a matter of debate), plasma insulin concentrations of at least 3 μU/mL (18 pmol/L), plasma C-peptide concentrations of at least 0.6 ng/L (0.2 nmol/L), and plasma proinsulin concentrations of at least 5.0 pmol/L (, ). The patients presented here fulfilled all these criteria. If hypoglycemia does not occur spontaneously, a 72-hour prolonged fasting test should be performed and blood should be drawn for measurements of insulin, and pro-insulin, or C-peptide measurements if hypoglycemia occurs. Some authors have advocated shorter fasting tests (, , ). There are data suggesting that a standardized evaluation of neurocognitive function during the fasting test (using the Mini-Mental test) can be helpful and more important than a glucose concentration threshold. Indeed, a median decrease in Mini-Mental score of ≥ 6 points has been shown to be superior for stopping the fasting test than a predefined glucose concentration (). For the differential diagnosis of hyperinsulinemic hypoglycemia not caused by insulinoma in individuals who have not undergone upper gastrointestinal surgery, such as persistent hyperinsulinemic hypoglycemia in infancy and noninsulinoma pancreatogenous hypoglycemia syndrome (nesidioblastosis), administration of exogenous insulin or sulfonylureas, insulinomatosis, and insulin (receptor) autoimmune syndrome (Hirata disease), the reader is referred to other publications ().
Incidence and Epidemiology
Insulinomas are the most common, yet still rare, hormone-producing pancreatic neuroendocrine neoplasms (panNEN) with a reported incidence of 0.7 to 4 cases per million per year (, , ). There is an age-specific incidence peak in the fifth decade of life in men and the sixth decade of life in women and the incidence is slightly higher in women than in men (). More than 99% of insulinomas are located in the pancreas, where its tumor locations are evenly distributed (, ). Extrapancreatic (occasionally metastatic) insulinomas are extremely rare and have been described in the lung, duodenum, ileum, jejunum, hilum of the spleen, and gastric antrum (). Approximately 10% of insulinomas present as multiple lesions (, ). Because the definitions for malignancy in insulinoma are ambiguous, nonmetastatic insulinomas are now referred to as “indolent” and metastatic insulinomas as “aggressive” (see Treatment of Metastatic or Inoperable Aggressive Insulinoma) (, , ). Approximately 10% to 15% of insulinomas belong to the aggressive category and 85% to 90% can be considered as indolent (, , , ). Patients with an aggressive insulinoma have lower survival compared with patients with an indolent insulinoma. The 5-year survival of patients with an indolent insulinoma has been reported to be 94% to 100% and for patients with an aggressive insulinoma this amounts to 24% to 67% (, , , , , , ). Secondary, or metachronous insulin secretion by panNEN that previously were nonsecreting, or secreting other peptide hormones, can also occasionally develop and is generally associated with a poor survival as compared with their non-insulin-secreting counterparts (, ).
A total of 5% to 10% of insulinomas are associated with the multiple endocrine neoplasia type 1 (MEN1) syndrome. The MEN1-related insulinomas may develop as multiple synchronous or metachronous lesions and are generally diagnosed at an earlier age than their sporadic counterparts because of application of the MEN1 screening recommendations (, , , ). Routine genetic testing for MEN1 is usually not recommended in patients with newly diagnosed insulinoma, but a thorough medical and family history should be obtained, and patients should be referred for appropriate testing if there is suspicion of the insulinoma being 1 of the manifestations of MEN1 (). A recent Delphi consensus recommends to consider MEN1 screening in patients aged 35 or younger who present with an apparently sporadic insulinoma, although this is not yet supported by the literature (, ). Other genetic syndromes associated with the development of insulinomas are neurofibromatosis type 1 () and tuberous sclerosis complex (, ).
Historical Perspective
“Harris’ syndrome” is a historical eponym for endogenous hyperinsulinemic hypoglycemia and named after the American surgeon Seale Harris (1870-1957) who was the first to notice that a few of his nondiabetic patients presented with the same symptoms as observed after insulin overdosing (“insulin shock”) (, , ). In 1926, the American surgeon William J Mayo (1861-1939) performed an exploratory laparotomy on a patient suffering from recurrent severe hypoglycemias caused by an unresectable pancreatic insulinoma with liver, lymph node, and mesenteric metastases (, , ). The first cure of hyperinsulinism following surgical removal of an insulinoma was reported in 1929 by the Canadian surgeon Roscoe R. Graham (1890-1948) (, ).
Localization
Localization of the insulinoma and exclusion or confirmation of metastatic disease by CT is still the preferred initial localization modality followed by EUS or magnetic resonance imaging (MRI) for indolent, localized insulinomas (Table 1) (Fig. 3). Glucagon-like peptide 1 receptor (GLP-1R) PET/CT or PET/MRI is a highly sensitive localization technique for seemingly occult, indolent, localized insulinomas and has become increasingly popular.
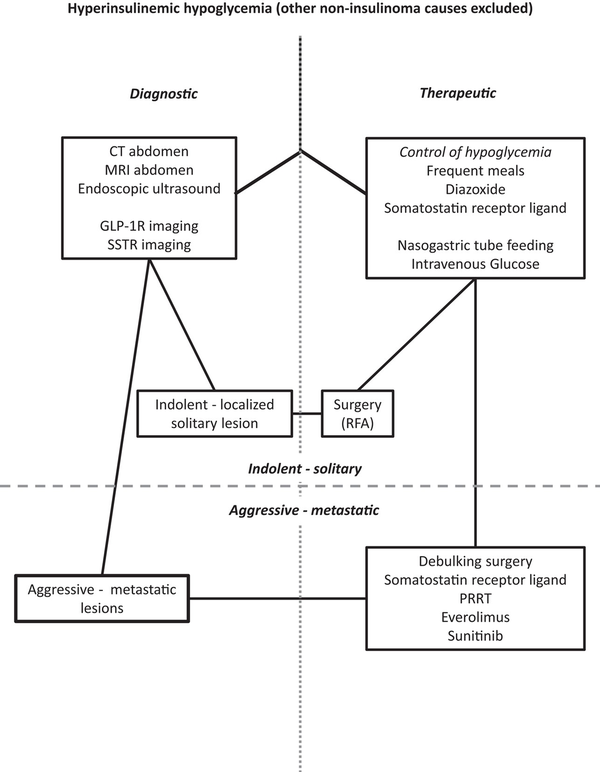
Figure 3
Algorithm for clinical suspicion of insulinoma. After the diagnosis of hyperinsulinemic hypoglycemia, imaging procedures should be performed to locate the source of hyperinsulinism and hypoglycemia should be prevented by dietary and medical interventions. Following the detection of the localized or metastatic insulinoma, tumor-directed surgical and/or medical therapy should be initiated. GLP-1R, glucagon-like peptide-1 receptor; PRRT, peptide receptor radionuclide therapy; RFA, radiofrequency ablation; SSTR, somatostatin receptor.
Preoperative localization of nonmetastatic insulinomas is important and sometimes difficult, because approximately 30% of insulinomas are less than 10 mm in diameter. Furthermore, particularly in patients with MEN1, 10% of insulinomas can be multiple (). The anatomical localization of nonmetastatic insulinomas is also important for the choice between laparoscopic, robot-assisted, and open pancreatic surgery and between enucleation or resection, or partial pancreatectomy (, ). The GLP-1R is mainly expressed on the pancreatic β cells, overexpressed on indolent insulinoma and is therefore an interesting target for imaging of (previously occult) indolent localized insulinomas (, ). In various studies, the radiolabeled GLP-1R agonists 111In-DOTA-exendin-4 single-photon emission computer tomography (SPECT) and/or 68Ga-DOTA-exendin-4 PET successfully detected localized indolent insulinomas. 68Ga-DOTA-exendin-4 PET/CT is more sensitive than 111In-DOTA-exendin-4 SPECT/CT (). In case 1, who had localized indolent insulinoma, the GLP-1R PET/CT was clearly positive in the pancreatic lesion (Fig. 1), whereas SSTR2 PET/CT was negative. However, as opposed to localized indolent insulinomas, aggressive malignant insulinomas often lack the expression of the GLP-1R. Conversely, malignant aggressive insulinomas usually express the SSTR2, which can be targeted with PET/CT or PET/MRI using 68Ga-DOTA-labeled somatostatin receptor ligands (SRLs), or in the past with somatostatin receptor scintigraphy and SPECT using 111In-pentetreotide (, ). In case 2, who had metastatic insulinoma, the SSTR PET/CT showed positive uptake in all tumor lesions (Fig. 2). In this case, no GLP-1R PET/CT was performed.
In the past, selective pancreatic angiography and selective intra-arterial injection of calcium with sampling of hepatic vein insulin were routinely used in high-volume centers. This invasive regionalization (an exact localization will be never given) procedure became less used because of the improved imaging procedures mentioned above ().
If all localization and regionalization techniques fail to localize a tumor, intraoperative palpation of the pancreas and intraoperative ultrasound might still prove to be successful ().
Pathophysiology and Pathology
Pathophysiology
Despite a low tumor mutational burden, insulinomas are enriched for genetic mutations and copy number variants in epigenetic modifiers (). This suggests a central role for chromatin remodeling in the pathogenesis of insulinomas. The predominant recurring genetic variant in up to 30% of insulinomas is an activating T372R mutation in the Yin Yang 1 (YY1) gene, which is absent in nonfunctioning panNEN (). YY1 is a transcriptional activator of the insulin gene. MEN1 mutations appear to be less common in insulinomas than nonfunctioning panNEN, but insulinomas do display loss of heterozygosity of the MEN1 region at chromosome 11q13.1 in 30% of cases (, ). Insulinomas are also associated with aberrant methylation patterns, including of the insulin promoter, where noncanonical transcription factors drive insulin expression ().
Pathology
The World Health Organization classification and grading of panNEN separates these tumors using the Ki67 index (MIB-1 antibody staining) into 4 broad categories: well-differentiated panNET grade 1, 2, or 3 (G1-2-3) and poorly differentiated pancreatic neuroendocrine carcinomas (NEC). Helpful for the distinction of panNEC from G3 panNET is the overexpression of p53 and loss of expression of Rb1. Insulin staining is not obligatorily positive in insulinomas and is usually not necessarily required once the clinical diagnosis has been made (, , , ).
Indolent and aggressive insulinomas are different entities. Aggressive insulinomas are characterized by rapid onset of symptoms, larger size, and expression of ARX and alpha-1-antitrypsin, and decreased or absent immunohistochemical expression of insulin, PDX1, and GLP-1R. Moreover, aggressive insulinomas often harbor ATRX and DAXX mutations, the alternative lengthening of telomeres phenotype and chromosomal instability. Tumor grade and somatic MEN1 or YY1 mutations are less useful for predicting clinical behavior. Aggressive insulinomas have similarities to normal α cells and nonfunctioning panNETs, whereas indolent insulinomas remain closely related to normal β cells (, ).
Treatment
Prevention of Hypoglycemia
Supportive measures ()
Dietary/glucose: regular meals or snacks rich in slow carbohydrates, also ante noctem, are generally recommended (Fig. 3). The inclusion of a bedtime or late night meal is sufficient in most patients, but nocturnal tube feeding might be required to avoid nocturnal hypoglycemia in severely symptomatic patients. IV glucose administered via a central IV indwelling catheter might be needed for the control of severe recurrent hypoglycemia. A continuous glucose monitoring system can support patients in recognizing hypoglycemic events and prevent serious complications, especially during the night ().
Medical treatment
Diazoxide is a nondiuretic benzothiadiazide vasodilator also known to control insulin secretion by blocking the adenosine triphosphate-dependent potassium channels of the pancreatic β cells (). Furthermore, it likely also has an extrapancreatic effect that increases the hepatic glucose output (, ). Generally, doses between 300 and 900 mg per day, usually divided into 3 equal and increasing doses every 8 hours, are rapidly successful in reversing hypoglycemia. The most frequent dose-limiting side effects of diazoxide occurring in up to 80% are fluid retention and edema, palpitations, nausea, anorexia, and hirsutism in female patients. Combination with a thiazide diuretic is generally recommended to prevent fluid retention, edema, and severe weight gain (, , ).
Somatostatin receptor ligands—first-generation
Expression of the SSTR2 and SSTR5 is generally low in indolent insulinoma cells, but usually high in aggressive insulinomas (). The currently commercially available first-generation SRLs, octreotide and lanreotide, both have a high affinity for the SSTR2, and some affinity to SSTR3 and SSTR5. The long-acting SRL, lanreotide Autogel, is the approved first-line therapy for control of tumor growth in low grade (G1-2) panNET. SRLs can adequately suppress the pathologic insulin hypersecretion in patients with insulinomas that express SSTR2 (, , , ). A challenge with subcutaneous administration of the short-acting SRL octreotide, is generally recommended before switching to a long-acting formulation. This test is required to avoid and timely intervene for a paradoxical aggravation of hypoglycemia via the suppression of counterregulatory glucagon secretion in patients with insulinomas which lack SSTR2 expression ().
Somatostatin receptor ligands—second-generation
Pasireotide is a second-generation multireceptor SRL that binds with high affinity to SSTR types 1, 2, 3, and 5 (). In Cushing disease, acromegaly and in phase 2-3 studies in NET (including panNET), hyperglycemia (79%), and type 2 diabetes were important side effects observed with pasireotide because of its inhibitory effects on incretin release (). However, until now, pasireotide has not been approved for the treatment of panNET. Anecdotal reports show successful control with pasireotide of hypoglycemia in patients with insulinoma (, ).
Other drugs
Glucocorticoids are sometimes used to control the hypoglycemia but their chronic use poses a significant risk for side effects (eg, weight gain, neuropsychiatric disturbances, hypertension, secondary [opportunistic] infections). Also β-adrenergic receptor blocking drugs, phenytoin, diltiazem, and verapamil have been tried with some success in selected cases ().
RZ358
RZ538 is a human monoclonal antibody that binds to a unique site on the insulin receptor and acts as negative allosteric modulator of insulin receptor. Currently, its effects have been studied in an open-label multiple-dose study in patients with congenital hyperinsulinism (NCT04538989). Recently, the successful use of RZ358 was reported in a patient with severe refractory hypoglycemia and a malignant insulinoma ().
Treatment of Localized, Nonmetastatic Insulinoma
Surgery and ablation
For single solitary insulinomas, curative surgical excision remains the treatment of choice. However, it should be performed only when the diagnosis is certain and by a surgeon who is skilled in pancreatic surgery. EUS with special focus on the relationship between the tumor and the pancreatic duct is an excellent tool to guide surgical resectability. Laparoscopic, or robot-assisted enucleation of an insulinoma, has been shown to be feasible, particularly if the lesion is visualized preoperatively on CT scan, or by EUS, and when there is sufficient distance to the pancreatic and/or common bile duct. Localized insulinomas at the head of the pancreas rarely require a pancreaticoduodenectomy (Whipple procedure), thereby avoiding considerable morbidity (, ).
Complications following pancreas resection include postoperative pancreatic fistula, postpancreatectomy hemorrhage, and delayed gastric emptying together with exocrine and endocrine pancreatic insufficiency. These complications seem to occur at higher rates than those observed in patients undergoing surgery for other solid pancreatic tumors (, ). Perioperative SRL administration may reduce the perioperative complications (). Enucleation is favored for small lesions (<2 cm) located >2 to 3 mm from the main pancreatic and/or common bile duct (, , ). In a large series of >1000 patients undergoing surgery for panNET, the complication rates as defined by a Clavien–Dindo score ≥3 was 32% for pancreaticoduodenectomy, 20% for distal pancreatectomy, and 25% for enucleation, whereas the rate of pancreatic fistula grade B/C was 23%, 29%, and 33%, respectively (, ). In case 1, the patient successfully underwent a robot-assisted laparoscopic enucleation of the insulinoma without any complications.
Nonsurgical therapy can be considered in patients who either have comorbidities precluding resection or do not want to undergo resection after being appropriately counseled about therapeutic options. In selected cases, curative EUS-guided radiofrequency ablation of a localized insulinoma can be feasible and safe, although long-term data are pending (, , , ). Stereotactic body radiotherapy is another promising noninvasive therapeutic modality that has also been shown to be able control hypoglycemia in selected patients with insulinoma (, ).
The approach to MEN1-associated (multiple) insulinomas differs from that in patients without MEN1 (, ). In patients with MEN1 and confirmed endogenous hyperinsulinism and multiple panNET at imaging, a selective intra-arterial injection of calcium with sampling of hepatic vein insulin or 68Ga-DOTA-exendin-4 PET/CT can regionalize or localize the insulinoma(s) and differentiate it/them from concurrent nonfunctional panNETs (, , ). In the past, the use of intraoperative intratumoral alcohol injection in patients with MEN1 with multiple panNET/insulinoma(s), in whom surgical resection was not feasible, induced good symptom control ().
Treatment of Metastatic or Inoperable Aggressive Insulinoma
Debulking
In aggressive malignant cases, debulking of the panNEN, including locoregional lymph nodes can be considered on a case-by-case basis, particularly in an attempt to control the endogenous hyperinsulinemic hypoglycemia (, , ). Also, liver metastases can be resected, or treated by trans-arterial bland or chemo-embolization, radioembolization, radiofrequency ablation, microwave and cryoablation, high-intensity focused ultrasound, laser, brachytherapy, and irreversible electroporation depending on the local availability (, ). If more than 90% of tumor load can be resected, palliative surgery can also be considered.
However, most aggressive malignant metastatic insulinomas cannot be cured by surgery or ablation procedures alone and require supportive measures, medical antihormonal, and antitumor treatment ().
Somatostatin receptor ligands
SRLs not only inhibit hormone release by functioning NENs, including insulin by a subset of insulinomas, but also suppress tumor growth (). Although no large case series on tumor stabilization of metastatic insulinoma by SRLs are available, SLRs can be considered as first-line therapy for metastatic insulinomas.
Peptide receptor radionuclide therapy
PRRT with radiolabeled SRL is an established second-line treatment for well-differentiated (tumor Ki67 index ≤ 20%) gastrointestinal tract and pancreas NET (, , , ). At present, β-radiation-emitting 177Lu-DOTATATE is the only approved theranostic therapy (, , ). It is imperative to demonstrate expression of SSTR on the tumors using pretreatment 68Ga DOTANOC / DOTATOC / DOTATATE PET/CT (). Favorable symptomatic and biochemical responses using PRRT with 177Lu-DOTATATE have been obtained in small patient series with functioning metastatic gastrointestinal tract and pancreas NEN like metastatic insulinomas, even when there was no visible tumor regression (, , , ). Acute side effects of PRRT are usually mild and include nausea and gastrointestinal upset, but these are probably more related to the amino acid infusions coadministered for kidney protection. However, rare (2%) but serious side effects include severe and irreversible bone marrow disease (pancytopenia, acute myelogenous leukemia, and myelodysplastic syndrome) (, , , ). In case 2, the patient underwent PRRT (4 cycles) and salvage PRRT (2 cycles) with 177Lu-DOTATATE.
Everolimus
Everolimus is a mammalian target of rapamycin (mTOR) inhibitor with antiproliferative activity in metastatic NET, including malignant insulinomas, via inhibition of signaling in the phosphoinositide 3-kinase/Akt/mTOR pathway. The drug is currently approved for the treatment of adult patients with advanced, progressive, and both functioning and nonfunctioning well-differentiated panNET (). Inhibition of the mTOR signaling pathway further results in impaired skeletal muscle and adipose tissue glucose uptake, impaired insulin-mediated suppression of hepatic gluconeogenesis, and impaired pancreatic β-cell insulin secretion (). The hyperglycemic effect of everolimus (and other mTOR inhibitors) is, therefore, a welcome side effect in the antiproliferative treatment of insulinomas (, ). Other major adverse effects of everolimus include skin rash, stomatitis, fatigue, gastrointestinal upset, pneumonitis, anemia, and opportunistic infections (, ). In case 2, the patient was treated with everolimus, which resulted in normalization of blood glucose levels.
Sunitinib
Sunitinib is an oral multitargeted receptor tyrosine kinase inhibitor that has antiangiogenic and antitumor activity by inhibiting a number of molecular pathways involved in angiogenesis. It is approved for the treatment of progressive, well-differentiated panNET in patients with unresectable locally advanced or metastatic disease (). In contrast to everolimus, sunitinib does not directly influence insulin secretion or insulin resistance. Conversely, sunitinib therapy in patients with advanced renal cell cancer resulted in a decrease of the blood glucose levels (). Further adverse events include mucositis, skin rash, hand-foot syndrome, diarrhea, nausea, vomiting, fatigue, hypertension, and neutropenia (, ).
Cytotoxic chemotherapy
Systemic chemotherapy is currently recommended in advanced high-grade pancreatic NEN (NET grade 3 and NEC) (, ). Historically, (combinations of) 5-fluorouracil, doxorubicin, and streptozotocin have been used for the treatment for inoperable functioning and nonfunctioning pancreatic high-grade NET, including malignant insulinomas (, ). However, these drugs and their combinations cause considerable toxicities. For NEC, the combination of cisplatin or carboplatin with etoposide is most commonly used (, , , , , , ). Recent trials show objective responses and good tolerance of temozolomide-based chemotherapy regimens like the capecitabine and temozolomide regimen in panNEN of all grades (). Again, it can be expected that considerable debulking following chemotherapy will be associated with less frequent and less severe hypoglycemias.
Disclosures
J.H. has received honoraria for speaker engagements and/or for advisory boards from Ipsen, Novartis, and Serb. J.C.R. declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. R.A.F. has received honoraria for speaker engagements and/or research grants from Recordati and Corcept. E.C. has received honoraria for speaker engagements from Novartis, Ipsen, Pfizer, HRA Pharma, Novo Nordisk, and AAA and for advisory boards from AAA, Pfizer, HRA Pharma, Ricordati Pharma GmBH, and Novo Nordisk. W.W.d.H. has received honoraria for speaker engagements and/or for advisory boards from Ipsen and Novartis.
Abbreviations
CT: computed tomography
EUS: endoscopic ultrasound
GLP-1R: glucagon-like peptide 1 receptor
MEN1: multiple endocrine neoplasia type 1
MRI: magnetic resonance imaging
mTOR: mammalian target of rapamycin
NEC: neuroendocrine carcinoma
NET: neuroendocrine tumor
panNEN: pancreatic neuroendocrine neoplasm
PET: positron emission tomography
PRRT: peptide radionuclide receptor therapy
SPECT: single-photon emission computer tomography
SRL: somatostatin receptor ligand
SSTR: somatostatin receptor subtype
References
- 1. Service FJ, Natt N. The prolonged fast. J Clin Endocrinol Metab. 2000;85(11):3973–3974.
- 2. Cryer PE, Axelrod L, Grossman AB, et al Evaluation and management of adult hypoglycemic disorders: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(3):709–728.
- 3. Whipple AO, Frantz VK. Adenoma of islet cells with hyperinsulinism: A review. Ann Surg. 1935;101(6):1299–1335.
- 4. Service FJ, McMahon MM, O'Brien PC, Ballard DJ. Functioning insulinoma–incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc. 1991;66(7):711–719.
- 5. Harrington MG, McGeorge AP, Ballantyne JP, Beastall G. A prospective survey for insulinomas in a neurology department. Lancet. 1983;1(8333):1094–1095.
- 6. Service FJ. Hypoglycemic disorders. N Engl J Med. 1995;332(17):1144–1152.
- 7. Hofland J, Falconi M, Christ E, et al European Neuroendocrine tumor society 2023 guidance paper for functioning pancreatic neuroendocrine tumour syndromes. J Neuroendocrinol. 2023;35(8):e13318.
- 8. Hirshberg B, Livi A, Bartlett DL, et al Forty-eight-hour fast: the diagnostic test for insulinoma. J Clin Endocrinol Metab. 2000;85(9):3222–3226.
- 9. Wiesli P, Schwegler B, Schmid B, Spinas GA, Schmid C. Mini-Mental state examination is superior to plasma glucose concentrations in monitoring patients with suspected hypoglycaemic disorders during the 72-hour fast. Eur J Endocrinol. 2005;152(4):605–610.
- 10. Bansal N, Weinstock RS. Non-Diabetic hypoglycemia. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. MDText.com, Inc.; 2000.
- 11. Dieterle MP, Husari A, Prozmann SN, et al Adult-Onset nesidioblastosis/non-insulinoma pancreatogenous hypoglycemia syndrome (NIPHS): review of the literature of a rare cause of hyperinsulinemic hypoglycemia. Biomedicines. 2023;11(6):1732.
- 12. de Herder WW, Klöppel G. One hundred years after the discovery of insulin and glucagon: the history of tumors and hyperplasias that hypersecrete these hormones. Endocr Relat Cancer. 2023;30(9):e220327.
- 13. Sempoux C, Klöppel G. Pathological features in non-neoplastic congenital and adult hyperinsulinism: from nesidioblastosis to current terminology and understanding. Endocr Relat Cancer. 2023;30(9):e230034.
- 14. Christ E, Iacovazzo D, Korbonits M, Perren A. Insulinomatosis: new aspects. Endocr Relat Cancer. 2023;30(6):e220327.
- 15. Hofland J, Kaltsas G, de Herder WW. Advances in the diagnosis and management of well-differentiated neuroendocrine neoplasms. Endocr Rev. 2020;41(2):371–403.
- 16. Svensson E, Muth A, Hedenström P, Ragnarsson O. The incidence of insulinoma in western Sweden between 2002 and 2019. Ann Gastroenterol. 2022;35(4):434–440.
- 17. Kurakawa KI, Okada A, Manaka K, et al Clinical characteristics and incidences of benign and malignant insulinoma using a national inpatient database in Japan. J Clin Endocrinol Metab. 2021;106(12):3477–3486.
- 18. Halfdanarson TR, Rubin J, Farnell MB, Grant CS, Petersen GM. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer. 2008;15(2):409–427.
- 19. Mehrabi A, Fischer L, Hafezi M, et al A systematic review of localization, surgical treatment options, and outcome of insulinoma. Pancreas. 2014;43(5):675–686.
- 20. La Rosa S, Hong SM, Ohike N, Brosens LAA, Mete O, de Herder W. Insulinoma. In: Klimstra DS, Osamura RY, eds. WHO Classification of Tumours. Endocrine and Neuroendocrine Tumours. 5 ed. International Agency for Research on Cancer; 2023.
- 21. La Rosa S, Pariani D, Calandra C, et al Ectopic duodenal insulinoma: a very rare and challenging tumor type. Description of a case and review of the literature. Endocr Pathol. 2013;24(4):213–219.
- 22. Shames JM, Dhurandhar NR, Blackard WG. Insulin-secreting bronchial carcinoid tumor with widespread metastases. Am J Med. 1968;44(4):632–637.
- 23. Adamson AR, Grahame-Smith DG, Bogomoletz V, Maw DS, Rothnie NG. Malignant argentaffinoma with carcinoid syndrome and hypoglycaemia. Br Med J. 1971;3(5766):93–94.
- 24. Pelletier G, Cortot A, Launay JM, et al Serotonin-secreting and insulin-secreting ileal carcinoid tumor and the use of in vitro culture of tumoral cells. Cancer. 1984;54(2):319–322.
- 25. Zhang X, Jia H, Li F, et al Ectopic insulinoma diagnosed by 68Ga-exendin-4 PET/CT: A case report and review of literature. Medicine (Baltimore). 2021;100(13):e25076.
- 26. Garg R, Memon S, Patil V, Bandgar T. Extrapancreatic insulinoma. World J Nucl Med. 2020;19(02):162–164.
- 27. Rindi G, Mete O, Uccella S, et al Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr Pathol. 2022;33(1):115–154.
- 28. Sada A, Habermann EB, Szabo Yamashita T, et al Comparison between sporadic and multiple endocrine neoplasia type 1-associated insulinoma. J Am Coll Surg. 2022;235(5):756–763.
- 29. Pieterman CRC, van Leeuwaarde RS, van den Broek MFM, van Nesselrooij BPM, Valk GD. Multiple endocrine neoplasia type 1. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. MDText.com, Inc.; 2000.
- 30. Hackeng WM, Brosens LAA, Dreijerink KMA. Aggressive versus indolent insulinomas: new clinicopathological insights. Endocr Relat Cancer. 2023;30(5):e220321.
- 31. Sada A, Yamashita TS, Glasgow AE, et al Comparison of benign and malignant insulinoma. Am J Surg. 2021;221(2):437–447.
- 32. de Mestier L, Hentic O, Cros J, et al Metachronous hormonal syndromes in patients with pancreatic neuroendocrine tumors: a case-series study. Ann Intern Med. 2015;162(10):682–689.
- 33. Crona J, Norlén O, Antonodimitrakis P, Welin S, Stålberg P, Eriksson B. Multiple and secondary hormone secretion in patients with metastatic pancreatic neuroendocrine tumours. J Clin Endocrinol Metab. 2016;101(2):445–452.
- 34. Thakker RV, Newey PJ, Walls GV, et al Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97(9):2990–3011.
- 35. Newey PJ, Newell-Price J. MEN1 Surveillance guidelines: time to (Re)Think?J Endocr Soc. 2022;6(2):bvac001.
- 36. Niederle B, Selberherr A, Bartsch DK, et al Multiple endocrine neoplasia type 1 and the pancreas: diagnosis and treatment of functioning and non-functioning pancreatic and duodenal neuroendocrine neoplasia within the MEN1 syndrome—an international consensus statement. Neuroendocrinology. 2021;111(7):609–630.
- 37. Klein Haneveld MJ, van Treijen MJC, Pieterman CRC, et al Initiating pancreatic neuroendocrine tumor (pNET) screening in young MEN1 patients: results from the DutchMEN study group. J Clin Endocrinol Metab. 2021;106(12):3515–3525.
- 38. Goudet P, Dalac A, Le Bras M, et al MEN1 Disease occurring before 21 years old: a 160-patient cohort study from the groupe d'étude des tumeurs endocrines. J Clin Endocrinol Metab. 2015;100(4):1568–1577.
- 39. de Laat JM, Tham E, Pieterman CRC, et al Predicting the risk of multiple endocrine neoplasia type 1 for patients with commonly occurring endocrine tumors. Eur J Endocrinol. 2012;167(2):181–187.
- 40. Perren A, Wiesli P, Schmid S, et al Pancreatic endocrine tumors are a rare manifestation of the neurofibromatosis type 1 phenotype: molecular analysis of a malignant insulinoma in a NF-1 patient. Am J Surg Pathol. 2006;30(8):1047–1051.
- 41. Dworakowska D, Grossman AB. Are neuroendocrine tumours a feature of tuberous sclerosis? A systematic review. Endocr Relat Cancer. 2009;16(1):45–58.
- 42. Kang MY, Yeoh J, Pondicherry A, Rahman H, Dissanayake A. Insulinoma and tuberous sclerosis: A possible mechanistic target of rapamycin (mTOR) pathway abnormality?J Endocr Soc. 2017;1(9):1120–1123.
- 43. Harris S. Hyperinsulinism and dysinsulinism. J Am Med Assoc. 1924;83(10):729–733.
- 44. Harris S. Hyperinsulinism, a definite disease entity. Etiology, pathology, symptoms, diagnosis, prognosis and treatment of spontaneous insulinogenic hypoglycemia (hyperinsulinism). J Am Med Assoc. 1933;101(25):1958–1965.
- 45. van Heerden JA, Churchward MM. Dr Dickinson Ober Wheelock–a case of sporadic insulinoma or multiple endocrine neoplasia type 1?Mayo Clin Proc. 1999;74(7):735–738.
- 46. Wilder RM, Allan FN, Power MH, Robertson HE. Carcinoma of the islands of the pancreas. J Am Med Assoc. 1927;89(5):348–355.
- 47. Howland G, Campbell WR, Maltby EJ, Robinson WL. Dysinsulinism: convulsions and coma due to islet-cell tumor of the pancreas with operation and cure. J Am Med Assoc. 1929;93(9):674–679.
- 48. Howland G, Campbell WR, Maltby EJ, Robinson WL. Dysinsulinism: convulsions and coma due to islet-cell tumor of pancreas: operation and cure. Trans Am Neurol Assoc. 1929;55:551–556.
- 49. Campbell WR, Graham RR, Robinson WL. Islet cell tumors of the pancreas. Am J Med Sci. 1939;198(4):445–454.
- 50. Yang Y, Shi J, Zhu J. Diagnostic performance of noninvasive imaging modalities for localization of insulinoma: A meta-analysis. Eur J Radiol. 2021;145:110016.
- 51. Wang H, Ba Y, Xing Q, Du JL. Diagnostic value of endoscopic ultrasound for insulinoma localization: A systematic review and meta-analysis. PLoS One. 2018;13(10):e0206099.
- 52. Wang H, Ba Y, Xing Q, Cai RC. Diagnostic value of ASVS for insulinoma localization: A systematic review and meta-analysis. PLoS One. 2019;14(11):e0224928.
- 53. Shah R, Garg R, Majmundar M, et al Exendin-4-based imaging in insulinoma localization: systematic review and meta-analysis. Clin Endocrinol (Oxf). 2021;95(2):354–364.
- 54. Sada A, McKenzie TJ, Vella A, Levy MJ, Halfdanarson TR. Interventional vs surgical procedures in localized/nonmetastatic insulinomas (ablation vs surgery). Endocr Relat Cancer. 2023;30(6):e220362.
- 55. Reubi JC, Perren A, Rehmann R, et al Glucagon-like peptide-1 (GLP-1) receptors are not overexpressed in pancreatic islets from patients with severe hyperinsulinaemic hypoglycaemia following gastric bypass. Diabetologia. 2010;53(12):2641–2645.
- 56. Wild D, Christ E, Caplin ME, et al Glucagon-like peptide-1 versus somatostatin receptor targeting reveals 2 distinct forms of malignant insulinomas. J Nucl Med. 2011;52(7):1073–1078.
- 57. Refardt J, Hofland J, Wild D, Christ E. Molecular imaging of neuroendocrine neoplasms. J Clin Endocrinol Metab. 2022;107(7):e2662–e2670.
- 58. Antwi K, Fani M, Nicolas G, et al Localization of hidden insulinomas with 68Ga-DOTA-exendin-4 PET/CT: A pilot study. J Nucl Med. 2015;56(7):1075–1078.
- 59. Christ E, Antwi K, Fani M, Wild D. Innovative imaging of insulinoma: the end of sampling? A review. Endocr Relat Cancer. 2020;27(4):R79–r92.
- 60. Christ E, Wild D, Ederer S, et al Glucagon-like peptide-1 receptor imaging for the localisation of insulinomas: a prospective multicentre imaging study. Lancet Diabetes Endocrinol. 2013;1(2):115–122.
- 61. Druce MR, Muthuppalaniappan VM, O'Leary B, et al Diagnosis and localisation of insulinoma: the value of modern magnetic resonance imaging in conjunction with calcium stimulation catheterisation. Eur J Endocrinol. 2010;162(5):971–978.
- 62. de Herder WW. GEP-NETS update: functional localisation and scintigraphy in neuroendocrine tumours of the gastrointestinal tract and pancreas (GEP-NETs). Eur J Endocrinol. 2014;170(5):R173–R183.
- 63. Morera J, Guillaume A, Courtheoux P, et al Preoperative localization of an insulinoma: selective arterial calcium stimulation test performance. J Endocrinol Invest. 2016;39(4):455–463.
- 64. Wang H, Bender A, Wang P, et al Insights into beta cell regeneration for diabetes via integration of molecular landscapes in human insulinomas. Nat Commun. 2017;8(1):767.
- 65. Cao Y, Gao Z, Li L, et alWhole exome sequencing of insulinoma reveals recurrent T372R mutations in YY1. Nat Commun. 2013;4(1):2810.
- 66. Karakose E, Wang H, Inabnet W, et al Aberrant methylation underlies insulin gene expression in human insulinoma. Nat Commun. 2020;11(1):5210.
- 67. Rindi G, Falconi M, Klersy C, et al TNM Staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104(10):764–777.
- 68. Rindi G, Klersy C, Albarello L, et al Competitive testing of the WHO 2010 versus the WHO 2017 grading of pancreatic neuroendocrine neoplasms: data from a large international cohort study. Neuroendocrinology. 2019;107(4):375–386.
- 69. Hackeng WM, Schelhaas W, Morsink FHM, et al Alternative lengthening of telomeres and differential expression of endocrine transcription factors distinguish metastatic and non-metastatic insulinomas. Endocr Pathol. 2020;31(2):108–118.
- 70. Baudin E, Caron P, Lombard-Bohas C, et alMalignant insulinoma: recommendations for characterisation and treatment. Ann Endocrinol (Paris). 2013; 74(5-6):523–533.
- 71. Aida A, Noto H. Diagnosis and treatment course of insulinoma presenting as hypoglycemia unawareness using a factory-calibrated continuous glucose monitoring system. Am J Case Rep. 2022;23:e936723.
- 72. Yamaguchi N, Yamada E, Matsumoto S, et al A case of insulinoma-induced hypoglycemia managed by dexcom G4 platinum. Neuro Endocrinol Lett. 2022;43(3):161–166.
- 73. Yuan T, Liu S, Zhu C, et al Continuous glucose monitoring in patients with insulinoma treated by endoscopic ultrasound-guided ethanol injection. Pancreas. 2021;50(2):183–188.
- 74. Kawasaki A, Suzuki K, Miyamoto M, et al Disruptive nocturnal behavior due to insulinoma revealed by continuous glucose monitoring. Eur J Neurol. 2014;21(5):e46–e47.
- 75. Brown E, Watkin D, Evans J, Yip V, Cuthbertson DJ. Multidisciplinary management of refractory insulinomas. Clin Endocrinol (Oxf). 2018;88(5):615–624.
- 76. Altszuler N, Moraru E, Hampshire J. On the mechanism of diazoxide-induced hyperglycemia. Diabetes. 1977;26(10):931–935.
- 77. Warren AM, Topliss DJ, Hamblin PS. Successful medical management of insulinoma with diazoxide for 27 years. Endocrinol Diabetes Metab Case Rep. 2020;2020:20-0132.
- 78. Gill GV, Rauf O, MacFarlane IA. Diazoxide treatment for insulinoma: a national UK survey. Postgrad Med J. 1997;73(864):640–641.
- 79. Goode PN, Farndon JR, Anderson J, Johnston ID, Morte JA. Diazoxide in the management of patients with insulinoma. World J Surg. 1986;10(4):586–592.
- 80. Alexandraki KI, Kaltsas GA, Grozinsky-Glasberg S. Emerging therapies for advanced insulinomas and glucagonomas. Endocr Relat Cancer. 2023;30(9):e230020.
- 81. Vezzosi D, Bennet A, Rochaix P, et al Octreotide in insulinoma patients: efficacy on hypoglycemia, relationships with octreoscan scintigraphy and immunostaining with anti-sst2A and anti-sst5 antibodies. Eur J Endocrinol. 2005;152(5):757–767.
- 82. Vezzosi D, Bennet A, Courbon F, Caron P. Short- and long-term somatostatin analogue treatment in patients with hypoglycaemia related to endogenous hyperinsulinism. Clin Endocrinol (Oxf). 2008; 68(6):904–911.
- 83. Gama R, Marks V, Wright J, Teale JD. Octreotide exacerbated fasting hypoglycaemia in a patient with a proinsulinoma; the glucostatic importance of pancreatic glucagon. Clin Endocrinol (Oxf). 1995; 43(1):117–120.
- 84. Stehouwer CD, Lems WF, Fischer HR, Hackeng WH, Naafs MA. Aggravation of hypoglycemia in insulinoma patients by the long-acting somatostatin analogue octreotide (sandostatin). Acta Endocrinol (Copenh). 1989; 121(1):34–40.
- 85. Kvols LK, Oberg KE, O'Dorisio TM, et al Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: results from a phase II study. Endocr Relat Cancer. 2012;19(5):657–666.
- 86. Cives M, Kunz PL, Morse B, et al Phase II clinical trial of pasireotide long-acting repeatable in patients with metastatic neuroendocrine tumors. Endocr Relat Cancer. 2015;22(1):1–9.
- 87. Feelders RA, de Herder WW, Neggers SJ, van der Lely AJ, Hofland LJ. Pasireotide, a multi-somatostatin receptor ligand with potential efficacy for treatment of pituitary and neuroendocrine tumors. Drugs Today (Barc). 2013;49(2):89–103.
- 88. Wolin EM, Jarzab B, Eriksson B, et al Phase III study of pasireotide long-acting release in patients with metastatic neuroendocrine tumors and carcinoid symptoms refractory to available somatostatin analogues. Drug Des Devel Ther. 2015;9:5075–5086.
- 89. Feelders RA, Hofland LJ, van Aken MO, et al Medical therapy of acromegaly: efficacy and safety of somatostatin analogues. Drugs. 2009;69(16):2207–2226.
- 90. Öberg K, Lamberts SW. Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: past, present and future. Endocr Relat Cancer. 2016;23(12):R551–R566.
- 91. Feelders RA, Newell-Price J, Pivonello R, Nieman LK, Hofland LJ, Lacroix A. Advances in the medical treatment of Cushing's Syndrome. Lancet Diabetes Endocrinol. 2019;7(4):300–312.
- 92. Henry RR, Ciaraldi TP, Armstrong D, Burke P, Ligueros-Saylan M, Mudaliar S. Hyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteers. J Clin Endocrinol Metab. 2013;98(8):3446–3453.
- 93. Hendren NS, Panach K, Brown TJ , et al Pasireotide for the treatment of refractory hypoglycaemia from malignant insulinoma. Clin Endocrinol (Oxf). 2018; 88(2):341–343.
- 94. Husni H, Khan SA, Alghaieb B, Abusamaan MS, Donner TW, Hamrahian AH. Pasireotide use for the treatment of endogenous hyperinsulinemic hypoglycemia refractory to conventional medical therapy: A case report and review of the literature. Clin Case Rep. 2022;10(3):e05650.
- 95. Ito T, Lee L, Jensen RT. Treatment of symptomatic neuroendocrine tumor syndromes: recent advances and controversies. Expert Opin Pharmacother. 2016;17(16):2191–2205.
- 96. Oziel-Taieb S, Maniry-Quellier J, Chanez B, Poizat F, Ewald J, Niccoli P. Pasireotide for refractory hypoglycemia in malignant insulinoma- case report and review of the literature. Front Endocrinol (Lausanne). 2022; 13:860614.
- 97. Siddiqui M, Vora A, Ali S, Abramowitz J, Mirfakhraee S. Pasireotide: A novel treatment for tumor-induced hypoglycemia due to insulinoma and non-islet cell tumor hypoglycemia. J Endocr Soc. 2021;5(1):bvaa171.
- 98. Blum I, Aderka D, Doron M, Laron Z. Suppression of hypoglycemia by DL-propranolol in malignant insulinoma. N Engl J Med. 1978;299(9):487.
- 99. Blum I, Rusecki Y, Doron M, Lahav M, Laron Z, Atsmon A. Evidence for a therapeutic effect of dl-propranolol in benign and malignant insulinoma: report of three cases. J Endocrinol Invest. 1983;6(1):41–45.
- 100. Stehouwer CD, Lems WF, Fischer HR, Hackeng WH. Malignant insulinoma: is combined treatment with verapamil and the long-acting somatostatin analogue octreotide (SMS 201-995) more effective than single therapy with either drug?Neth J Med. 1989;35(1-2):86–94.
- 101. Imanaka S, Matsuda S, Ito K, Matsuoka T, Okada Y. Medical treatment for inoperable insulinoma: clinical usefulness of diphenylhydantoin and diltiazem. Jpn J Clin Oncol. 1986;16(1):65–71.
- 102. Osataphan S, Vamvini M, Rosen ED, et al Anti-Insulin receptor antibody for malignant insulinoma and refractory hypoglycemia. N Engl J Med. 2023;389(8):767–769.
- 103. Howe JR, Merchant NB, Conrad C, et al The north American neuroendocrine tumor society consensus paper on the surgical management of pancreatic neuroendocrine tumors. Pancreas. 2020;49(1):1–33.
- 104. Dusch N, Lietzmann A, Barthels F, Niedergethmann M, Rückert F, Wilhelm TJ. International study group of pancreatic surgery definitions for postpancreatectomy complications: applicability at a high-volume center. Scand J Surg. 2017;106(3):216–223.
- 105. van Beek DJ, Takkenkamp TJ, Wong-Lun-Hing EM, et al Risk factors for complications after surgery for pancreatic neuroendocrine tumors. Surgery. 2022;172(1):127–136.
- 106. Gurusamy KS, Koti R, Fusai G, Davidson BR. Somatostatin analogues for pancreatic surgery. Cochrane Database Syst Rev. 2013;2013(4):Cd008370.
- 107. Heidsma CM, Tsilimigras DI, van Dieren S, et al Indications and outcomes of enucleation versus formal pancreatectomy for pancreatic neuroendocrine tumors. HPB (Oxford). 2021;23(3):413–421.
- 108. Sallinen VJ, Le Large TYS, Tieftrunk E, et al Prognosis of sporadic resected small (≤2 cm) nonfunctional pancreatic neuroendocrine tumors—a multi-institutional study. HPB (Oxford). 2018;20(3):251–259.
- 109. Hofland J, de Herder WW, Kann PH. Turning up the heat: endoscopic ablation of pancreatic neuroendocrine neoplasms. J Clin Endocrinol Metab. 2019;104(11):5053–5055.
- 110. Oleinikov K, Dancour A, Epshtein J, et al Endoscopic ultrasound-guided radiofrequency ablation: A new therapeutic approach for pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2019;104(7):2637–2647.
- 111. Crinò SF, Napoleon B, Facciorusso A, et al Endoscopic ultrasound-guided radiofrequency ablation versus surgical resection for treatment of pancreatic insulinoma. Clin Gastroenterol Hepatol. 2023;21(11):2834–2843.
- 112. Kann PH. Relevance of endoscopic ultrasound in endocrinology today: multiple endocrine neoplasia type 1, insulinoma, primary aldosteronism-an Expert's Perspective based on three decades of scientific and clinical experience. Cancers (Basel). 2023;15(13):3494.
- 113. Myrehaug S, Hallet J, Chu W, et al Proof of concept for stereotactic body radiation therapy in the treatment of functional neuroendocrine neoplasms. J Radiosurg SBRT. 2020;6(4):321–324.
- 114. van Vliembergen ENM, Eijkelenkamp H, Valk GD, et al Precision radiotherapy using MR-linac for pancreatic neuroendocrine tumors in MEN1 patients (PRIME): a protocol for a phase I-II trial, and systematic review on available evidence for radiotherapy of pNETs. Front Endocrinol (Lausanne). 2023;14:994370.
- 115. van Beek DJ, Nell S, Vorselaars WMCM, et al Complications after Major surgery for duodenopancreatic neuroendocrine tumors in patients with MEN1: results from a nationwide cohort. Ann Surg Oncol. 2021;28(8):4387–4399.
- 116. Antwi K, Nicolas G, Fani M, et al 68Ga-Exendin-4 PET/CT detects insulinomas in patients with endogenous hyperinsulinemic hypoglycemia in MEN-1. J Clin Endocrinol Metab. 2019;104(12):5843–5852.
- 117. Levy MJ, Thompson GB, Topazian MD, Callstrom MR, Grant CS, Vella A. US-guided ethanol ablation of insulinomas: a new treatment option. Gastrointest Endosc. 2012;75(1):200–206.
- 118. Halfdanarson TR, Strosberg JR, Tang L, et al The north American neuroendocrine tumor society consensus guidelines for surveillance and medical management of pancreatic neuroendocrine tumors. Pancreas. 2020;49(7):863–881.
- 119. Habibollahi P, Bai HX, Sanampudi S, Soulen MC, Dagli M. Effectiveness of liver-directed therapy for the management of intractable hypoglycemia in metastatic insulinoma. Pancreas. 2020;49(6):763–767.
- 120. Caplin ME, Pavel M, Ćwikła JB, et al Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–233.
- 121. Brabander T, van der Zwan WA, Teunissen JJM, et al Long-Term efficacy, survival, and safety of [(177)Lu-DOTA(0), tyr(3)]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res. 2017;23(16):4617–4624.
- 122. Hofland J, Brabander T, Verburg FA, Feelders RA, de Herder WW. Peptide receptor radionuclide therapy. J Clin Endocrinol Metab. 2022;107(12):3199–3208.
- 123. Becx MN, Minczeles NS, Brabander T, de Herder WW, Nonnekens J, Hofland J. A clinical guide to peptide receptor radionuclide therapy with (177)Lu-DOTATATE in neuroendocrine tumor patients. Cancers (Basel). 2022;14:23.
- 124. van Schaik E, van Vliet EI, Feelders RA, et al Improved control of severe hypoglycemia in patients with malignant insulinomas by peptide receptor radionuclide therapy. J Clin Endocrinol Metab. 2011;96(11):3381–3389.
- 125. Veltroni A, Cosaro E, Spada F, et al Clinico-pathological features, treatments and survival of malignant insulinomas: a multicenter study. Eur J Endocrinol. 2020;182(4):439–446.
- 126. Zandee WT, Brabander T, Blažević A, et al Symptomatic and radiological response to 177Lu-DOTATATE for the treatment of functioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2019;104(4):1336–1344.
- 127. Yao JC, Shah MH, Ito T, et al Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523.
- 128. Bourcier ME, Sherrod A, DiGuardo M, Vinik AI. Successful control of intractable hypoglycemia using rapamycin in an 86-year-old man with a pancreatic insulin-secreting islet cell tumor and metastases. J Clin Endocrinol Metab. 2009;94(9):3157–3162.
- 129. Kulke MH, Bergsland EK, Yao JC. Glycemic control in patients with insulinoma treated with everolimus. N Engl J Med. 2009;360(2):195–197.
- 130. de Herder WW, van Schaik E, Kwekkeboom D, Feelders RA. New therapeutic options for metastatic malignant insulinomas. Clin Endocrinol (Oxf). 2011; 75(3):277–284.
- 131. Raymond E, Dahan L, Raoul JL, et al Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–513.
- 132. Billemont B, Medioni J, Taillade L, et al Blood glucose levels in patients with metastatic renal cell carcinoma treated with sunitinib. Br J Cancer. 2008;99(9):1380–1382.
- 133. Pavel M, Öberg K, Falconi M, et al Gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(7):844–860.
- 134. Garcia-Carbonero R, Anton-Pascual B, Modrego A. Del carmen riesco-martinez M, Lens-pardo A, carretero-puche C, rubio-cuesta B, Soldevilla B. Advances in the treatment of gastroenteropancreatic neuroendocrine carcinomas: are we moving forward?Endocr Rev. 2023;44(4):724–736.
- 135. Garcia-Carbonero R, Rinke A, Valle JW, et al ENETS Consensus guidelines for the standards of care in neuroendocrine neoplasms. Systemic therapy 2: chemotherapy. Neuroendocrinology. 2017;105(3):281–294.
- 136. Strosberg JR, Coppola D, Klimstra DS, et al The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39(6):799–800.
- 137. Jensen RT, Cadiot G, Brandi ML, et al ENETS Consensus guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95(2):98–119.
- 138. Kulke MH, Anthony LB, Bushnell DL, et al North American neuroendocrine tumor S. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39(6):735–752.
- 139. Eads JR, Halfdanarson TR, Asmis T, et al Expert consensus practice recommendations of the north American neuroendocrine tumor society for the management of high grade gastroenteropancreatic and gynecologic neuroendocrine neoplasms. Endocr Relat Cancer. 2023;30(8):e220206.
- 140. Garcia-Carbonero R, Sorbye H, Baudin E, et al ENETS Consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology. 2016;103(2):186–194.
- 141. Strosberg JR, Fine RL, Choi J, et al First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117(2):268–275.
- 142. de Mestier L, Walter T, Evrard C, et al Temozolomide alone or combined with capecitabine for the treatment of advanced pancreatic neuroendocrine tumor. Neuroendocrinology. 2020;110(1-2):83–91.
- 143. Chan DL, Bergsland EK, Chan JA, et al Temozolomide in grade 3 gastroenteropancreatic neuroendocrine neoplasms: A multicenter retrospective review. Oncologist. 2021;26(11):950–955.
- 144. Kunz PL, Graham NT, Catalano PJ, et al Randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors (ECOG-ACRIN E2211). J Clin Oncol. 2023;41(7):1359–1369.