INTRODUCTION
Quadruplet regimens, consisting of a CD38 monoclonal antibody (mAb) with a proteasome inhibitor, immunomodulatory (IMiD) agent, and steroids, have shown improved efficacy over triplet therapies in patients with newly diagnosed multiple myeloma (NDMM). Isatuximab (Isa) is an immunoglobulin G1 mAb that targets CD38 and is approved in combination with lenalidomide-bortezomib-dexamethasone (RVd) to treat transplant-ineligible patients with NDMM on the basis of the phase III IMROZ trial., Previously, we showed that 18-week induction with Isa-RVd significantly improved minimal residual disease negativity (MRD–) rates in patients with NDMM eligible for autologous hematopoietic stem-cell transplant (ASCT; odds ratio [OR], 1.82 [95% CI, 1.33 to 2.48]; P < .001) in the GMMG-HD7 (ClinicalTrials.gov identifier: NCT03617731) phase III trial, part 1. In this study, we report a prespecified analysis of part 1, including response and MRD– rates after transplant, progression-free survival (PFS) from first random assignment, PFS comparing Isa-RVd and RVd induction followed by lenalidomide maintenance, and landmark analyses exploring the impact of MRD status on PFS.
METHODS
GMMG-HD7 is an open-label, randomized, multicenter, active-controlled, phase III trial involving 67 sites in Germany (Data Supplement, online only). Briefly, patients were aged 18-70 years with NDMM requiring systemic treatment and eligible for ASCT (Te).,
This two-part trial has two random assignments (Data Supplement, Fig A1). In part 1, patients were randomly assigned 1:1 for induction with Isa-RVd or RVd and given three 6-week cycles of a standard RVd dosing schedule. Patients on Isa-RVd also received 10 mg/kg Isa intravenously once per day on days 1, 8, 15, 22, and 29 in cycle 1 and on days 1, 15, and 29 in cycles 2 and 3.
Patients subsequently underwent mobilization of autologous hematopoietic stem cells, followed by high-dose melphalan and ASCT. A tandem ASCT was recommended if patients achieved < complete response (CR) or had high-risk disease. In part 2, patients were randomly reassigned 1:1 to maintenance with lenalidomide alone or Isa-lenalidomide.
Kaplan-Meier estimates were calculated with pointwise 95% CIs for analysis of induction treatment effect on time-to-event end points (PFS, overall survival [OS]). To evaluate each induction therapy's effect, we calculated PFS adjusted for induction treatment followed by lenalidomide maintenance and calculated survival by applying the weighted risk set estimator to account for the second random assignment.,
The trial was approved by ethics committees at all study sites. All patients provided written informed consent. Detailed methodology is provided in the Data Supplement.
RESULTS
Between October 23, 2018, and September 22, 2020, 662 patients were randomly assigned. The intention-to-treat and safety populations comprised 331 and 330 patients on Isa-RVd and 329 and 328 patients on RVd, respectively (Data Supplement, Fig A2). Patient disposition during induction was previously described (Data Supplement, Fig A3). A total of 304 patients (92%) on Isa-RVd and 278 (84%) on RVd received at least 1 ASCT while 79 (24%) and 99 (30%) received tandem ASCT. The most common reason for tandem ASCT was < CR after first ASCT (Data Supplement, Table A1). Two hundred eighty-nine (87%) and 271 (82%) patients in the Isa-RVd and RVd arms, respectively, underwent second random assignment for maintenance therapy (Isa-lenalidomide or lenalidomide).
Demographic and disease characteristics were balanced (Table 1). The median number of days from stem-cell reinfusion to leukocyte and platelet recovery was similar in both arms (Data Supplement, Table A2).
TABLE 1.
Baseline Patient Characteristics
| Characteristic | Isa-RVd (n = 331) | RVd (n = 329) |
|---|---|---|
| Age at random assignment, years | ||
| Median (IQR) | 59 (54-64) | 60 (54-65) |
| Sex, No. (%) | ||
| Female | 127 (38) | 123 (37) |
| Male | 204 (62) | 206 (63) |
| Ethnicity, No. (%) | ||
| White | 327 (99) | 327 (99) |
| African | 1 (<1) | 1 (<1) |
| Arabic | 1 (<1) | 0 |
| Asian | 2 (<1) | 1 (<1) |
| WHO PS, No. (%) | ||
| 0 | 158 (48) | 168 (51) |
| 1 | 137 (41) | 130 (40) |
| 2 | 35 (11) | 29 (9) |
| 3 | 0 | 1 (<1) |
| Unknown | 1 (<1) | 1 (<1) |
| 0-1 | 295 (89) | 298 (91) |
| >1 | 35 (11) | 30 (9) |
| Heavy chain type, No. (%) | ||
| IgG | 194 (59) | 192 (58) |
| IgA | 68 (21) | 69 (21) |
| Light chain only | 59 (18) | 61 (19) |
| Other | 10 (3) | 7 (2) |
| ISS disease stage, No. (%) | ||
| I | 124 (37) | 149 (45) |
| II | 129 (39) | 114 (35) |
| III | 78 (24) | 66 (20) |
| High-risk cytogenetics, No. (%) | ||
| No | 254 (77) | 234 (71) |
| Yes | 58 (18) | 66 (20) |
| Unknown | 19 (6) | 29 (9) |
| Elevated lactate dehydrogenase, No. (%) | ||
| No | 268 (81) | 286 (87) |
| Yes | 63 (19) | 43 (13) |
| R-ISS disease stage, No. (%) | ||
| I | 77 (23) | 98 (30) |
| II | 219 (66) | 185 (56) |
| III | 27 (8) | 26 (8) |
| Not classified | 9 (3) | 20 (6) |
| Renal impairment, No. (%) | ||
| No | 312 (94) | 307 (93) |
| Yes | 19 (6) | 22 (7) |
More patients in the Isa-RVd versus RVd arm achieved CR (144 [43.5%] v 112 [34.0%]; OR, 1.49 [95% CI, 1.08 to 2.07]; P = .013; Data Supplement, Table A3) and MRD– (219 [66.2%] v 157 [47.7%]; OR, 2.13 [95% CI, 1.56 to 2.92]; P < .0001) after transplant, whereas 126 patients (38.1%) on Isa-RVd and 85 (25.8%) on RVd achieved CR and MRD– (OR, 1.76 [95% CI, 1.25 to 2.50]; P = .001). Preplanned exploratory analyses after transplant demonstrated higher MRD– rates in the Isa-RVd arm in most subgroups, except in patients with WHO performance status (WHO PS) >1, Revised International Staging System (R-ISS) stage III, and high-risk cytogenetics (Data Supplement, Fig A4).
PFS from first random assignment independent of maintenance revealed a statistically significant benefit for Isa-RVd versus RVd (hazard ratio [HR], 0.70 [95% CI, 0.52 to 0.95]; stratified log-rank test P = .0184; Fig 1A, Data Supplement). Median PFS was not reached in either arm.
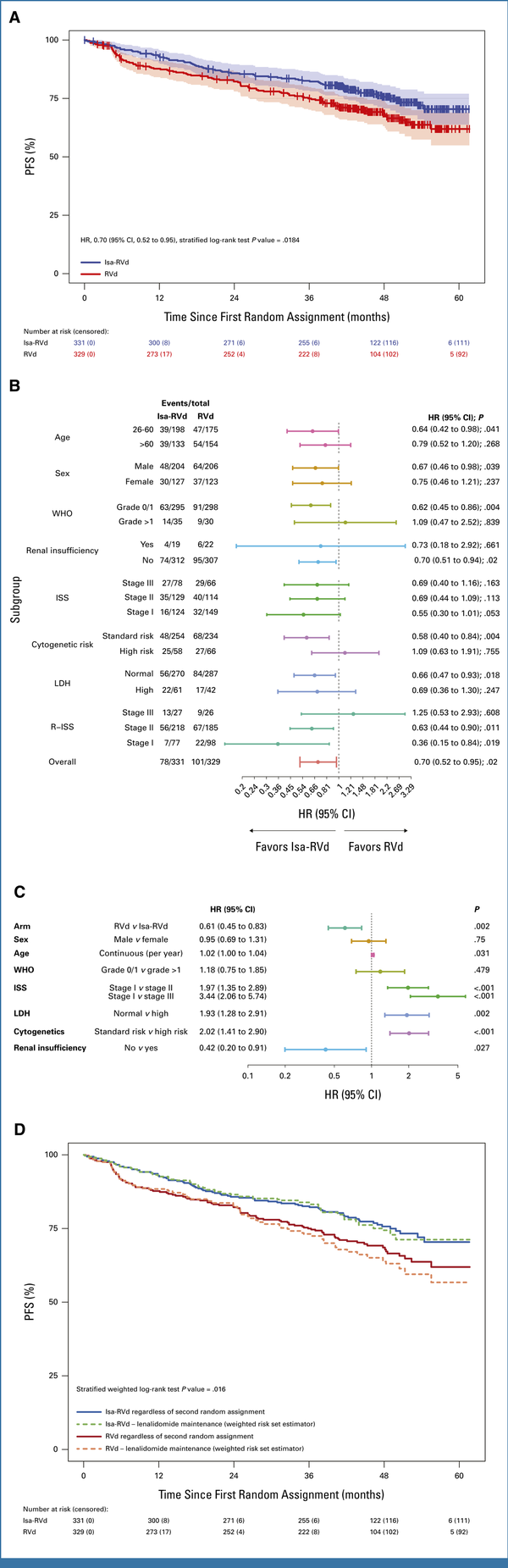
FIG 1.
Analyses of PFS from part 1 of the GMMG-HD7 trial comparing Isa-RVd with RVd induction. (A) Kaplan-Meier estimates for PFS from the first random assignment. PFS rates at 3 and 4 years were 83% (95% CI, 79 to 87) and 76% (95% CI, 71 to 81) with Isa-RVd versus 75% (95% CI, 70 to 80) and 69% (95% CI, 63 to 74) with RVd, respectively. (B) Subgroup analyses for PFS from the first random assignment. (C) Multivariable model on PFS. (D) Weighted risk set estimator analysis for PFS accounting for second random assignment. Estimated PFS rates at 3 and 4 years were 84% (95% CI, 79 to 89) and 74% (95% CI, 68 to 81) with Isa-RVd versus 73% (95% CI, 67 to 79) and 64% (95% CI, 56 to 71) with RVd, respectively. The numbers at risk counts are raw counts of the 331 and 329 patients who were randomly assigned. d, dexamethasone; HR, hazard ratio; Isa, isatuximab; ISS, International Staging System; LDH, lactate dehydrogenase; PFS, progression-free survival; R, lenalidomide; R-ISS, Revised International Staging System; V, bortezomib.
Preplanned subgroup analyses showed a PFS benefit with Isa-RVd versus RVd in most subgroups, except in patients with WHO PS >1, R-ISS stage III, and high-risk cytogenetics (Fig 1B). PFS benefit for Isa-RVd versus RVd was confirmed using a multivariable model (HR, 0.61 [95% CI, 0.45 to 0.83]; P = .002) including established prognostic factors (Fig 1C).
Preplanned weighted risk set estimator analysis accounting for second random assignment and maintenance revealed a statistically significant benefit for Isa-RVd versus RVd, both followed by lenalidomide maintenance (stratified weighted log-rank test P = .016; Fig 1D).
Landmark analyses revealed significantly longer PFS from end of induction (HR, 0.38 [95% CI, 0.26 to 0.55]; P < .001; Fig 2A) and end of transplant (HR, 0.42 [95% CI, 0.28 to 0.63]; P < .001; Fig 2B) in all patients achieving MRD– compared with MRD positivity (MRD+). PFS from end of induction was similar among MRD– patients in both arms (P = .72) but significantly longer in MRD+ patients treated with Isa-RVd versus RVd (HR, 0.64 [95% CI, 0.43 to 0.96]; P = .03; Fig 2C). PFS from end of transplant was similar in both arms whether patients were MRD– (P = .882) or MRD+ (P = .314; Fig 2D).
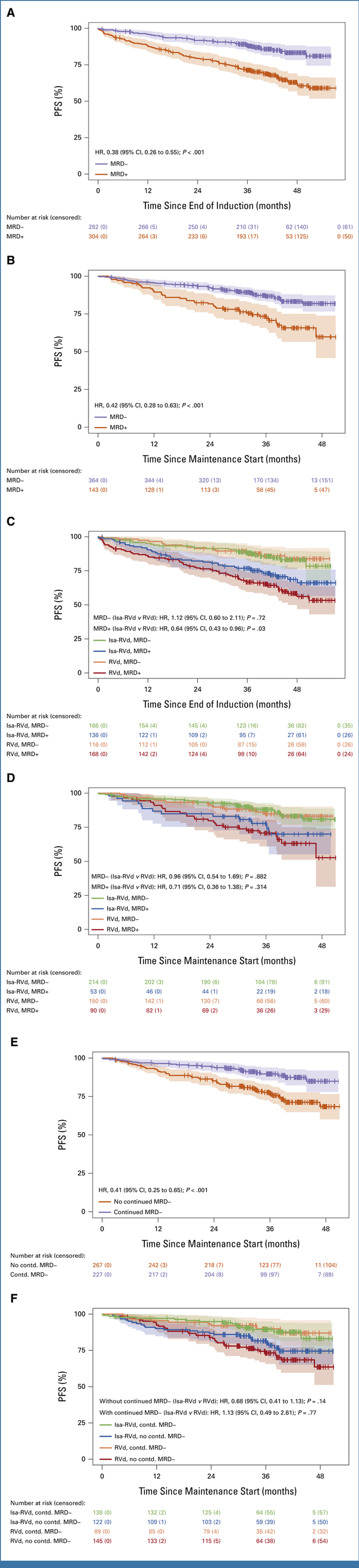
FIG 2.
Impact of MRD status on PFS in part 1 of the GMMG-HD7 trial. (A) PFS from end of induction with respect to MRD status. Estimated 3-year PFS rates were 88% (95% CI, 85 to 92) in the Isa-RVd group and 71% (95% CI, 66 to 77) in the RVd group. (B) PFS from start of maintenance with respect to MRD status at the end of transplant. Estimated 3-year PFS rates after transplant were 87% (95% CI, 83 to 91) in the Isa-RVd group and 73% (95% CI, 66 to 81) in the RVd group. (C) PFS from end of induction with respect to induction treatment arm and MRD status. (D) PFS from start of maintenance with respect to induction treatment arm and MRD status at the end of transplant. (E) PFS from start of maintenance with respect to continued MRD–.a Estimated 3-year PFS rates from maintenance start were 90% (95% CI, 86 to 94) in the Isa-RVd group and 77% (95% CI, 72 to 83) in the RVd group. (F) PFS from start of maintenance with respect to induction treatment arm and continued MRD–.a The median time from random assignment to MRD measurements (after induction) was 4.57 and 4.47 months in the Isa-RVd and RVd arms, respectively, and the corresponding post-transplant time from random assignment to MRD measurements was 9.72 and 9.81 months, respectively. The median time between continued MRD measurements (between end of induction and end of transplant) was 5.04 and 5.17 months, respectively. aContinued MRD– was defined as MRD– persisting from end of induction to post-transplant. d, dexamethasone; HR, hazard ratio; Isa, isatuximab; MRD, minimal residual disease; PFS, progression-free survival; R, lenalidomide; V, bortezomib.
PFS from maintenance start was significantly prolonged in patients with continued MRD– versus those without (HR, 0.41 [95% CI, 0.25 to 0.65]; P < .001; Fig 2E). Patients in both arms with continued MRD– had similar PFS from maintenance start (P = .77). Among patients without continued MRD–, PFS from maintenance start tended to be longer in the Isa-RVd versus RVd arm (HR, 0.68 [95% CI, 0.41 to 1.13]; P = .14; Fig 2F). Additional details are in the Data Supplement.
OS data were not mature at this cutoff (stratified log-rank test P = .548; Data Supplement, Fig A5).
DISCUSSION
At a median follow-up of approximately 4 years from the first random assignment, the addition of Isa to RVd during 18-week induction, without post-transplant consolidation, translated into a significant and clinically meaningful PFS benefit, regardless of subsequent maintenance with Isa-lenalidomide. The GMMG-HD7 trial design reflects real-world clinical practice, where patients usually receive a transplant without consolidation.,
Deep and sustained responses in patients with multiple myeloma correlate with improved survival, underscoring MRD– as a key prognostic factor., The PFS observed with Isa-RVd corroborates the previously reported MRD– benefit after induction along with the increased depth of response after transplant shown here. Landmark analyses from end of induction showed significantly longer PFS in patients achieving MRD– than MRD+ and continued MRD– than without. Owing to decreasing sample size when analyzing by MRD and treatment arm, longer follow-up is needed to assess PFS differences between arms in MRD– patients; however, PFS tended to be longer with Isa-RVd over RVd in MRD+ patients.
At this final analysis of Part 1, Isa-RVd demonstrated consistent PFS benefit and higher MRD– rates as compared to RVd across clinically relevant subgroups, except in patients with WHO PS >1, R-ISS stage III, and high-risk cytogenetics. As the trial was not powered to detect a difference in these small subpopulations, these results should be interpreted with caution. The previous GMMG-HD7 interim analysis showed a manageable and consistent safety profile with Isa-RVd in Te patients with NDMM.
In the phase III PERSEUS trial, the addition of daratumumab (Dara) to RVd induction (four 4-week cycles), RVd consolidation (two 4-week cycles), and lenalidomide maintenance yielded significant PFS improvements (estimated 4-year PFS 84.3% [Dara-RVd] v 67.7% [RVd]; HR, 0.42 [95% CI, 0.30 to 0.59]; P < .001]) in Te patients with NDMM. Comparisons between PERSEUS and GMMG-HD7 trials are limited because of study design differences (dose intensity, induction duration, use of consolidation, and random assignment for maintenance), which may explain differences between HRs, although both trials demonstrated benefits with a quadruplet regimen. Dara-RVd is approved in the United States and Europe for induction and Dara-R is approved in Europe for maintenance in Te patients with NDMM, with other trials investigating various NDMM maintenance therapies.
Limitations of this analysis include PFS results from the first random assignment may be diluted by the tandem ASCT and second random assignment. However, second random assignment was accounted for by the weighted risk set estimator analysis and yielded similar results to the unweighted PFS analysis. Moreover, fewer patients on Isa-RVd versus RVd received tandem ASCT, partially explained by a higher proportion of patients on Isa-RVd achieving CR. Despite more tandem ASCT in the control arm, a consistent improvement in MRD– and CR rates after transplant in favor of Isa-RVd was observed. This analysis is also limited by the lack of maintenance data, to be reported when data are mature.
In conclusion, 18-week induction with Isa-RVd in Te patients with NDMM results in higher rates of MRD– after induction and transplant that translate into a significant PFS benefit versus RVd regardless of maintenance therapy. Our results support the use of Isa quadruplet regimens as the current standard of care in Te patients with NDMM.
PRIOR PRESENTATION
Parts of this manuscript will be presented at the American Society of Hematology Meeting 2024, San Diego, CA.
CLINICAL TRIAL INFORMATION
NCT03617731 (GMMG-HD7)
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO-24-02266. The GMMG-HD7 trial is ongoing. Data from published parts of the GMMG-HD7 trial can be made available upon request to and decision of the principal investigator (Hartmut Goldschmidt; [email protected]) and the board of directors of the German-speaking Myeloma Multicenter Group (GMMG) after finalization of the trial.
AUTHOR CONTRIBUTIONS
Conception and design: Elias K. Mai, Uta Bertsch, Igor W. Blau, Steffen Luntz, Katja C. Weisel, Hartmut Goldschmidt
Financial support: Hartmut Goldschmidt
Administrative support: Elias K. Mai, Hartmut Goldschmidt
Provision of study materials or patients: Elias K. Mai, Roland Schroers, Mathias Hänel, Christoph Mann, Lisa B. Leypoldt, Stefanie Huhn, Christof Scheid, Igor W. Blau, Karolin Trautmann-Grill, Maika Klaiber-Hakimi, Martin Müller, Evgenii Shumilov, Wolfgang Knauf, Christian S. Michel, Thomas Geer, Marc S. Raab, Martin Hoffmann, Katja C. Weisel, Hans J. Salwender, Hartmut Goldschmidt, Ivana von Metzler
Collection and assembly of data: Elias K. Mai, Uta Bertsch, Roland Fenk, Britta Besemer, Roland Schroers, Christoph Mann, Lisa B. Leypoldt, Bernhard Heilmeier, Stefanie Huhn, Sabine K. Vogel, Christof Scheid, Steffen Luntz, Tobias A.W. Holderried, Karolin Trautmann-Grill, Deniz Gezer, Maika Klaiber-Hakimi, Martin Müller, Evgenii Shumilov, Wolfgang Knauf, Thomas Geer, Hendrik Riesenberg, Christoph Lutz, Marc S. Raab, Martin Hoffmann, Katja C. Weisel, Hans J. Salwender, Hartmut Goldschmidt, Ivana von Metzler
Data analysis and interpretation: Elias K. Mai, Uta Bertsch, Ema Pozek, Christine Hanoun, Roland Schroers, Mathias Hänel, Bernhard Heilmeier, Michael Hundemer, Christof Scheid, Niels Weinhold, Diana Tichy, Tobias A.W. Holderried, Martin Müller, Wolfgang Knauf, Christian S. Michel, Marc S. Raab, Axel Benner, Katja C. Weisel, Hartmut Goldschmidt
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Isatuximab, Lenalidomide, Bortezomib, and Dexamethasone Induction Therapy for Transplant-Eligible Newly Diagnosed Multiple Myeloma: Final Part 1 Analysis of the GMMG-HD7 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Elias K. Mai
Honoraria: Janssen, Takeda, Bristol Myers Squibb/Celgene, Sanofi, GlaxoSmithKline, Stemline Therapeutics, Oncopeptides
Consulting or Advisory Role: Janssen, Bristol Myers Squibb/Celgene, Takeda, Sanofi, GlaxoSmithKline, Stemline Therapeutics, Oncopeptides
Research Funding: Janssen, Bristol Myers Squibb/Celgene, Takeda, Sanofi, GlaxoSmithKline
Travel, Accommodations, Expenses: Janssen, Bristol Myers Squibb/Celgene, Takeda, GlaxoSmithKline, Sanofi, Stemline Therapeutics
Roland Fenk
Honoraria: BMS/Celgene, Janssen, Sanofi, Amgen, Takeda, Pfizer
Travel, Accommodations, Expenses: Janssen, BMS/Celgene, GlaxoSmithKline
Britta Besemer
Honoraria: Janssen-Cilag (Inst), AMGEN (Inst), GlaxoSmithKline (Inst), Sanofi (Inst), Oncopeptides (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Janssen-Cilag, AMGEN (Inst), Janssen Cilag (Inst)
Ivana von Metzler
Honoraria: Sanofi
Consulting or Advisory Role: Janssen, Pfizer, Oncopeptides, Amgen, GlaxoSmithKline, BMS GmbH & Co KG, Sanofi, AbbVie, Stemline Therapeutics
Travel, Accommodations, Expenses: Janssen
Mathias Hänel
Honoraria: SOBI, Novartis, Gilead Sciences, Falk Foundation, SOBI, BMS GmbH & Co KG, Kite, a Gilead company
Consulting or Advisory Role: Sanofi/Aventis, Amgen, SOBI, Janssen, Kite/Gilead, Amgen, Janssen, Sanofi/Aventis, BMS GmbH & Co KG, Kite/Gilead, Amgen, Janssen, BeiGene
Travel, Accommodations, Expenses: AbbVie
Christoph Mann
Consulting or Advisory Role: Sanofi, BMS GmbH & Co KG, Janssen
Lisa B. Leypoldt
Honoraria: Janssen, Sanofi, Sanofi, Adaptive Biotechnologies, AbbVie, Pfizer, GlaxoSmithKline, Bristol Myers Squibb/Celgene
Consulting or Advisory Role: Sanofi, GlaxoSmithKline, Janssen, Bristol Myers Squibb/Celgene
Research Funding: GlaxoSmithKline (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Sanofi, GlaxoSmithKline, AbbVie, Oncopeptides
Bernhard Heilmeier
Consulting or Advisory Role: Janssen
Travel, Accommodations, Expenses: SOBI
Sabine K. Vogel
Stock and Other Ownership Interests: Sanofi (I), Bristol Myers Squibb (I)
Michael Hundemer
Employment: MSD (I)
Consulting or Advisory Role: BeiGene (Inst), Becton Dickinson (Inst)
Research Funding: Novartis (Inst), BeiGene (Inst), Sanofi (Inst), Janssen (Inst), Alexion Pharmaceuticals (Inst), Roche (Inst), Celgene (Inst)
Travel, Accommodations, Expenses: BeiGene (Inst)
Christof Scheid
Employment: University of Cologne
Honoraria: Amgen, Bristol Myers Squibb/Celgene, Janssen Oncology, Novartis, Pfizer, Takeda, Sanofi/Aventis, GlaxoSmithKline, Stemline Therapeutics, Oncopeptides, Adaptive Biotechnologies
Consulting or Advisory Role: Amgen, Roche, Janssen Oncology, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Sanofi/Aventis
Research Funding: Janssen Oncology (Inst), Takeda (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb/Celgene, Janssen Oncology, Amgen
Niels Weinhold
Honoraria: GlaxoSmithKline
Consulting or Advisory Role: Sanofi
Research Funding: Celgene/Bristol Myers Squibb
Tobias A.W. Holderried
Consulting or Advisory Role: GlaxoSmithKline, Amgen, Novartis, Jazz Pharmaceuticals, Kite/Gilead, Bristol Myers Squibb/Celgene, Pfizer, Sanofi
Travel, Accommodations, Expenses: AbbVie, Medac, Janssen Oncology, Kite/Gilead, BeiGene, Sanofi, Jazz Pharmaceuticals, Immatics
Karolin Trautmann-Grill
Honoraria: Novartis, GlaxoSmithKline, Takeda
Consulting or Advisory Role: SOBI, Grifols, Amgen, GlaxoSmithKline, Roche, Takeda
Speakers' Bureau: GlaxoSmithKline
Research Funding: Amgen, Novartis, Sobi, Grifols
Travel, Accommodations, Expenses: CSL Behring
Deniz Gezer
Honoraria: GlaxoSmithKline
Consulting or Advisory Role: Amgen, BMS GmbH & Co KG, Janssen Oncology, Sanofi Aventis GmbH, Stemline Therapeutics, Pfizer
Travel, Accommodations, Expenses: Pfizer, Amgen
Evgenii Shumilov
Honoraria: Amgen, Sanofi, Stemline, BMS, Incyte, Takeda, Pfizer, Oncopeptides
Consulting or Advisory Role: Sanofi, Stemline, Amgen, Takeda, Pfizer, Sobi, BMS, Oncopeptides
Travel, Accommodations, Expenses: Amgen, Sanofi, Takeda, Oncopeptides
Wolfgang Knauf
Consulting or Advisory Role: Sanofi, Janssen Oncology, Celgene
Travel, Accommodations, Expenses: Sanofi, Janssen Oncology, Celgene
Christian S. Michel
Employment: Sanofi (I)
Stock and Other Ownership Interests: Sanofi (I)
Honoraria: Bristol Myers Squibb, Johnson & Johnson/Janssen, Oncopeptides, GlaxoSmithKline
Consulting or Advisory Role: Johnson & Johnson/Janssen, GlaxoSmithKline, Sanofi, Oncopeptides
Travel, Accommodations, Expenses: Johnson & Johnson/Janssen
Christoph Lutz
Consulting or Advisory Role: Novartis, Eisai, GlaxoSmithKline, Ipsen, BMS, Janssen
Speakers' Bureau: Novartis
Marc S. Raab
Honoraria: AbbVie, Bristol Myers Squibb/Celgene, Janssen, Sanofi
Consulting or Advisory Role: Bristol Myers Squibb/Celgene (Inst), Amgen (Inst), GlaxoSmithKline (Inst), Janssen (Inst), Sanofi (Inst), Pfizer (Inst)
Research Funding: Bristol Myers Squibb/Celgene (Inst), Janssen (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: AbbVie, Bristol Myers Squibb/Celgene, Amgen, Janssen, Sanofi, Pfizer
Martin Hoffmann
Travel, Accommodations, Expenses: Janssen Oncology, Pfizer
Katja C. Weisel
Honoraria: Amgen, Bristol Myers Squibb, Janssen-Cilag, GlaxoSmithKline, Adaptive Biotechnologies, Karyopharm Therapeutics, Takeda, Sanofi, AbbVie, GlaxoSmithKline, Novartis, Pfizer, Celgene, Janssen (Inst), Oncopeptides, Roche, Menarini, Stemline Therapeutics, AstraZeneca, BeiGene
Consulting or Advisory Role: Amgen, Adaptive Biotechnologies, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen-Cilag, Karyopharm Therapeutics, Sanofi, Takeda, Oncopeptides, Roche, Menarini, Regeneron, AbbVie, BeiGene
Research Funding: Amgen (Inst), Celgene (Inst), Sanofi (Inst), Janssen-Cilag (Inst), Bristol Myers Squibb/Celgene (Inst), GlaxoSmithKline (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Amgen, Celgene, Bristol Myers Squibb, Janssen-Cilag, GlaxoSmithKline, Takeda, Menarini
Hans J. Salwender
Honoraria: Janssen, BMS GmbH & Co KG, Amgen, AbbVie, Stemline Therapeutics, Oncopeptides, AstraZeneca, Sanofi, Genzyme, GlaxoSmithKline, Pfizer, Roche
Consulting or Advisory Role: Pfizer, Janssen Oncology, Sanofi, Oncopeptides, GlaxoSmithKline, Amgen, AbbVie, Bristol Myers Squibb/Celgene, Roche, Genzyme, Stemline Therapeutics, AstraZeneca
Travel, Accommodations, Expenses: Amgen, BMS GmbH & Co KG, Janssen, Pfizer, Stemline Therapeutics, Sanofi
Hartmut Goldschmidt
Honoraria: Janssen-Cilag, Novartis, Bristol Myers Squibb, Chugai Pharma, Sanofi, Amgen, GlaxoSmithKline, Pfizer
Consulting or Advisory Role: Janssen-Cilag (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), Adaptive Biotechnologies (Inst), Sanofi (Inst)
Research Funding: Bristol Myers Squibb (Inst), Janssen (Inst), Novartis (Inst), Celgene (Inst), Amgen (Inst), Sanofi (Inst), Takeda (Inst), Molecular Partners (Inst), MSD (Inst), Incyte (Inst), GlycoMimetics Inc (Inst), GlaxoSmithKline (Inst), Heidelberg Pharma (Inst), Roche (Inst), Karyopharm Therapeutics (Inst), Millennium Pharmaceuticals (Inst), MorphoSys (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Janssen-Cilag, Sanofi, Amgen, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Novartis, Pfizer
Other Relationship: Amgen (Inst), Celgene/Bristol Myers Squibb (Inst), Chugai Pharma Europe (Inst), Janssen (Inst), Sanofi (Inst), Mundipharma (Inst), Array BioPharma/Pfizer (Inst)
No other potential conflicts of interest were reported.
Elias K. Mai
Honoraria: Janssen, Takeda, Bristol Myers Squibb/Celgene, Sanofi, GlaxoSmithKline, Stemline Therapeutics, Oncopeptides
Consulting or Advisory Role: Janssen, Bristol Myers Squibb/Celgene, Takeda, Sanofi, GlaxoSmithKline, Stemline Therapeutics, Oncopeptides
Research Funding: Janssen, Bristol Myers Squibb/Celgene, Takeda, Sanofi, GlaxoSmithKline
Travel, Accommodations, Expenses: Janssen, Bristol Myers Squibb/Celgene, Takeda, GlaxoSmithKline, Sanofi, Stemline Therapeutics
Roland Fenk
Honoraria: BMS/Celgene, Janssen, Sanofi, Amgen, Takeda, Pfizer
Travel, Accommodations, Expenses: Janssen, BMS/Celgene, GlaxoSmithKline
Britta Besemer
Honoraria: Janssen-Cilag (Inst), AMGEN (Inst), GlaxoSmithKline (Inst), Sanofi (Inst), Oncopeptides (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Janssen-Cilag, AMGEN (Inst), Janssen Cilag (Inst)
Ivana von Metzler
Honoraria: Sanofi
Consulting or Advisory Role: Janssen, Pfizer, Oncopeptides, Amgen, GlaxoSmithKline, BMS GmbH & Co KG, Sanofi, AbbVie, Stemline Therapeutics
Travel, Accommodations, Expenses: Janssen
Mathias Hänel
Honoraria: SOBI, Novartis, Gilead Sciences, Falk Foundation, SOBI, BMS GmbH & Co KG, Kite, a Gilead company
Consulting or Advisory Role: Sanofi/Aventis, Amgen, SOBI, Janssen, Kite/Gilead, Amgen, Janssen, Sanofi/Aventis, BMS GmbH & Co KG, Kite/Gilead, Amgen, Janssen, BeiGene
Travel, Accommodations, Expenses: AbbVie
Christoph Mann
Consulting or Advisory Role: Sanofi, BMS GmbH & Co KG, Janssen
Lisa B. Leypoldt
Honoraria: Janssen, Sanofi, Sanofi, Adaptive Biotechnologies, AbbVie, Pfizer, GlaxoSmithKline, Bristol Myers Squibb/Celgene
Consulting or Advisory Role: Sanofi, GlaxoSmithKline, Janssen, Bristol Myers Squibb/Celgene
Research Funding: GlaxoSmithKline (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Sanofi, GlaxoSmithKline, AbbVie, Oncopeptides
Bernhard Heilmeier
Consulting or Advisory Role: Janssen
Travel, Accommodations, Expenses: SOBI
Sabine K. Vogel
Stock and Other Ownership Interests: Sanofi (I), Bristol Myers Squibb (I)
Michael Hundemer
Employment: MSD (I)
Consulting or Advisory Role: BeiGene (Inst), Becton Dickinson (Inst)
Research Funding: Novartis (Inst), BeiGene (Inst), Sanofi (Inst), Janssen (Inst), Alexion Pharmaceuticals (Inst), Roche (Inst), Celgene (Inst)
Travel, Accommodations, Expenses: BeiGene (Inst)
Christof Scheid
Employment: University of Cologne
Honoraria: Amgen, Bristol Myers Squibb/Celgene, Janssen Oncology, Novartis, Pfizer, Takeda, Sanofi/Aventis, GlaxoSmithKline, Stemline Therapeutics, Oncopeptides, Adaptive Biotechnologies
Consulting or Advisory Role: Amgen, Roche, Janssen Oncology, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Sanofi/Aventis
Research Funding: Janssen Oncology (Inst), Takeda (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb/Celgene, Janssen Oncology, Amgen
Niels Weinhold
Honoraria: GlaxoSmithKline
Consulting or Advisory Role: Sanofi
Research Funding: Celgene/Bristol Myers Squibb
Tobias A.W. Holderried
Consulting or Advisory Role: GlaxoSmithKline, Amgen, Novartis, Jazz Pharmaceuticals, Kite/Gilead, Bristol Myers Squibb/Celgene, Pfizer, Sanofi
Travel, Accommodations, Expenses: AbbVie, Medac, Janssen Oncology, Kite/Gilead, BeiGene, Sanofi, Jazz Pharmaceuticals, Immatics
Karolin Trautmann-Grill
Honoraria: Novartis, GlaxoSmithKline, Takeda
Consulting or Advisory Role: SOBI, Grifols, Amgen, GlaxoSmithKline, Roche, Takeda
Speakers' Bureau: GlaxoSmithKline
Research Funding: Amgen, Novartis, Sobi, Grifols
Travel, Accommodations, Expenses: CSL Behring
Deniz Gezer
Honoraria: GlaxoSmithKline
Consulting or Advisory Role: Amgen, BMS GmbH & Co KG, Janssen Oncology, Sanofi Aventis GmbH, Stemline Therapeutics, Pfizer
Travel, Accommodations, Expenses: Pfizer, Amgen
Evgenii Shumilov
Honoraria: Amgen, Sanofi, Stemline, BMS, Incyte, Takeda, Pfizer, Oncopeptides
Consulting or Advisory Role: Sanofi, Stemline, Amgen, Takeda, Pfizer, Sobi, BMS, Oncopeptides
Travel, Accommodations, Expenses: Amgen, Sanofi, Takeda, Oncopeptides
Wolfgang Knauf
Consulting or Advisory Role: Sanofi, Janssen Oncology, Celgene
Travel, Accommodations, Expenses: Sanofi, Janssen Oncology, Celgene
Christian S. Michel
Employment: Sanofi (I)
Stock and Other Ownership Interests: Sanofi (I)
Honoraria: Bristol Myers Squibb, Johnson & Johnson/Janssen, Oncopeptides, GlaxoSmithKline
Consulting or Advisory Role: Johnson & Johnson/Janssen, GlaxoSmithKline, Sanofi, Oncopeptides
Travel, Accommodations, Expenses: Johnson & Johnson/Janssen
Christoph Lutz
Consulting or Advisory Role: Novartis, Eisai, GlaxoSmithKline, Ipsen, BMS, Janssen
Speakers' Bureau: Novartis
Marc S. Raab
Honoraria: AbbVie, Bristol Myers Squibb/Celgene, Janssen, Sanofi
Consulting or Advisory Role: Bristol Myers Squibb/Celgene (Inst), Amgen (Inst), GlaxoSmithKline (Inst), Janssen (Inst), Sanofi (Inst), Pfizer (Inst)
Research Funding: Bristol Myers Squibb/Celgene (Inst), Janssen (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: AbbVie, Bristol Myers Squibb/Celgene, Amgen, Janssen, Sanofi, Pfizer
Martin Hoffmann
Travel, Accommodations, Expenses: Janssen Oncology, Pfizer
Katja C. Weisel
Honoraria: Amgen, Bristol Myers Squibb, Janssen-Cilag, GlaxoSmithKline, Adaptive Biotechnologies, Karyopharm Therapeutics, Takeda, Sanofi, AbbVie, GlaxoSmithKline, Novartis, Pfizer, Celgene, Janssen (Inst), Oncopeptides, Roche, Menarini, Stemline Therapeutics, AstraZeneca, BeiGene
Consulting or Advisory Role: Amgen, Adaptive Biotechnologies, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Janssen-Cilag, Karyopharm Therapeutics, Sanofi, Takeda, Oncopeptides, Roche, Menarini, Regeneron, AbbVie, BeiGene
Research Funding: Amgen (Inst), Celgene (Inst), Sanofi (Inst), Janssen-Cilag (Inst), Bristol Myers Squibb/Celgene (Inst), GlaxoSmithKline (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Amgen, Celgene, Bristol Myers Squibb, Janssen-Cilag, GlaxoSmithKline, Takeda, Menarini
Hans J. Salwender
Honoraria: Janssen, BMS GmbH & Co KG, Amgen, AbbVie, Stemline Therapeutics, Oncopeptides, AstraZeneca, Sanofi, Genzyme, GlaxoSmithKline, Pfizer, Roche
Consulting or Advisory Role: Pfizer, Janssen Oncology, Sanofi, Oncopeptides, GlaxoSmithKline, Amgen, AbbVie, Bristol Myers Squibb/Celgene, Roche, Genzyme, Stemline Therapeutics, AstraZeneca
Travel, Accommodations, Expenses: Amgen, BMS GmbH & Co KG, Janssen, Pfizer, Stemline Therapeutics, Sanofi
Hartmut Goldschmidt
Honoraria: Janssen-Cilag, Novartis, Bristol Myers Squibb, Chugai Pharma, Sanofi, Amgen, GlaxoSmithKline, Pfizer
Consulting or Advisory Role: Janssen-Cilag (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), Adaptive Biotechnologies (Inst), Sanofi (Inst)
Research Funding: Bristol Myers Squibb (Inst), Janssen (Inst), Novartis (Inst), Celgene (Inst), Amgen (Inst), Sanofi (Inst), Takeda (Inst), Molecular Partners (Inst), MSD (Inst), Incyte (Inst), GlycoMimetics Inc (Inst), GlaxoSmithKline (Inst), Heidelberg Pharma (Inst), Roche (Inst), Karyopharm Therapeutics (Inst), Millennium Pharmaceuticals (Inst), MorphoSys (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Janssen-Cilag, Sanofi, Amgen, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Novartis, Pfizer
Other Relationship: Amgen (Inst), Celgene/Bristol Myers Squibb (Inst), Chugai Pharma Europe (Inst), Janssen (Inst), Sanofi (Inst), Mundipharma (Inst), Array BioPharma/Pfizer (Inst)
No other potential conflicts of interest were reported.
APPENDIX. LIST OF GMMG-HD7 INVESTIGATORS AND STAFF
The following local investigators/centers (in alphabetical order) participated in the GMMG-HD7 trial as main trial sites:
Miriam Ahlborn, Medizinische Klinik III, Hämatologie und Onkologie, Städtisches Klinikum Braunschweig, Braunschweig
Joachim Behringer, Onkologische Schwerpunktpraxis Speyer, Speyer
Helga Bernhard, Onkologisches Zentrum, Medizinische Klinik V, Klinikum Darmstadt, Darmstadt
Britta Besemer, Innere Medizin II—Hämatologie, Onkologie, Klinische Immunologie und Rheumatologie, Universitätsklinikum Tübingen, Tübingen
Jörg Bittenbring, Klinik für Innere Medizin I (Onkologie, Hämatologie, Klinische Immunologie und Rheumatologie), Universitätsklinikum des Saarlandes, Homburg
Igor Wolfgang Blau, Klinik für Hämatologie, Onkologie und Tumorimmunologie, Charité Campus Benjamin Franklin und Campus Virchow—Universitätsmedizin Berlin, Berlin
Claus Bolling, Agaplesion Markus Krankenhaus, Frankfurt am Main
Amelie Boquoi, Klinik für Hämatologie und Stammzelltransplantation, Universitätsklinikum, Universitätsmedizin Essen, Essen
Daniel Debatin, Onkologische Schwerpunktpraxis Heidelberg Gerrit Dingeldein, Onkologische Schwerpunktpraxis Darmstadt, Darmstadt
Gerlinde Egerer, Krankenhaus St Vincentius der Evangelische Stadtmission Heidelberg, Heidelberg
Roland Fenk, Klinik für Hämatologie, Onkologie und Klinische Immunologie, Universitätsklinikum Düsseldorf, Düsseldorf
Barbara Ferstl, Medizinische Klinik 5—Hämatologie und Internistische Onkologie, Universitätsklinikum Erlangen, Erlangen
Stefan Fronhoffs, Zentrum für Ambulante Hämatologie und Onkologie (ZAHO), Standort Siegburg
Tobias Gaska, Klinik für Hämatologie und Onkologie, Brüderkrankenhaus St. Josef Paderborn, Paderborn
Thomas Geer, Klinik für Innere Medizin III—Klinik für Onkologie, Hämatologie und Palliativmedizin, Diakonie-Klinikum Schwäbisch Hall, Schwäbisch Hall
Deniz Gezer, Klinik für Hämatologie, Onkologie, Hämostaseologie und Stammzelltransplantation, Uniklinik RWTH Aachen, Aachen
Hartmut Goldschmidt, Medizinische Klinik V, Universitätsklinikum Heidelberg und Nationales Centrum für Tumorerkrankungen (NCT), Heidelberg
Martin Görner, Klinik für Hämatologie, Onkologie und Palliativmedizin, Klinikum Bielefeld, Bielefeld
Ullrich Graeven, Klinik für Hämatologie, Onkologie und Gastroenterologie, Kliniken Maria Hilf, Mönchengladbach
Mathias Hänel, Klinik für Innere Medizin III—Hämatologie, Onkologie, und Zelltherapie, Klinikum Chemnitz, Chemnitz
Bernhard Heilmeier, Klinik für Onkologie und Hämatologie, Krankenhaus Barmherzige Brüder, Regensburg
Michael Heinsch, Fachbereich Onkologie und Hämatologie, Helios St. Johannes Klinik Duisburg, Duisburg
Gerhard Held, Klinik für Innere Medizin 1, Westpfalz-Klinikum, Kaiserslautern
Martin Hoffmann, Medizinische Klinik A, Klinikum Ludwigshafen, Ludwigshafen
Tobias A.W. Holderried, Medizinische Klinik und Poliklinik III—Innere Medizin mit den Schwerpunkten Onkologie, Hämatologie, Stammzeltransplantationen, Zell-und Immuntherapien, Klinische Immunologie und Rheumatologie, Universitätsklinikum Bonn, Bonn
Olaf Hopfer, Medizinische Klinik I, Hämatologie/internistische Onkologie/Hämostaseologie, Klinikum Frankfurt (Oder), Frankfurt (Oder)
Snjezana Janjetovic, Klinik für Hämatologie und Stammzelltransplantation, Helios Klinikum Berlin-Buch, Berlin
Maika Klaiber-Hakimi, Klinik für Onkologie, Hämatologie und Palliativmedizin, Marien Hospital Düsseldorf, Düsseldorf
Martine Klausmann, Studienzentrum Aschaffenburg, Aschaffenburg
Stefan Klein, III. Medizinischen Klinik, Universitätsklinikum Mannheim, Mannheim
Wolfgang Knauf, Centrum für Hämatologie und Onkologie, Agaplesion Bethanien Krankenhaus, Frankfurt am Main
Yon-Dschun Ko, Abteilung Hämatologie, Internistische Onkologie, Johanniter-Krankenhaus Bonn Philippe Kostrewa, Klinisches Studienzentrum GmbH, Klinikum Fulda, Fulda
Doris Maria Kraemer, Klinik für Hämatologie und Onkologie, Katholisches Krankenhaus Hagen, Hagen
Stephan Kremers, Gemeinschaftspraxis für Hämatologie und Onkologie, Lebach
Martin Kropff, MVZ Hämatologie und Onkologie, Bluttransfusionswesen, Klinikum Osnabrück
Paul La Rosée, Klinik für Innere Medizin II (Onkologie, Hämatologie, Immunologie, Infektiologie und Palliativmedizin), Schwarzwald-Baar Klinikum Villingen-Schwenningen, Villingen-Schwenningen
Rolf Mahlberg, Innere Medizin 1 (Hämato-Onkologie, Infektiologie, Gastroenterologie), Klinikum Mutterhaus der Borromäerinnen, Trier
Christoph Mann, Hämatologie, Onkologie und Immunologie, Universitätsklinikum Marburg, Marburg
Uwe Martens, Klinik für Innere Medizin III (Hämatologie, Onkologie und Palliativmedizin), SLK- Kliniken Heilbronn, Heilbronn
Ivana von Metzler, Klinik für Innere Medizin, Hämatologie und Onkologie, Universitätsklinikum Frankfurt, Frankfurt am Main
Christian Michel, III. Medizinische Klinik und Poliklinik—Hämatologie, und Medizinische Onkologie und Pneumologie, Universitätsklinikum Mainz, Mainz
Martin Müller, Klinik für Hämatologie, Onkologie und Immunologie, KRH Klinikum Siloah, Hannover
Wolfram Pönisch, Medizinische Klinik und Poliklinik I—Hämatologie und Zelltherapie, Internistische Onkologie, Hämostaseologie, Universitätsklinikum Leipzig, Leipzig
Peter Reimer, Klinik für Hämatologie, Onkologie und Stammzelltransplantation, KEM | Evang. Kliniken Essen-Mitte, Essen-Werden, Essen
Claudia Riechel, Klinik für Hämatologie, Onkologie und Palliativmedizin, Klinikum Stuttgart, Stuttgart
Armin Riecke, Hämatologie und internistische Onkologie, Bundeswehrkrankenhaus Ulm, Ulm
Hendrik Riesenberg, Onkologische Schwerpunktpraxis Bielefeld, Bielefeld
Mark Ringhoffer, Städtisches Klinikum Karslruhe gGmbH, Karlsruhe
Mathias Rummel, Medizinische Klinik IV, Schwerpunktabteilung für Hämatologie, Universitätsklinikum Gießen, Gießen
Volker Runde, Klinik für Innere Medizin—Abteilung Hämatologie und internistische Onkologie, Katholisches Karl-Leisner-Klinikum, Wilhelm-Anton-Hospital, Goch
Hans Jürgen Salwender, Abteilung für Hämatologie, Onkologie und Stammzelltransplantation, Asklepios Klinik St Georg, Hamburg sowie 2. Medizinische Abteilung, Onkologie mit Sektion Hämatologie, Asklepios Klinik Altona, Hamburg
Markus Schaich, Klinik für Hämatologie, Onkologie und Palliativmedizin, Rems-Murr-Kliniken, Winnenden
Christof Scheid, Klinik I für Innere Medizin—Onkologie, Hämatologie, Klinische Infektiologie, Klinische Immunologie, Hämostaseologie, Internistische Intensivmedizin, Universitätsklinikum Köln, Köln
Martin Schmidt-Hieber, 2. Medizinische Klinik (Onkologische Tagesklinik), Carl-Thiem-Klinikum Cottbus, Cottbus
Daniel Schöndube, Klinik für Hämatologie, Onkologie und Palliativmedizin, Helios Klinikum Bad Saarow, Bad Saarow
Roland Schroers, Klinik für Hämatologie, Onkologie, Stammzelltransplantation und Zelltherapie, Universitätsklinikum, Knappschaftskrankenhaus Bochum, Bochum
Hans-Joachim Schütte, Überörtliche Berufsausübungsgemeinschaft Prof. Dr. med. Hans-Joachim Schütte
Evgenii Shumilov, Medizinische Klinik A—Hämatologie, Hämostaseologie, Onkologie und Pneumologie, Universitätsklinikum Münster, Münster
Peter Staib, Klinik für Hämatologie, Onkologie und Palliativmedizin, St Antonius-Hospital, Eschweiler
Dirk Strumberg, Medizinische Klinik III—Hämatologie/Onkologie, St Elisabeth Gruppe—Katholische Kliniken Rhein-Ruhr, Marien Hospital Herne, Herne
Hans-Joachim Tischler, Abt. Hämatologie, Onkologie, Gerinnungsstörungen und Palliativmedizin, Johannes Wesling Klinikum der Mühlenkreiskliniken, Minden
Karolin Trautmann-Grill, Medizinische Klinik und Poliklinik I—Hämatologie, Zelltherapie und Medizinische Onkologie, Universitätsklinikum Dresden an der TU Dresden
Walter Verbeek, Zentrum für Ambulante Hämatologie und Onkologie (ZAHO), Standort Bonn
Rudolf Weide, Institut für Versorgungsforschung in der Onkologie, Koblenz
Eckhart Weidmann, Institut für Klinisch-Onkologische Forschung am Krankenhaus Nordwest, Frankfurt am Main
Katja C. Weisel, II. Medizinische Klinik und Poliklinik, Universitätsklinikum Hamburg-Eppendorf, Hamburg
Maike de Wit, Klinik für Innere Medizin—Hämatologie, Onkologie und Palliativmedizin, Vivantes Klinikum Neukölln, Berlin
The following local investigators/centers (in alphabetical order) participated in the GMMG-HD7 trial as associated trial sites:
Christiane Bernhardt, Gefos Dortmund mbH, Dortmund
Thomas Fietz, Onkologische Schwerpunktpraxis, Singen
Jochen Grassinger, MVZ Onkologie Klinikum St. Elisabeth Straubing GmbH, Straubing
Peter Immenschuh, Klinikum Hanau, Medizinische Klinik III, Hanau
Georg Köchling, Onkologie Schwarzwald-Alb, Villingen-Schwenningen
Michael Koenigsmann, Mediprojekt GbR, Hannover
Michael Neise, MVZ West GmbH, Hämatologie und Onkologie, Krefeld
Holger Nückel, Hämatologisch-onkologische Schwerpunktpraxis, Bochum
Maria Procaccianti, Onkologische Schwerpunktpraxis, Karlsruhe
Stefan Schmitt, Klinikum Mittelbaden gGmbH inkl. MVZ Onkologie gGmbH, Baden-Baden
Andreas Schwarzer, Praxis für Hämatologie, Onkologie und Palliativmedizin, Leipzip
Heike Steiniger, Überörtliche Berufsausübungsgemeinschaft Dr. med. Heike Steiniger, Axel Schneider, Oberhausen
Barbara Tschechne, MVZ Onko Medical GmbH Neustadt, Neustadt am Rübenberge
Michael Varvenne, Onkologische Schwerpunktpraxis Celle, Celle
Bettina Whitlock, Zollernalb-Klinikum/Onkologische Tagesklinik, Balingen
Matthias Zaiß, PIO—Praxis für Interdisziplinäre Onkologie und Hämatologie, Freiburg
Carsten Ziske, Überörtliche Berufsausübungsgesellschaft Dr. H. Forstbauer/PD Dr. C. Ziske/Dr. R. Reihs/Dr. E. Rodermann/A. Thiel, Troisdorf
ACKNOWLEDGMENT
The authors thank all participating patients and their families/caregivers; all GMMG members and employees who helped to initiate, conduct, and analyze the study; the Coordination Centre for Clinical Trials (KKS), Heidelberg and Leipzig (Germany), and participating employees for monitoring the trial; as well as all participating centers, investigators, and study nurses. The Heidelberg University Hospital (Heidelberg, Germany) was the sponsor of the study. The authors thank all members and employees at the Department of Internal Medicine V, Hematology, Oncology and Rheumatology, Heidelberg University Hospital (Heidelberg, Germany), involved in any aspect of the study. The authors thank the three members of the Data and Safety Monitoring Board, Thierry Facon (France), Tom Martin (United States), and Lutz Edler (Germany), for their continued review during the trial. The investigational medicinal products isatuximab and lenalidomide were provided by Sanofi and Bristol Myers Squibb/Celgene, respectively. Sanofi funded this collaborative trial. E.K.M., H.G., and U.B. had unrestricted access to all trial data and had final responsibility for the decision to submit for publication. Coordination of the development of this manuscript, facilitation of author discussion, and critical review were provided by Wendell Lamar Blackwell, PhD, Associate Sci Comms Director, at Sanofi. Editorial support was provided by Kirsty Lee, MPH, and Camile Semighini Grubor, PhD, both of Envision Pharma Group, contracted by Sanofi, and Mareike Hampel, contracted by the GMMG (Heidelberg University Hospital, Heidelberg, Germany). Sanofi and Bristol Myers Squibb/Celgene reviewed the manuscript.
The GMMG-HD7 investigators are listed in the Appendix (online only).
REFERENCES
1.
Channar A, Naqvi SAA, Khan MA, et al.: Quadruplet therapy for transplant-eligible newly diagnosed multiple myeloma (TENDMM): A systematic review and meta-analysis. J Clin Oncol 42, 2024 (suppl 16; abstr e19534)2.
Facon T, Dimopoulos MA, Leleu XP, et al.: Isatuximab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 391:1597-1609, 20243.
US Food and Drug Administration: FDA approves Isatuximab-Irfc with bortezomib, lenalidomide, and dexamethasone for newly diagnosed multiple myeloma. 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-isatuximab-irfc-bortezomib-lenalidomide-and-dexamethasone-newly-diagnosed-multiple4.
Goldschmidt H, Mai EK, Bertsch U, et al.: Addition of isatuximab to lenalidomide, bortezomib, and dexamethasone as induction therapy for newly diagnosed, transplantation-eligible patients with multiple myeloma (GMMG-HD7): Part 1 of an open-label, multicentre, randomised, active-controlled, phase 3 trial. Lancet Haematol 9:e810-e821, 20225.
Rajkumar SV, Dimopoulos MA, Palumbo A, et al.: International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15:e538-e548, 20146.
Kidwell KM, Wahed AS: Weighted log-rank statistic to compare shared-path adaptive treatment strategies. Biostatistics 14:299-312, 20137.
Guo X, Tsiatis A: A weighted risk set estimator for survival distributions in two-stage randomization designs with censored survival data. Int J Biostatistics 1:1-15, 20058.
Cote J, LeBlanc R, Mian H, et al.: Real-world results of autologous stem cell transplantation in newly diagnosed multiple myeloma: A report from the Canadian Myeloma Research Group database. Blood Cancer J 13:137, 20239.
Richter J, Pan D, Salinardi T, et al.: Real-world multiple myeloma front-line treatment and outcomes by transplant in the United States. EJHaem 4:984-994, 202310.
Paiva B, van Dongen JJ, Orfao A: New criteria for response assessment: Role of minimal residual disease in multiple myeloma. Blood 125:3059-3068, 201511.
Munshi NC, Avet-Loiseau H, Rawstron AC, et al.: Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: A meta-analysis. JAMA Oncol 3:28-35, 201712.
Sonneveld P, Dimopoulos MA, Boccadoro M, et al.: Daratumumab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 390:301-313, 202413.
US Food and Drug Administration: FDA approves daratumumab and hyaluronidase-fihj with bortezomib, lenalidomide, and dexamethasone for multiple myeloma. 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-daratumumab-and-hyaluronidase-fihj-bortezomib-lenalidomide-and-dexamethasone-multiple14.
Tariq SM, Abdallah AO, Goodman A, et al.: The landscape of currently enrolling maintenance trials in multiple myeloma. Clin Hematol Int 5:170-176, 202315.
Johnson & Johnson: DARZALEX® (daratumumab)-SC based quadruplet regimen approved by the European Commission for patients with newly diagnosed multiple myeloma who are transplant-eligible. 2024. https://jnj.com/media-center/press-releases/darzalex-daratumumab-sc-based-quadruplet-regimen-approved-by-the-european-commission-for-patients-with-newly-diagnosed-multiple-myeloma-who-are-transplant-eligible#:~:text=BEERSE%2C%20BELGIUM%20(23%20October%202024,and%20dexamethasone%20(daratumumab-VRd)16.
Gay F, Roeloffzen W, Dimopoulos MA, et al.: Results of the phase III randomized Iskia trial: Isatuximab-Carfilzomib-Lenalidomide-Dexamethasone vs carfilzomib-lenalidomide-dexamethasone as pre-transplant induction and post-transplant consolidation in newly diagnosed multiple myeloma patients. Blood 142:4, 202317.
Leypoldt LB, Tichy D, Besemer B, et al.: Isatuximab, carfilzomib, lenalidomide, and dexamethasone for the treatment of high-risk newly diagnosed multiple myeloma. J Clin Oncol 42:26-37, 202418.
O'Donnell E, Mo C, Yee AJ, et al.: Isatuximab, carfilzomib, lenalidomide, and dexamethasone in patients with newly diagnosed, transplantation-eligible multiple myeloma (SKylaRk): A single-arm, phase 2 trial. Lancet Haematol 11:e415-e424, 2024
