INTRODUCTION
DB-020 (sodium thiosulfate pentahydrate) is being developed for intratympanic (IT) injection to protect hearing in patients receiving cisplatin-based chemotherapy without interfering with cisplatin's anticancer activity, by chelating and inactivating cisplatin locally in the cochlea. IT administration enables the release of otoprotectants into the middle ear, where the drug can cross the round window membrane to enter the inner ear and cochlea. Cisplatin is a cornerstone chemotherapeutic agent that is widely used for a broad range of solid tumors. Cisplatin administration is associated with severe dose-limiting toxicities, including the risk of ototoxicity from sensory hair cell loss in the cochlea of the inner ear. The incidence of cisplatin-induced ototoxicity in adult patients with cancer has been estimated to be 36%. Previous efforts to reduce cisplatin ototoxicity with IT injections failed to demonstrate benefit. More recently, protective effects of IT steroids were demonstrated in preclinical studies. Given cisplatin's foundational role in chemotherapy regimens, a reduction in cisplatin ototoxicity is needed to protect patients with cancer from permanent hearing loss.,
CONTEXT
Key Objective
This study evaluated the safety, tolerability, and efficacy of intratympanic (IT) DB-020 administered to adult patients with cancer receiving cisplatin.
Knowledge Generated
DB-020 IT injections resulted in significant reductions in cisplatin ototoxicity with no apparent impact on plasma concentrations of free cisplatin. No persistent tympanic perforations and no serious adverse events related to ear and labyrinth disorders were reported. These findings support DB-020 25% as an appropriate dose for future clinical trials.
Relevance (R.G. Maki)
While execution on such an injection plan beyond this trial will require training, this well-designed trial confirmed that hearing can be protected through use of transtympanic thiosulfate injections. These data appear to demonstrate a material and genuine advance to help protect organ function in patients who receive high-dose cisplatin with curative intent.*
*Relevance section written by JCO Associate Editor Robert G. Maki, MD, PhD, FACP, FASCO.
There are currently no approved drugs to protect against cisplatin ototoxicity in adults. Intravenously (IV) administered thiosulfate (Pedmark, Fennec Pharmaceuticals, Durham, NC) has received approval from the US Food and Drug Administration for use in pediatric patients to reduce the risk of cisplatin-associated ototoxicity. Thiosulfate requires a high dose (16-20 g/m2) administered IV, whereas DB-020 is delivered by IT injection. IT injection offers the potential to reduce ototoxicity without diminishing the intended antitumor effects provided by systemic cisplatin exposure.
The safety, tolerability, and systemic pharmacokinetics (PK) of DB-020 were previously evaluated in a phase I study in healthy volunteers (Study DB-020-001) in which no serious treatment-emergent adverse events (TEAEs) or discontinuations occurred due to adverse events (AEs). To our knowledge, the current study (DB-020-002) was the first evaluation of DB-020 in chemo-naïve patients with cancer receiving cisplatin. The primary objective was to evaluate safety and tolerability through the incidence of AEs; vital signs; electrocardiogram results; clinical laboratory assessments; otoscopy examination results; and follow-up disease status. Secondary objectives included evaluation of efficacy in DB-020–treated compared with placebo-treated ears using the incidence of American Speech-Language-Hearing Association (ASHA)–defined ototoxicity, followed by other changes in pure tone audiometry, Speech Intelligibility Index (SII), distortion product otoacoustic emissions (DPOAEs), Words in Noise (WIN) test, tympanometry, and Hearing Handicap Inventory for Adults (HHIA; Data Supplement, Appendix S1, online only). Plasma levels of DB-020 and PK of free (unbound) cisplatin were also assessed.
METHODS
Study Design and Eligibility
This was a phase Ib, randomized, double-blind, placebo-controlled clinical trial conducted at five centers in Australia and the United States. Patients were over 18 years of age and were scheduled to receive a total cumulative cisplatin dose of ≥280 mg/m2 over at least three cycles (once every 21 days or once every 28 days) for the treatment of cancer of any type. All patients had an Eastern Cooperative Oncology Group performance status of 0 to 2, and an anticipated survival of >1 year. Concomitant use of other chemotherapy and radiation was permitted except for investigational agents and/or radiation >35 Gy involving the cochlear region. Patients with hearing loss of >45 dB averaged over 6 and 8 kHz in either ear were excluded. Additional inclusion and exclusion criteria are provided in the Data Supplement (Appendices S2 and S3).
The study complied with the Declaration of Helsinki, International Council for Harmonisation Good Clinical Practice, local laws, and regulatory requirements. Independent ethics committees or institutional review boards approved the protocol. Patients provided written informed consent.
Study Procedures and Treatment
Eligible patients were randomly assigned to receive double-blinded, bilateral IT injections with DB-020 in one ear and placebo (a solution of sodium hyaluronate in 0.9% sodium chloride) in the other, allowing each patient to serve as their own control. The randomization scheme included a block size of 4. Randomization assignments included DB-020 12% w/v (0.5 M) in the right ear, DB-020 12% w/v (0.5 M) in the left ear, DB-020 25% w/v (1 M) in the right ear, and DB-020 25% w/v (1 M) in the left ear, with placebo assigned to the non–DB-020 ear for each patient (Data Supplement, Table S7). The same ear assignments (left or right) for DB-020 and placebo were maintained throughout the treatment period. The dose levels of 0.5 and 1.0 M were selected because they were well tolerated in the phase I DB-020-001 study and were supported by results from nonclinical toxicology studies. Additionally, these doses were predicted to achieve efficacious (ie, protective) DB-020 concentrations on the basis of preclinical pharmacology and PK results.
IT injections were administered once every 3 or 4 weeks, according to the cisplatin dosing schedule (entirely in single-day infusion regimens given as close as possible before cisplatin administration). Additional details about the clinical procedure of DB-020 administration are provided in the Data Supplement (Appendix S4).
All AEs were recorded, regardless of their relationship to study drug. Investigators rated AEs on a study-specific severity scale of mild (awareness of sign or symptom, but easily tolerated), moderate (discomfort sufficient to interfere with normal activities), and severe (incapacitating, with inability to perform normal activities); they also assessed AE relationship to the IT injection and/or study drug. Additional safety measures and various efficacy assessments were performed at each clinic visit.
The key time point was the end-of-treatment (EOT) outcome after completion of all chemotherapy, examined with the last observation carried forward (LOCF) approach (Data Supplement, Appendix S5). This was chosen because patients were expected to differ in the total number of cycles of chemotherapy, with toxicity progressing over multiple cycles.
Ototoxicity was defined by ASHA criteria. ASHA defines an ototoxic change if one of the following criteria are met: (1) a ≥20-dB increase in audiometric threshold at any one test frequency, (2) a ≥10-dB increase in threshold at any two adjacent frequencies, or (3) loss of response at three consecutive frequencies where responses were previously obtained. ASHA criteria were chosen because of their broad acceptance and clinical relevance. Severe ototoxicity was defined as a ≥20-dB increase in measured threshold at any two adjacent frequencies.
Approximately 50 patients were planned to be randomly assigned to provide at least 25 patients with total cumulative cisplatin exposure of ≥280 mg/m2 at each dose level with a 70% power to detect a difference in the proportion of ototoxicity between DB-020–treated and placebo-treated ears at a two-sided significance level of 0.05, assuming a 0.60 ototoxicity rate for placebo and a 0.24 ototoxicity rate for DB-020.
An interim analysis was planned to take place when about 20 patients completed both baseline and follow-up audiograms (after ≥1 cisplatin cycle). The interim analysis was done when 19 patients had available data, because a blinded review indicated higher rates of discordance in ototoxicity than expected between ears. The interim analysis was performed by an independent statistician at the contract research organization (CRO). Only select sponsor personnel were unblinded to the results; all other sponsor personnel, the CRO staff, site staff, and patients remained blinded until study completion.
Recruitment was stopped after the interim analysis because study objectives were met. Three patients who were already in the study completed their participation, leading to 22 patients enrolled overall.
Pure tone threshold assessments were carried out at frequencies ranging from 250 to 16,000 Hz for air conduction audiometry and 250 to 4,000 Hz for bone conduction audiometry.
Statistics
For measurement of safety outcomes, all patients who received at least one dose of DB-020 were considered evaluable. For efficacy outcomes, patients were defined as evaluable if they had at least one baseline and one follow-up hearing assessment. LOCF ototoxicity values from the DB-020–treated ears were compared with placebo-treated ears using McNemar's test (Data Supplement, Appendix S5). To account for multiplicity, this key efficacy assessment of ototoxicity was tested sequentially, first with the DB-020 25% dose versus placebo, and then with the DB-020 12% dose versus placebo.
Ototoxicity between ears was also examined using the Wilcoxon signed-rank test. Other efficacy assessments were analyzed using mixed model with repeated measures (MMRM) and analysis of covariance (ANCOVA). Specifically, both MMRM and ANCOVA methods were used for air conduction threshold change from baseline, shift results, and SII results; MMRM was used for bone conduction threshold results; and ANCOVA was used for DPOAEs and WIN results (Data Supplement, Appendix S6). All efficacy assessments were summarized using descriptive statistics.
RESULTS
Disposition and Demographics
The completed study included 22 patients enrolled between February 17, 2020, and May 17, 2022.
An overview of patient disposition is shown in Figure 1. All patients received DB-020 within 3 hours of their cisplatin treatment, except for one who received DB-020 almost 4 hours before cisplatin treatment. This patient experienced ototoxicity in their placebo-treated ear and not in the DB-020–treated ear during this cycle. Eleven patients (50.0%) did not complete at least three doses of the study drug. One patient only received one injection of DB-020 in one ear and withdrew before receiving the injection of placebo in the other ear. This patient is included in all summaries because they had one treated ear and one untreated ear. All patients completed the EOT visit. Fourteen patients (63.6%) completed the 1-year follow up. Four patients (18.2%) withdrew from the study before the 1-year follow-up. One death occurred outside the safety reporting period (Data Supplement, Appendix S7).
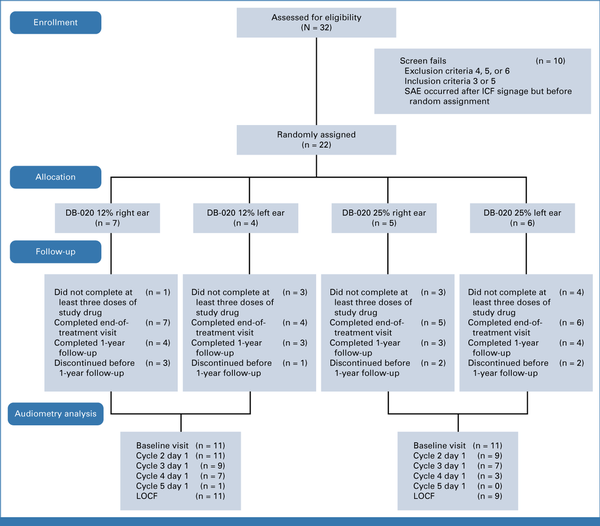
FIG 1.
CONSORT diagram. ICF, informed consent form; LOCF, last observation carried forward; SAE, serious adverse event.
The median patient age was 55.1 years; 86.4% were male. All patients were White. There was consistency in demographics and other baseline characteristics across randomized treatment groups (Table 1).
TABLE 1.
Demographics and Other Baseline Characteristics
| Demographic Characteristic | DB-020 12% Right Ear (n = 7) | DB-020 12% Left Ear (n = 4) | DB-020 25% Right Ear (n = 5) | DB-020 25% Left Ear (n = 6) | Overall (N = 22) |
|---|---|---|---|---|---|
| Age at consent, years | |||||
| Mean (SD) | 56.7 (12.76) | 56.0 (6.78) | 53.4 (6.23) | 54.0 (12.95) | 55.1 (10.12) |
| Median | 62.0 | 53.5 | 54.0 | 53.0 | 54.0 |
| Min, max | 37, 71 | 51, 66 | 46, 62 | 35, 69 | 35, 71 |
| Sex, No. (%) | |||||
| Male | 5 (71.4) | 4 (100) | 5 (100) | 5 (83.3) | 19 (86.4) |
| Female | 2 (28.6) | 0 | 0 | 1 (16.7) | 3 (13.6) |
| Race, No. (%) | |||||
| White | 7 (100) | 4 (100) | 5 (100) | 6 (100) | 22 (100) |
| Ethnicity, No. (%) | |||||
| Not Hispanic or Latino | 6 (85.7) | 3 (75.0) | 5 (100) | 5 (83.3) | 19 (86.4) |
| Unknown | 1 (14.3) | 1 (25.0) | 0 | 1 (16.7) | 3 (13.6) |
| ECOG performance status, No. (%) | |||||
| 0 | 7 (100) | 4 (100) | 5 (100) | 5 (83.3) | 21 (95.5) |
| 1 | 0 | 0 | 0 | 1 (16.7) | 1 (4.5) |
| Neoplasms benign, malignant, and unspecified, No. (%) | 6 (85.7) | 4 (100) | 5 (100) | 6 (100) | 21 (95.5) |
| Tonsil cancer | 3 (42.9) | 2 (50.0) | 4 (80.0) | 3 (50.0) | 12 (54.5) |
| Squamous cell carcinoma of the tongue | 1 (14.3) | 1 (25.0) | 1 (20.0) | 1 (16.7) | 4 (18.2) |
| Oropharyngeal squamous cell carcinoma | 0 | 1 (25.0) | 0 | 1 (16.7) | 2 (9.1) |
| Squamous cell carcinoma | 1 (14.3) | 0 | 0 | 1 (16.7) | 2 (9.1) |
| Basal cell carcinoma | 0 | 0 | 0 | 1 (16.7) | 1 (4.5) |
| Lung neoplasm malignant | 1 (14.3) | 0 | 0 | 0 | 1 (4.5) |
| Malignant melanoma | 0 | 0 | 0 | 1 (16.7) | 1 (4.5) |
| Oral cavity cancer metastatic | 0 | 0 | 0 | 1 (16.7) | 1 (4.5) |
| Oropharyngeal cancer | 1 (14.3) | 0 | 0 | 0 | 1 (4.5) |
Safety
All patients experienced at least one TEAE. The most common TEAEs by preferred term were ear pain (n = 18, 81.8%), nausea (n = 16, 72.7%), constipation (n = 13, 59.1%), tinnitus (n = 11, 50.0%), and dysgeusia (n = 9, 40.9%). See the Data Supplement (Table S1) for a full description.
For all occurrences of ear pain, the date of onset and the date of resolution were the same, with the exception of one patient. This patient reported mild pain in the left ear (assigned to placebo), with onset 2 weeks after IT injection and no resolution date. For the other patients, ear pain related to injections lasted a median duration of 10 minutes after injection (range, 2-65 minutes).
AE reports for ear pain and tinnitus (except for one case of tinnitus, which was included below as affecting both ears) indicated which ear was involved, allowing incidences in placebo-treated ears to be determined. The incidence of ear pain was 13.6% in placebo-treated ears and 77.3% in DB-020–treated ears. The incidence of tinnitus was greater in placebo-treated ears (50.0%) than in DB-020–treated ears (13.6%; Table 2).
TABLE 2.
Ear Pain and Tinnitus Treatment-Emergent Adverse Events by Treatment
| Preferred Term | Placebo (N = 22), No. (%) | DB-020 12% (n = 11), No. (%) | DB-020 25% (n = 11), No. (%) | DB-020 (N = 22), No. (%) |
|---|---|---|---|---|
| Ear pain | 3 (14) | 8 (73) | 9 (82) | 17 (77) |
| Tinnitus | 11 (50) | 0 (0) | 3 (27) | 3 (14) |
Eight patients (36.4%) experienced a total of 14 serious AEs (SAEs), all of which were considered not related to study drug or IT injection (Data Supplement, Table S2). The most frequent SAE was acute kidney injury (two patients [9.1%]); no other SAEs occurred in more than one patient. There were no clinically significant findings for clinical laboratory assessments or vital signs. Otoscopy results showed no tympanic membrane perforations. No AEs led to death during this study.
Exposure
The mean total cumulative cisplatin dose for all patients was 255 mg/m2, and the mean number of cisplatin cycles was 2.3. DB-020 exposure by assessment time point is summarized in the Data Supplement (Table S3). On cycle 1 day 1, all patients (22/22) were exposed to DB-020; this decreased to 72.7% (16/22) on cycle 2 day 1 and 50.0% (11/22) on cycle 3 day 1, and only one patient received a fourth DB-020 treatment (4.5% [1/22] on cycle 4 day 1; Data Supplement, Table S3). The number of patients in the four treatment groups was similar at seven, four, five, and six patients for DB-020 12% in the right ear, DB-020 in the 12% left ear, DB-020 in the 25% right ear, and DB-020 in the 25% left ear, respectively. The progressive reduction in patient numbers at each cycle was due to patients ceasing cisplatin treatment rather than withdrawing from DB-020 treatment, and it reflects the challenges of tolerating cisplatin therapy. Two patients withdrew because of ear pain after receiving one dose of DB-020 25%.
Pharmacokinetics
DB-020 did not affect endogenous plasma concentrations of thiosulfate. Before DB-020 and placebo administration, mean endogenous thiosulfate plasma concentrations were 400 and 570 ng/mL in patients treated with DB-020 12% and DB-020 25%, respectively. Fifteen minutes before the cisplatin dose, mean thiosulfate plasma concentrations were 393 and 530 ng/mL in patients treated with DB-020 12% and DB-020 25%, respectively.
Mean unbound cisplatin concentrations were 1,928 and 2,061 ng/mL with DB-020 12% and DB-020 25% treatment at the end of infusion on cycle 1 day 1, respectively.
Ototoxicity
Across various time points, the highest incidence of ototoxicity was in placebo-treated ears, with an intermediate incidence in ears treated with DB-020 12.5% and the lowest incidence in ears treated with DB-020 25%. ASHA ototoxicity rates at LOCF for the 250-8,000 Hz frequency range showed a dose response, with 85.0%, 54.5%, and 22.2% ototoxicity incidence in ears treated with placebo, DB-020 12%, and DB-020 25%, respectively (P = .0454 for DB-020 12% v placebo and P = .0253 for DB-020 25% v placebo using McNemar's test; Fig 2). A reduction in ototoxicity was observed after single treatment with DB-020 at the beginning of cycle 2, with an ototoxicity rate of 70.0% for placebo-treated ears versus 15.0% for DB-020–treated ears (ASHA criteria, 250-8,000 Hz; Data Supplement, Tables S4 and S5). Significantly lower incidences of ototoxicity and severe ototoxicity were seen with DB-020 in the frequency ranges of 250-8,000 Hz and 9,000-16,000 Hz, respectively (Table 3). Between-ear ototoxicity using Wilcox signed-rank test showed a similar trend (Data Supplement, Table S6). The average threshold shift averaged over 4,000-8,000 Hz was greater in placebo-treated ears (least-squares mean of 30.22 dB) than in ears treated with DB020 12% (10.24 dB) or DB-020 25% (5.23 dB). Mean audiogram plots by treatment show that the change in hearing levels from baseline to LOCF was greater for patients treated with placebo (Fig 3).
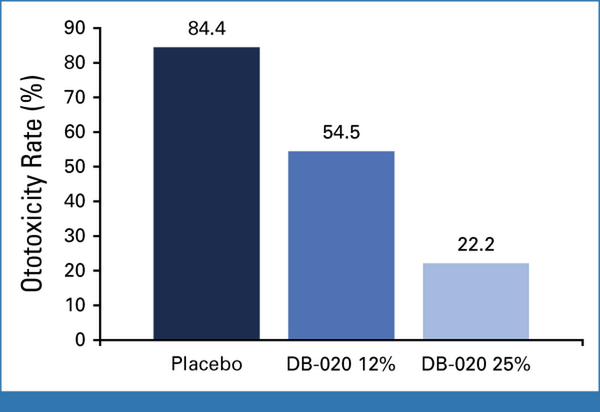
FIG 2.
Ototoxicity at LOCF: ASHA criteria applied to the 250-8,000 Hz frequency range. P = .0454 for DB-020 12% versus placebo and P = .0253 for DB-020 25% versus placebo using McNemar's test. ASHA, American Speech-Language-Hearing Association; LOCF, last observation carried forward.
TABLE 3.
Summary of Ototoxicity Assessments for Combined DB-020 Treatment Versus Placebo at End of Treatment Visit (LOCF)
| Assessment | Placebo Ears (N = 20) | DB-020 Ears (N = 20) | P DB-020 v Placebo |
|---|---|---|---|
| Ototoxicity (250-8,000 Hz), % | 85 | 40 | .0027 |
| Ototoxicity (9,000-16,000 Hz), % | 90 | 60 | .0143 |
| Severe ototoxicity (250-8,000 Hz), % | 70 | 15 | .0009 |
| Severe ototoxicity (9,000-16,000 Hz), % | 80 | 35 | .0027 |
| Average threshold shift (4,000-8,000 Hz), LS mean dB | 30.22 | 7.99 | <.0001 |
| Average threshold shift (9,000-16,000 Hz), LS mean dB | 21.38 | 9.19 | .0022 |
| Speech intelligibility index, LS mean | –0.15 | –0.03 | .0001 |
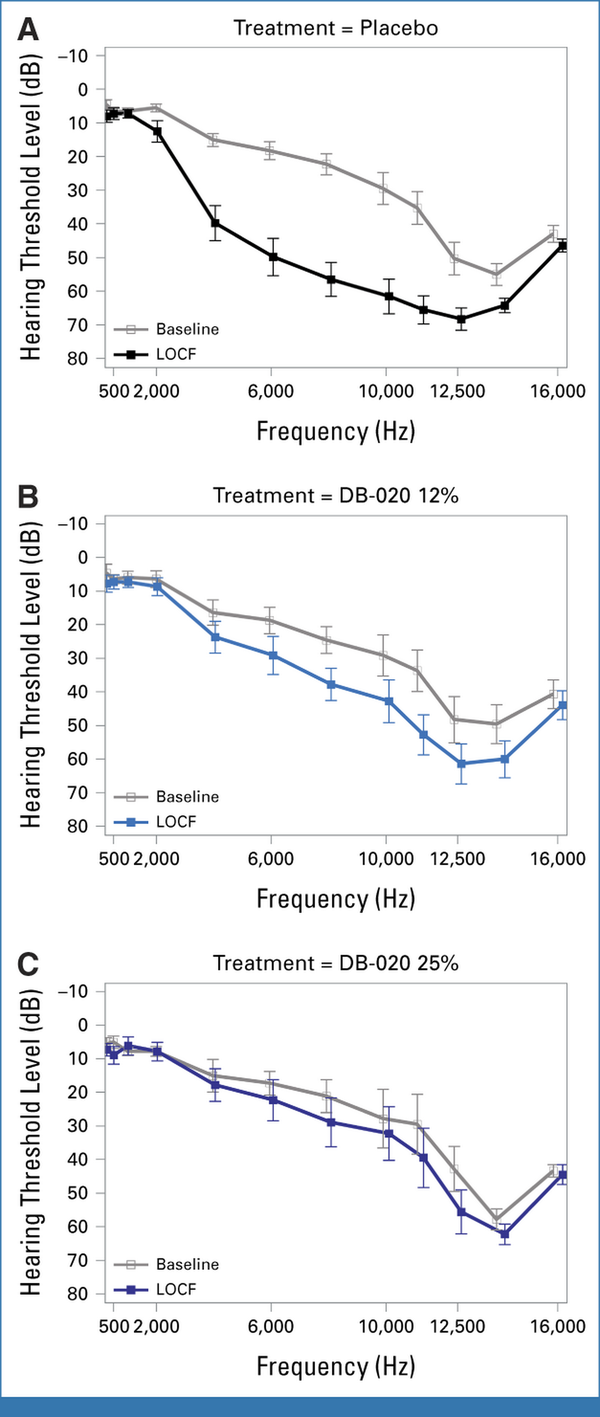
FIG 3.
Mean audiogram plot for (A) placebo, (B) DB-020 12%, and (C) DB-020 25%. Mean hearing levels at baseline and LOCF for frequencies of 250-16,000 Hz. LOCF, last observation carried forward.
The reduction in ototoxicity in DB-020–treated ears was also evident in the results from additional average threshold shift in air conduction and SII results. These effects were dose-responsive (Data Supplement, Tables S7-S9).
Results from DPOAEs, WIN, tympanometry, bone conduction threshold shifts, and HHIA assessments support the efficacy of DB-020 treatment for patients receiving high-dose cisplatin chemotherapy. A shift from DPOAEs being present to absent was generally more common in placebo-treated ears than in DB-020–treated ears, suggesting that DB-020 was effective in preserving hearing (Data Supplement, Table S10). Changes from baseline in WIN threshold results were greatest on placebo, lower on DB-020 12.5%, and lowest on DB-020 25% at 2.83, 2.22, and –0.28 dB, respectively (Data Supplement, Table S11). Tympanometry results indicated little-to-no middle ear function change after the IT injection. Only one ear each for placebo, DB-020 12.5%, and DB-020 25% shifted from category A (normal middle ear function) to C (eustachian tube dysfunction, or early or resolving middle ear infection; Data Supplement, Table S12). Bone conduction threshold shifts mirrored the air conduction changes (Data Supplement, Table S13). HHIA score results showed little change (Data Supplement, Table S14).
DISCUSSION
Cisplatin ototoxicity was very common in placebo-treated ears and often severe, indicating a significant unmet need because of cisplatin-induced hearing loss. Ototoxicity was evident with the first cisplatin cycle and progressed over time, indicating the importance of treating the ear before every cisplatin administration.
Ototoxicity was reduced in DB-020–treated ears compared with placebo-treated ears. The difference was shown by a clear separation between ears for both the 250-8,000 Hz and 9,000-16,000 Hz frequency ranges at LOCF, respectively. There was clear evidence of a dose response.
Even after cycle 1, patterns of ototoxicity were similar to those at EOT. The reduction in ototoxicity with DB-020 treatment was clinically meaningful, determined according to relevant thresholds prospectively defined by the ASHA, and a difference was evident in speech reception on the WIN test. The consistency across ototoxicity assessments, the dose-dependent responses, and the large effect size provide robust evidence that DB-020 IT treatment reduced cisplatin ototoxicity. In line with results from recent studies, these encouraging results provide further insight into cisplatin-related ototoxicity and suggest that DB-020 could be an alternative treatment option for patients receiving cisplatin-based chemotherapy.
In all but one case, the ear(s) affected by tinnitus were specified, allowing a comparison of placebo and DB-020. This showed that tinnitus was more common in placebo-treated ears than in DB-020–treated ears, suggesting tinnitus was related to ototoxicity.
HHIA assessments showed that a new handicap affected only one patient. Because this is a whole-person measure rather than an individual ear assessment, it is possible that preservation of hearing in only one ear with DB-020 avoided the development of a worsening handicap.
Administration of thiosulfate in the clinical setting requires close coordination between the otolaryngologist and the oncology team. In this study, administration logistics were feasible at multiple study sites, and all IT injections except one were administered within 3 hours of cisplatin chemotherapy.
A strength of this study was that each patient was their own control with one ear treated with DB-020 and the other with placebo. This enabled a clear treatment effect to become evident with a small sample size. Multiple assessments per patient and analyses across different frequencies also contributed to the robustness of the results (Data Supplement, Tables S4 and S6-S9). However, this design limited the extent to which some efficacy, particularly for the HHIA assessments, and safety data could be interpreted, as no patients received placebo in both ears.
Safety results indicated that DB-020 IT injections were well tolerated. The 14 SAEs were all determined to be unrelated to study drug, with nine occurring in DB-020 12%-treated patients and five in DB-020 25%-treated patients. Although ear pain was the most common TEAE related to DB-020 and IT injection, leading to the withdrawal of two patients, ear pain associated with IT injection resolved quickly (within approximately 10 minutes post injection). Overall, the safety profile was generally acceptable to patients who are otherwise faced with a very-high incidence of permanent hearing loss and risk of tinnitus.
Thiosulfate is a well-known and relatively safe treatment, and the observed safety profile in this study was entirely consistent with the safety profile of cisplatin and radiation. Although previous studies have shown thiosulfate to provide moderate hearing protection, the systemic administration methods that were used required a high intravenous thiosulfate dose (16 g/m2 or 20 g/m2), resulting in high systemic levels of thiosulfate., In this study, fifteen minutes before cisplatin administration, plasma concentrations of thiosulfate were below those that would be expected to affect cisplatin antitumor efficacy on the basis of nonclinical in vitro tumor cell antiproliferation studies. These concentrations (≤530 ng/mL) were significantly lower than concentrations seen with systemic administration (approximately 2 million ng/mL). Additionally, free cisplatin levels in this study were consistent with values expected in the absence of a cisplatin-chelating agent,, suggesting that DB-020 had no systemic impact on free cisplatin plasma concentrations. These results highlight the advantages of IT sodium thiosulfate injection, which only chelates cisplatin in the ear, leaving cisplatin free in other tissues for systemic antitumor effects.
In conclusion, overall, this clinical trial with DB-020 IT injections showed clear and meaningful reductions in cisplatin ototoxicity, low plasma concentration of thiosulfate, no apparent impact on plasma concentrations of free cisplatin, and a safety profile that supports continued development of the highest dose, DB-020 25%.
See accompanying Editorial, p. 2139
PRIOR PRESENTATION
Presented in part at ASCO 2023, Chicago, IL, June 2-6, 2023; and in full at ASCO 2024, Chicago, IL, May 31-June 4, 2024.
SUPPORT
Supported by Decibel Therapeutics Inc.
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.24.00905.
AUTHOR CONTRIBUTIONS
Conception and design: Benedict J. Panizza, Pablo Lapuerta, John Lee, Shane Raines, Heather M Wolff, John Keilty, Danny Rischin
Financial support: John Lee
Provision of study materials or patients: Benedict J. Panizza, Christopher David Hart, Chandra Sai Diwakarla, Catherine Barnett, Pablo Lapuerta, John Lee, Sandro V. Porceddu, Margaret McGrath, Nagashree Seetharamu, Danny Rischin
Collection and assembly of data: Benedict J. Panizza, Stephen John O'Leary, Christopher David Hart, Catherine Barnett, Pablo Lapuerta, Shane Raines, Tera Quigley, Heather M. Wolff, John Keilty, Rahul Ladwa, Sandro V. Porceddu, Margaret McGrath, Nagashree Seetharamu, Danny Rischin
Data analysis and interpretation: Benedict J. Panizza, Stephen John O'Leary, Chandra Sai Diwakarla, Catherine Barnett, Pablo Lapuerta, John Lee, Shane Raines, Tera Quigley, Heather M. Wolff, John Keilty, Rahul Ladwa, Sandro V. Porceddu, Nagashree Seetharamu, Tsien Fua, Danny Rischin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase Ib Clinical Trial of DB-020 Intratympanic Injections to Reduce High-Dose Cisplatin Ototoxicity
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Benedict J. Panizza
Stock and Other Ownership Interests: Cochlear Ltd, Ramsay Health Care, Sonic Healthcare
Speakers' Bureau: Sanofi-Aventis Australia Pty Ltd
Research Funding: Decibel Therapeutics (Inst), QBiotics (Inst)
Stephen O'Leary
Honoraria: Cochlear Ltd
Speakers' Bureau: Cochlear Ltd, Sensorion
Research Funding: Cochlear Ltd (Inst)
Patents, Royalties, Other Intellectual Property: Named inventor on patents arising from my work at the University of Melbourne arising from research grants to the Institution from Cochlear. None are delivering royalties (Inst)
Chandra Sai Diwakarla
Travel, Accommodations, Expenses: Novartis
Pablo Lapuerta
Employment: 4M Therapeutics Inc (I)
Leadership: 4M Therapeutics Inc
Stock and Other Ownership Interests: 4M Therapeutics Inc, 4M Therapeutics Inc (I), Lexicon, Sarepta Therapeutics, Pelagos, Youngene Therapeutics, EncuraGen
Consulting or Advisory Role: Pelagos, EncuraGen, Youngene Therapeutics, Regeneron, Decibel Therapeutics
Travel, Accommodations, Expenses: Regeneron, Decibel Therapeutics, Youngene Therapeutics
John Lee
Employment: Decibel Therapeutics, Regeneron
Leadership: Decibel Therapeutics
Stock and Other Ownership Interests: Decibel Therapeutics
Consulting or Advisory Role: Source Bio, Matrisome Bio
Research Funding: Decibel Therapeutics, Regeneron
Patents, Royalties, Other Intellectual Property: Patents from work at Decibel Therapeutics associated with work on delivery, formulation, and programs, including DB-020
Travel, Accommodations, Expenses: Decibel Therapeutics, Regeneron
Shane Raines
Consulting or Advisory Role: Decibel Therapeutics (Inst)
Tera Quigley
Employment: Regeneron, Decibel Therapeutics
Stock and Other Ownership Interests: Regeneron, Decibel Therapeutics
Travel, Accommodations, Expenses: Regeneron, Decibel Therapeutics
John Keilty
Stock and Other Ownership Interests: Pfizer, Amgen, Thermo Fisher Scientific, MOMA Therapeutics
Rahul Ladwa
Honoraria: MSD (Inst), Sanofi (Inst)
Consulting or Advisory Role: Merck Sharp & Dohme (Inst), La Roche-Posay (Inst)
Research Funding: MSD
Travel, Accommodations, Expenses: Sanofi, MSD
Sandro V. Porceddu
Stock and Other Ownership Interests: Icon Cancer Care (I)
Consulting or Advisory Role: Regeneron
Patents, Royalties, Other Intellectual Property: UpToDate Royalties as Chapter author
Travel, Accommodations, Expenses: Merck Serono, Merck Sharp & Dohme
Margaret McGrath
Honoraria: Medison
Consulting or Advisory Role: Medison
Speakers' Bureau: La Roche-Posay
Travel, Accommodations, Expenses: MSD
Nagashree Seetharamu
Honoraria: AstraZeneca/MedImmune, Pfizer, Janssen Oncology, Mirati Therapeutics, Blueprint Medicines, Takeda, Boehringer Ingelheim, Amgen, Genentech, Array BioPharma, Jazz Pharmaceuticals
Consulting or Advisory Role: AstraZeneca, Genentech, Takeda, Amgen, Boehringer Ingelheim, Mirati Therapeutics, Blueprint Medicines, Regeneron
Other Relationship: Regeneron
Danny Rischin
Research Funding: Merck (Inst), Regeneron (Inst), Decibel Therapeutics (Inst), ALX Oncology (Inst), AstraZeneca (Inst), Erasca, Inc (Inst), Bicara Therapeutics (Inst)
Uncompensated Relationships: Regeneron, Merck, GlaxoSmithKline, Eisai
No other potential conflicts of interest were reported.
Benedict J. Panizza
Stock and Other Ownership Interests: Cochlear Ltd, Ramsay Health Care, Sonic Healthcare
Speakers' Bureau: Sanofi-Aventis Australia Pty Ltd
Research Funding: Decibel Therapeutics (Inst), QBiotics (Inst)
Stephen O'Leary
Honoraria: Cochlear Ltd
Speakers' Bureau: Cochlear Ltd, Sensorion
Research Funding: Cochlear Ltd (Inst)
Patents, Royalties, Other Intellectual Property: Named inventor on patents arising from my work at the University of Melbourne arising from research grants to the Institution from Cochlear. None are delivering royalties (Inst)
Chandra Sai Diwakarla
Travel, Accommodations, Expenses: Novartis
Pablo Lapuerta
Employment: 4M Therapeutics Inc (I)
Leadership: 4M Therapeutics Inc
Stock and Other Ownership Interests: 4M Therapeutics Inc, 4M Therapeutics Inc (I), Lexicon, Sarepta Therapeutics, Pelagos, Youngene Therapeutics, EncuraGen
Consulting or Advisory Role: Pelagos, EncuraGen, Youngene Therapeutics, Regeneron, Decibel Therapeutics
Travel, Accommodations, Expenses: Regeneron, Decibel Therapeutics, Youngene Therapeutics
John Lee
Employment: Decibel Therapeutics, Regeneron
Leadership: Decibel Therapeutics
Stock and Other Ownership Interests: Decibel Therapeutics
Consulting or Advisory Role: Source Bio, Matrisome Bio
Research Funding: Decibel Therapeutics, Regeneron
Patents, Royalties, Other Intellectual Property: Patents from work at Decibel Therapeutics associated with work on delivery, formulation, and programs, including DB-020
Travel, Accommodations, Expenses: Decibel Therapeutics, Regeneron
Shane Raines
Consulting or Advisory Role: Decibel Therapeutics (Inst)
Tera Quigley
Employment: Regeneron, Decibel Therapeutics
Stock and Other Ownership Interests: Regeneron, Decibel Therapeutics
Travel, Accommodations, Expenses: Regeneron, Decibel Therapeutics
John Keilty
Stock and Other Ownership Interests: Pfizer, Amgen, Thermo Fisher Scientific, MOMA Therapeutics
Rahul Ladwa
Honoraria: MSD (Inst), Sanofi (Inst)
Consulting or Advisory Role: Merck Sharp & Dohme (Inst), La Roche-Posay (Inst)
Research Funding: MSD
Travel, Accommodations, Expenses: Sanofi, MSD
Sandro V. Porceddu
Stock and Other Ownership Interests: Icon Cancer Care (I)
Consulting or Advisory Role: Regeneron
Patents, Royalties, Other Intellectual Property: UpToDate Royalties as Chapter author
Travel, Accommodations, Expenses: Merck Serono, Merck Sharp & Dohme
Margaret McGrath
Honoraria: Medison
Consulting or Advisory Role: Medison
Speakers' Bureau: La Roche-Posay
Travel, Accommodations, Expenses: MSD
Nagashree Seetharamu
Honoraria: AstraZeneca/MedImmune, Pfizer, Janssen Oncology, Mirati Therapeutics, Blueprint Medicines, Takeda, Boehringer Ingelheim, Amgen, Genentech, Array BioPharma, Jazz Pharmaceuticals
Consulting or Advisory Role: AstraZeneca, Genentech, Takeda, Amgen, Boehringer Ingelheim, Mirati Therapeutics, Blueprint Medicines, Regeneron
Other Relationship: Regeneron
Danny Rischin
Research Funding: Merck (Inst), Regeneron (Inst), Decibel Therapeutics (Inst), ALX Oncology (Inst), AstraZeneca (Inst), Erasca, Inc (Inst), Bicara Therapeutics (Inst)
Uncompensated Relationships: Regeneron, Merck, GlaxoSmithKline, Eisai
No other potential conflicts of interest were reported.
ACKNOWLEDGMENT
Editorial support was provided by Alpha (a division of Prime, Knutsford, United Kingdom), supported by Regeneron Pharmaceuticals, Inc.
REFERENCES
1.
Viglietta V, Shi F, Hu QY, et al.: Phase 1 study to evaluate safety, tolerability and pharmacokinetics of a novel intra-tympanic administered thiosulfate to prevent cisplatin-induced hearing loss in cancer patients. Invest New Drugs 38:1463-1471, 20202.
Hamid M, Trune D: Issues, indications, and controversies regarding intratympanic steroid perfusion. Curr Opin Otolaryngol Head Neck Surg 16:434-440, 20083.
Paken J, Govender CD, Pillay M, et al.: Cisplatin-associated ototoxicity: A review for the Health professional. J Toxicol 2016:1809394, 20164.
Karasawa T, Sibrian-Vazquez M, Strongin RM, et al.: Identification of cisplatin-binding proteins using agarose conjugates of platinum compounds. PLoS One 8:e66220, 20135.
Chattaraj A, Syed MP, Low CA, et al.: Cisplatin-induced ototoxicity: A concise review of the burden, prevention, and interception strategies. JCO Oncol Pract 19:278-283, 20236.
Taş BM, Şimşek G, Azman M, et al.: Efficacy of 2 different intratympanic steroid regimen on prevention of cisplatin ototoxicity: An experimental study. Ear Nose Throat J 100:417-422, 20217.
Brock PR, Maibach R, Childs M, et al.: Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med 378:2376-2385, 20188.
Dhillon S: Sodium thiosulfate: Pediatric first approval. Paediatr Drugs 25:239-244, 20239.
Freyer DR, Chen L, Krailo MD, et al.: Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): A multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 18:63-74, 201710.
American Speech-Language-Hearing Association: Audiologic management of individuals receiving cochleotoxic drug therapy [Guidelines]. 1994. www.asha.org/policy11.
Kates JM, Arehart KH: Coherence and the speech intelligibility index. J Acoust Soc Am 117:2224-2237, 200512.
Jedrzejczak WW, Pilka E, Ganc M, et al.: Ultra-high frequency distortion product otoacoustic emissions for detection of hearing loss and tinnitus. Int J Environ Res Public Health 19:2123, 202213.
Wilson RH: Clinical experience with the words-in-noise test on 3430 veterans: Comparisons with pure-tone thresholds and word recognition in quiet. J Am Acad Audiol 22:405-423, 201114.
Lous J, Hansen JG, Felding JU: [Tympanometry]. Ugeskr Laeger 162:1908-1911, 200015.
Newman CW, Weinstein BE, Jacobson GP, et al.: The hearing handicap inventory for adults: Psychometric adequacy and audiometric correlates. Ear Hear 11:430-433, 199016.
Oken MM, Creech RH, Tormey DC, et al.: Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649-655, 198217.
Berglin CE, Pierre PV, Bramer T, et al.: Prevention of cisplatin-induced hearing loss by administration of a thiosulfate-containing gel to the middle ear in a Guinea pig model. Cancer Chemother Pharmacol 68:1547-1556, 201118.
Andersson A, Fagerberg J, Lewensohn R, et al.: Pharmacokinetics of cisplatin and its monohydrated complex in humans. J Pharm Sci 85:824-827, 199619.
Rajkumar P, Mathew BS, Das S, et al.: Cisplatin concentrations in long and short duration infusion: Implications for the optimal time of radiation delivery. J Clin Diagn Res 10:Xc01-xc04, 2016
