INTRODUCTION
This article emanates from a gathering of head and neck medical oncologists, radiation oncologists, and surgeons who are members of the Brazilian Head and Neck Cancer Group (Grupo Brasileiro de Cabeça e Pescoço [GBCP]) and the Latin America Cooperative Oncology Group—Head and Neck (LACOG-HN). The assembly focused on clinical research in Head and Neck Oncology, with Professor Hisham Mehanna (Birmingham, UK) participating in the deliberations as an invited international expert. The overarching goal of the meeting was to exchange insights, foster collaboration, and stimulate clinical and translational research. Specifically, we aimed to discuss significant challenges in the field in developing countries, and then pose pivotal questions, and explore avenues for Brazilian investigators to make meaningful contributions to global head and neck cancer (HNC) research. In this manuscript, we share the knowledge generated from this gathering.
The following questions were formulated in advance to guide the discussion, although they were not strictly adhered to:
1.What are the primary unmet needs of patients with HNC?
2.What are the key challenges in HNC research in developing countries?
3.Should the research focus in developing nations be aligned with that of developed nations?
4.How to become a researcher in a developing country?
5.Are Brazilian research centers prepared for phase I and II clinical trials?
6.How can patient recruitment and retention be improved in HNC research studies?
7.What is the role of a team science approach to clinical research?
8.In what ways can collaboration among researchers, government agencies, and industry partners be enhanced?
9.How can collaboration between Brazilian researchers and counterparts from developed countries be strengthened?
We present below the deliberations from the meeting, enriched with a brief literature review of each topic.
What Are the Primary Unmet Needs of Patients With HNC?
Among several unmet needs in HNC, we highlighted the need to reduce treatment side effects and personalize therapy, with a focus on biomarker-based approaches.
A major toxicity burden for patients with HNC relates to delivery of radiation therapy, especially when combined with systemic therapy. Recent discussions have centered on the possibility of deintensifying radiation therapy, particularly for human papillomavirus (HPV)–positive oropharyngeal cancers (OPSCCs), to minimize adverse effects without compromising disease control. Technological advancements, including intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT), have demonstrated improvements in toxicity outcomes. However, challenges persist in balancing treatment deintensification with disease control. Presently, treatment deintensification remains experimental and should not be implemented outside of a clinical trial. This is illustrated by the recent results from the NRG-HN005 study, which demonstrated excellent efficacy outcomes with standard concurrent cisplatin and radiation therapy (2-year progression-free survival [PFS] of 98.1%), and failure of the two experimental arms to achieve noninferior results (88.6% 2-year PFS for reduced-dose radiation delivered concurrently with cisplatin and 90.3% 2-year PFS for reduced-dose radiation delivered concurrently with nivolumab). This reinforces the importance of developing evidence-based criteria for appropriate patient selection and deintensification strategies before modifying standard treatment protocols.
Future studies could explore several promising avenues: (1) optimizing dose volume, including optimal reductions in elective dose volumes in the cervical region to minimize toxicity without compromising regional control; (2) using functional imaging, such as positron emission tomography (PET)-FMISO and other predictive biomarkers, to identify radiosensitive tumors, potentially allowing for personalized, deintensified treatment approaches,; (3) reducing radiation dose in the tumor bed following robotic surgery resection; and (4) adaptive replanning throughout the treatment course to account for anatomical changes and to optimize dose distribution.
Challenges to personalized care stem from the fact that head and neck squamous cell carcinoma (HNSCC) is composed of a heterogeneous group of diseases defined fundamentally by the primary tumor site and, more complexly, by the underlying molecular alterations driving carcinogenesis. These features determine distinct tumor biology, influencing patterns of locoregional dissemination, response to treatments, and survival. Various treatment options exist, which can be delivered as a single modality or as part of a multimodality approach sequenced in different ways. Historically, the individualization of treatment according to anatomical subsites has been based on clinical factors determined by staging and clinical presentation. However, with advances in precision medicine and the search for prognostic factors, biomarkers have gained prominence in defining the best treatment sequence for HNC.
Biomarkers are crucial tools for diagnosis, assessing disease progression, and predicting treatment response. Numerous HNSCC biomarkers have been proposed to have a significant impact on diagnosis and prognosis, including HPV, immune markers, somatic gene mutations, and copy number alterations. However, only a few have been validated for clinical use.
HPV-related OPSCCs are increasing, including in developing countries, with HPV16 present in approximately 90% of HPV-associated OPSCCs. Salivary HPV DNA has shown predictive value for recurrence: detected in 54% of patients at diagnosis but only 5% after treatment., Most post-treatment HPV-positive patients relapsed, and persistent infection correlated with worse disease-free survival and overall survival (OS). Prospective trials are needed to define how post-treatment HPV testing could guide personalized surveillance or treatment strategies.
Increasing evidence underscores the necessity for the development of immunological biomarkers to provide prognostic insights and aid clinical decision making. Tumor-infiltrating immune cells, such as T and B lymphocytes, macrophages, or neutrophils, can represent either a negative or positive influence on tumor progression. Cytotoxic CD8+ tumor-infiltrating lymphocytes (TILs) are believed to be the primary effector immune cells targeting tumor cells and have demonstrated prognostic significance across numerous solid tumors. HNSCC is characterized by profound immunosuppression. Several studies have documented a significantly higher density of TILs in HPV-positive compared with HPV-negative OPSCC, suggesting a more robust antitumoral immune response in HPV-associated OPSCCs. Additionally, elevated levels of both CD8+ and CD3+ T cells have been linked to improved OS following definitive chemoradiotherapy in both HPV-positive and HPV-negative HNSCC cases. Immune checkpoints modulate signaling and either inhibit or enhance T-cell response. Cytotoxic T-lymphocyte antigen 4 and PD-1 are distinct examples of coinhibitory molecules. In laryngeal SCC, high PD-L1 expression was associated with better outcomes, whereas in oral cavity cancers, it correlated with poorer survival. Although PD-L1 guides anti-PD1 therapy selection, it alone is not a reliable predictor of benefit. Integrating PD-L1 with other molecular markers may better distinguish responders from nonresponders to immunotherapy.
Over the past few years, next-generation sequencing (NGS) has provided the opportunity for molecular characterization, significantly advancing our understanding of genetic profiles across various solid tumors. In HNSCC, several retrospective studies have identified mutations in genes critical for disease progression. Particularly notable are mutations in TP53, PIK3CA, CDKN2A, and TERT promoter and alterations in NOTCH pathway genes. Although NGS remains an emerging biomarker modality, its true value for (targeted) treatment selection remains to be clinically demonstrated and should continue to be the focus of intense clinical investigations.
What Are the Key Challenges in HNC Research in Developing Countries?
In addition to the inherent complexities of the neoplasm, patients with HNSCC frequently present with comorbidities such as anemia, liver disease, malnutrition, and cachexia., Particularly when these conditions are decompensated, they are often used as exclusion criteria from clinical trials. Furthermore, a poor performance status similarly renders these patients ineligible for research studies. This condition further complicates eligibility for clinical trials in this patient group. These factors are prevalent in underserved populations in developing countries and underscore the need for inclusive research approaches that ensure diversity representation in clinical trials and development of management strategies that are applicable to patients in the real (developing) world.
Additionally, in countries like Brazil, limited access to early diagnosis and optimal treatment is a critical issue. The majority of patients with HNSCC are diagnosed with locally advanced disease (stages III and IV), which significantly reduces the opportunity for timely intervention and effective treatment. The lack of infrastructure and funding for head and cancer research significantly impedes advancements in this field. This limitation affects the capacity to establish and conduct clinical trials, which require substantial financial investments. In addition, the involvement of a multidisciplinary team (MDT) is crucial for managing head and neck tumors. However, the availability of multidisciplinary consultations varies across different countries. As a result, limited access to health care services and resources directly affects clinical outcomes. Enhancing funding opportunities and increasing collaborative networks are fundamental steps to overcome these challenges.
Another important issue in Brazil's public health care system is the limited access to modern radiotherapy technologies. Although IMRT and IGRT are considered standard of care in many countries due to their ability to minimize toxicity (and for some tumors to increase survival), these technologies are still not widely available for patients with HNC treated under the public system. This limitation is largely due to insufficient investment in radiotherapy infrastructure. For instance, the linear accelerators distributed through the PER-SUS program (Radiotherapy Expansion Plan in the Brazilian Public System) do not include IMRT capabilities, reflecting a persistent gap in technological access essential for reducing treatment-related morbidity.
Moreover, the integration of supportive care, particularly dental care and laser therapy during radiotherapy, also remains a significant challenge in the public sector. Oral mucositis is a common and debilitating side effect of radiation, and although laser therapy has shown benefits in symptom control and treatment adherence, access is scarce or nonexistent in many public cancer centers.
These limitations underscore a broader concern: although precision oncology and personalized treatments are the focus of many research efforts, in low- and middle-income countries, the most pressing priority remains ensuring equitable access to basic and adequate care for all patients.
Is (or should) the Research Focus in Developing Nations (be) Aligned With Those of Developed Nations?
It is essential to recognize that cancer research needs and priorities vary significantly across countries or regions. This diversity must be carefully considered during both the planning and implementation phases of a study. Strategies for conducting scientific research must be tailored to the existing realities of each region, with a primary focus on assessing factors scientifically relevant to their research hypotheses while considering data generated elsewhere. This requires a diverse participant pool that accurately reflects the disease population, which is critical for the global implementation of therapeutic interventions.
Developing nations encounter unique obstacles in the realm of oncology, including a higher incidence of certain cancers, restricted access to early diagnosis and treatment options, and higher mortality rates. Consequently, research initiatives in these countries should focus on exploring and addressing mitigation of these particular challenges. The constrained economic resources, health care infrastructure, and professional expertise in developing countries necessitate a pragmatic research approach. This involves concentrating on cost-effective measures and strategies that are feasible within the existing resources. Furthermore, cultural perceptions, health care–seeking behaviors, and societal norms play a pivotal role in shaping cancer research priorities. In developing countries, it is critical to account for cultural perspectives on cancer, traditional healing practices, and the significance of engagement to ensure that interventions are relevant and widely accepted.
Despite variations in research priorities, cancer remains a pressing global health issue that transcends borders. Therefore, it is imperative to foster partnerships between researchers in developed and developing nations for the collective advancement of knowledge, sharing resources, and creating impactful interventions that are applicable to diverse populations. Capacity building is often a significant component of research in developing countries, aimed at enhancing local research capabilities, educating health care workers, and mobilizing communities toward proactive engagement in cancer prevention and management. Such initiatives could benefit from the exchange of experiences with developed countries but may necessitate customized strategies that address distinct challenges and make optimal use of existing resources.
In essence, although the focal points of cancer research may vary between developed and developing regions, the importance of collaborative efforts, contextual sensitivity, and capacity building remains crucial for global advancements in cancer prevention, early detection, treatment, and care.
Pathways and Challenges for Emerging Researchers in Developing Countries
Embarking on the path to becoming a researcher in a developing country involves navigating a complex process that requires a dynamic interplay of collaboration, awareness, communication, and active participation. Conducting research anywhere in the world poses unique challenges. These challenges may come from the complexities of the research process itself, such as formulating the right question or hypothesis, selecting appropriate methods, and determining suitable analyses. To become a researcher, professionals—whether they are physicians or nonphysicians—must possess critical thinking skills and a commitment to lifelong learning. Creativity and a desire for innovation are also essential traits. In developing countries, aspiring researchers face additional hurdles that require a greater degree of resilience and persistence. A collaborative spirit and the ability to work in teams are equally fundamental.
Are Brazilian Research Centers Prepared for Phase I and II Clinical Trials?
Clinical research centers in Brazil have gradually increased in number and gained personnel capacitation over the last few years at various levels. Oncology clinical trials are complex, and many centers have developed the infrastructure to conduct such trials with high-quality standards. One of the largest hurdles faced locally is the time needed for regulatory approvals, which did not favor the inclusion of Brazilian sites in early-phase clinical trials. However, recently, Brazil's central institutional review board, Comissão Nacional de Ética em Pesquisa, has been restructured to shorten regulatory timelines. This process has empowered local IRBs, which now may issue a national approval for a given clinical trial in a decentralized way. Such an initiative, although still limited to a few Brazilian regions, is currently expanding and has made it possible to increase the number of early-phase trials.
It is important to underscore the need to build a solid infrastructure and adequately train all the study team members. Observational studies and later phase trials, such as phase III, phase IV, and even expanded access programs, can aid the development of a quality program framework. Once the center is able to adequately conduct such trials and gain experience with daily routines and requirements, conducting early-phase trials is possible. It is essential for the research center to have a competent leader who can motivate a group of individuals interested in research.
Currently, only a few fully equipped and staffed centers exist in Brazil, which does not facilitate the inclusion of patients from different regions of the country. Moreover, given the expansion of oncology research centers, there is a growing need for trained and experienced staff to conduct and oversee complex trials, not only at the center level but also at clinical research organizations. There is also the need to considered language barriers and local practices that sometimes might affect the course of an early-phase trial.
On the other hand, Brazil offers a large and diverse population, which is frequently treatment-naïve, allowing studies to explore disease biology. These studies offer the possibility for engaged investigators to participate in early-stage clinical development and also include patients with HNC, which are typically not the focus of the early stages of drug development.
How Can Patient Recruitment and Retention be Improved in HNC Research Studies?
Recruiting patients to trials is a very challenging process. Although HNC research has advanced in the past two decades, with many important trials published, data related to recruitment issues in this disease are still scant. Rogers et al reported details of trial recruitment in a head and neck Patient Concerns Inventory intervention trial from two UK head and neck tertiary centers. The authors showed that older age and low income were independent predictors of clinical trial participation refusal. Kaur et al aimed to identify perceived barriers to recruitment among three pioneer HNC trials: SEND, PET-Neck, and HOPON. The most commonly identified barriers were expressed treatment preferences, aversion to random assignment, excess complexity, amount of information provided to patients, and lack of time in clinic to accommodate research. Brazilian data in this field are lacking, but the experiences reported above suggest that appropriate communication, education, and awareness may enhance patient recruitment to clinical trials. These goals might be reached with innovative approaches, such as creating videos that explain the trial, its objectives, risks and benefits, and why participation is essential.
What Is the Role of an MDT Approach to Clinical Research?
Contemporary treatment of patients with HNSCC is multifaceted and relies on many uniquely qualified professionals. Despite the availability of resources, patients often experience fragmented and uncoordinated care that leads to delays in treatment, compromised outcomes, severe distress in patients and families, and dissatisfaction with care. Patients experience numerous stressors such as severe symptoms due to the disease and the aggressive treatments, body image concerns, loss of speech, oral complications, difficulty in swallowing, nutritional issues, and respiratory problems that affect their quality of life and ability to function on a day-to-day basis. Additionally, patients experience barriers to accessing quality, timely care throughout all stages of the cancer care continuum, from diagnosis through survivorship. This leads to decreased treatment compliance, intensified psychosocial distress, financial hardship, and poor oncological outcomes.,
A well-coordinated MDT approach is the present standard of care for patients with HNSCC. Studies show that an integrated team approach yields better 5-year survival outcomes, increased rates of completion of planned therapy, and higher compliance with recommendations.,, A comprehensive assessment and monitoring of patients with HNSCC by specialized MDTs will result in better treatment adherence and tolerance, reduction in long-term side effects, improved quality of life, and ultimately improved treatment outcome and survival. However, we still lack a clear definition of the most format, scope of practice, and criteria according to which individual health care institutions could assess effective MDTs.
The core function of MDTs to improve patient prognosis is holding tumor boards, where all new HNSCC cases are reviewed and personalized treatment plans are created., These boards integrate expertise across disciplines, enhancing diagnosis and treatment. Challenges include coordinating schedules, workflow disruptions, and billing management. Effective design that preserves workflow, billing practices, and patient volume is essential. Involving clinic administrators and billing specialists early supports implementation and helps prevent revenue loss.
The extension of an integrated MDT to include translational research can help reduce the existing gap between current clinical practice and basic science. The reciprocal interaction and feedback from researchers and physicians will also contribute to improving prospective studies and determining the feasibility of the correlative analysis. Therefore, collaboration and timely coordination between research laboratories and clinics are crucial to conducting these studies smoothly and successfully. Proposed recommendations to succeed in the integration of clinical and translational research within the MDT and HNSCC units are (1) assign a coordinator for clinical and translational research within the unit, (2) promote periodic meetings to update projects and explain novel proposals, (3) ensure access to all members of the unit so that everyone can contribute with new ideas and lead projects, (4) include educational programs for young members and trainees, (5) create working groups to deliver projects with appointed project leaders among the MDT members, and (6) use MDT tumor boards as a source of active participation in clinical trials and scientific projects. Considering this, some institutions have established Research Boards dedicated to deliberating and facilitating the implementation of collaborative studies.
In What Ways Can Collaboration Among Researchers, Government Agencies, and Industry Partners be Enhanced?
Governmental agencies play a crucial role in actively supporting and facilitating clinical trials that may answer important questions but may not necessarily interest or be the focus of pharmaceutical companies. These trials include, for example, pragmatic studies and those involving low-cost drugs or reduction in the duration of treatment. Brazilian researchers receive financial support for scientific, technological, and innovation research projects mainly from four official governmental agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Financiadora de Estudos e Projetos (FINEP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundações de Apoio à Pesquisa (FAPs).
CNPq is a Brazilian governmental agency under the Ministry of Science, Technology, and Innovation that supports scientific research and human resource development. It funds basic, translational, and clinical research projects and offers scholarships for undergraduate, master's, and PhD students, as well as grants for established researchers.
FINEP is another agency linked to the Brazilian Ministry of Science, Technology, and Innovation, which promotes science (basic research, applied research), technology, and innovation and the development of products, services, and processes in universities, institutes, and other public or private institutions. FINEP supports the development of technology companies and the implementation of technology parks, and is also responsible for identifying, negotiating, and monitoring research cooperation with international partners in priority areas for our country.
CAPES, an agency linked to the Ministry of Education of Brazil, promotes the training of highly qualified human resources through the granting of scholarships for postgraduate courses (master's and doctorate). CAPES alone provides financial resources for 55% of masters and doctoral scholarships in our country.
FAPs are regional funding agencies that support individual and institutional research projects by helping develop infrastructure in public or private institutions. Their resources vary by region, depending on local development levels, and are typically managed through state departments of science and technology.
Guidance for researchers on the types of financial support that can be obtained from official governmental agencies can contribute to obtaining resources for their research projects. Furthermore, considering that scientific and technological knowledge is more complex and that the skills of each researcher are limited, collaboration between researchers from different areas of knowledge and across the country makes research projects more interesting, innovative, and worthy of financial support from official governmental agencies. Governmental participation in trials that are not of interest to pharmaceutical industries may be possible if cancer is seen as a public health problem and if the studies are seen as potential solutions to reduce the costs of treatment of patients with cancer by the governmental funding agencies or by the Brazilian Ministry of Science and Technology, to which these agencies report.
BRAZILIAN SUCCESS EXAMPLES
Latin American Cooperative Oncology Group
LACOG is a nonprofit organization founded in 2009 in Porto Alegre, Brazil. LACOG was the Latin America's first multinational cooperative group dedicated to clinical and translational cancer research. It currently has more than 540 investigator members in 259 institutions in 16 Latin American countries. Over the course of its existence, LACOG has included more than 20,000 participants in observational or interventional studies. Remarkably, among the cooperative groups in Latin America, LACOG is the only one that develops studies in HNC. The LACOG 0720—SMART-KEY study (ClinicalTrials.gov identifier: NCT04943445), a phase 2 multicenter trial, recently completed enrollment and is awaiting data maturation. It evaluates an immunotherapy-based approach for newly diagnosed patients with squamous cell larynx carcinoma eligible for larynx preservation. The primary end point is 2-year laryngectomy-free survival; secondary end points include larynx dysfunction–free survival, overall survival, response rate, toxicities, and quality of life. Despite the rarity of this population, 41 patients were enrolled across 10 sites in 18 months, highlighting Latin America's recruitment potential in HNC trials.
HEADSpAcE
HEADSpAcE is a multicentric study on HNC funded by International Agency for Research on Cancer/EUROPEAN COMMISSION—Horizon 2020/FAPESP/Brazil. The objectives of HEADSpAcE are to understand reasons for late diagnosis and reduce the proportion of HNC that are diagnosed at a very late stage. It will identify the most appropriate method to diagnose cancers caused by HPV and provide genomic evidence for strong predictors of prognosis that may have the potential to improve care and reduce treatment-related morbidity. The work of HEADSpAcE is based on seven clinical cohorts from different countries, the majority of which have already been recruited. The cohorts have detailed follow-up information, risk factor data, HPV evaluation, and extensive biological samples. Participating centers are in Argentina, Brazil, Colombia, Czech Republic, and France, among others.
Brazilian Group of Head and Neck Cancer
GBCP is a nonprofit organization founded in October 2017. Its members include oncologists, head and neck surgeons, radiation oncologists, nutritionists, voice therapists, dentists, psychologists, and others. The organization's mission is to support health care professionals who treat patients with HNC at all stages of care. GBCP operates in four main areas: health professional education, community awareness, patient and caregiver support, and research in partnership with LACOG. Since 2018, it has organized webinars, virtual tumor boards, an Annual Symposium focused on treatment, and a Multidisciplinary Forum focused on support and rehabilitation. It also partners with the Brazilian Society of Clinical Oncology to create guidelines for treating HNSCC, salivary gland, and thyroid cancer, with annual updates. GBCP has written two books—the HNC Oncology Manual (2021) and the HNC Oncology Manual—Support and Rehabilitation (2022), as well as many articles. In addition, GBCP partners with the European HNC Society for the MakeSense Campaign and the Brazilian Society of Head and Neck Surgery for the Green July Campaign to raise awareness in the community.
These Brazilian initiatives—LACOG, HEADSpAcE, and GBCP—serve as valuable success stories that demonstrate how collaborative frameworks, international partnerships, and multidisciplinary engagement can overcome structural and financial challenges in HNC research. Their accomplishments highlight essential elements for success, including strong local leadership, effective patient recruitment strategies, integrated research and care pathways, and sustained commitment to professional education and community engagement. Embedding these examples into structured tools can serve as a roadmap for other low- and middle-income countries facing similar barriers (Table 1).
TABLE 1
Key Success Stories in Brazilian HNC Research
| Initiative | Main Focus | Key Strengths | Why It Worked |
|---|---|---|---|
| LACOG | Multinational cooperative group conducting interventional trials (industry-sponsored), epidemiological studies, and investigator-initiated research across Latin America | Strong governance; extensive network; diversified funding; experience in HNC; capacity building across the region | Versatile research portfolio; sustained collaboration; regional relevance; ability to recruit and retain diverse patient populations |
| HEADSpAcE study | Late-stage diagnosis, HPV-related HNC, and prognostic biomarkers | International funding (Horizon 2020 from the European Union); integration of epidemiology + genomics | Strategic partnerships, protocol harmonization, high recruitment despite LMIC constraints |
| GBCP initiatives | Education, guidelines, patient support, awareness campaigns | Multidisciplinary engagement; alliance with international and national societies | Continuous activities (webinars, symposia); high visibility and broad stakeholder engagement |
In conclusion, Brazil and other developing nations have a huge opportunity to significantly advance the management of HNSCC. Its high incidence in some areas of the world poses this disease as a public health care issue, and investments in research could not only bring significant benefits to individual patients but also help mitigate resource constraints that ultimately threaten population-wide outcomes. Nonetheless, the development of a national research program is challenging, albeit possible, as discussed in this document and illustrated in a Strength, Weaknesses, Opportunities, and Threats analysis in Figure 1. We strongly believe that through concerted efforts, internal and external collaborations, Brazilian investigators should be able to increasingly contribute to developing novel strategies to improve HNSCC management in the coming years in ways that are relevant, not only to the region but also to the worldwide community of patients with HNCs.
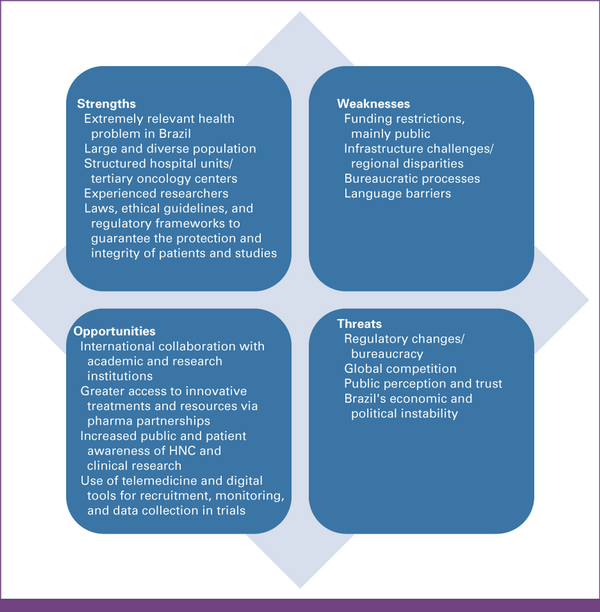
FIG 1
SWOT analysis for HNC clinical research in Brazil. This figure summarizes key SWOT related to HNC research in Brazil. Strengths include experienced multidisciplinary professionals, established research networks, and motivated investigators. Weaknesses highlight limitations in funding, infrastructure, and training. Opportunities point to international collaborations, a large treatment-naïve patient population, and emerging clinical trial capacity. Threats include regulatory hurdles, regional disparities, and limited access to advanced diagnostics and technology. HNC, head and neck cancer; SWOT, strengths, weaknesses, opportunities, and threats.
AUTHOR CONTRIBUTIONS
Conception and design: Aline Lauda Freitas Chaves, William Nassib William Jr
Administrative support: Aline Lauda Freitas Chaves, William Nassib William Jr
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Aline Lauda Freitas Chaves
Consulting or Advisory Role: Merck Sharp & Dohme, Merck Serono, Knight Pharmaceuticals, Dr Reddy's, Danone
Speakers' Bureau: Merck Serono, Merck Sharp & Dohme, Knight Therapeutics, Dr Reddy's
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Merck Serono, Knight Pharmaceuticals, Dr Reddy's
Luiz Paulo Kowalski
Employment: University of São Paulo Medical School, A.C. Camargo Cancer Center
Research Funding: Ministry of Health, Brazil
Milena Perez Mak
Honoraria: Merck KGaA, Sanofi, Lilly, Bristol Myers Squibb, United Medical, Johnson & Johnson/Janssen
Travel, Accommodations, Expenses: Knight Pharmaceuticals
Dalvaro Oliveira de Castro Junior
Honoraria: Janssen Oncology, Merck Serono
Consulting or Advisory Role: Janssen Oncology
Speakers' Bureau: Janssen Oncology
Travel, Accommodations, Expenses: Bayer, Janssen Oncology
Thiago Bueno de Oliveira
Consulting or Advisory Role: MSD Oncology, AstraZeneca, Janssen Oncology, Knight Therapeutics, Lilly, Daiichi Sankyo/Astra Zeneca
Speakers' Bureau: MSD Oncology, BMS Brazil, AstraZeneca, Janssen Oncology, Merck Serono, Sanofi, Knight Therapeutics, Lilly, Daiichi Sankyo/Astra Zeneca, Roche/Genentech
Research Funding: MSD Oncology
Travel, Accommodations, Expenses: MSD Oncology, Janssen Oncology
Ligia Traldi Macedo
Stock and Other Ownership Interests: Bristol Myers Squibb (BMY)
Guilherme Harada
Honoraria: Lilly, Novartis, AstraZeneca, Merck Sharp Dohme, Bristol Myers Squibb, BeiGene, Daiichi Sankyo, Johnson & Johnson/Janssen, Takeda
Malu Viter da Rosa Barbosa
Employment: AstraZeneca
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: AstraZeneca, Merck
Travel, Accommodations, Expenses: AstraZeneca, Daiichi Sankyo/Lilly
Gilberto De Castro Junior
Honoraria: AstraZeneca, Pfizer, Merck Sharp & Dohme, Bristol Myers Squibb, Novartis, Roche, Amgen, Janssen, Merck Serono, Lilly, Takeda, Daiichi Sankyo/UCB Japan
Consulting or Advisory Role: Boehringer Ingelheim, Pfizer, Bayer, Roche, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Yuhan, Merck Serono, Janssen, Libbs, Sanofi, Novartis, Lilly, Takeda, Daiichi Sankyo/UCB Japan
Speakers' Bureau: AstraZeneca, Bayer, Novartis, Roche, Merck Serono, Bristol Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, Pfizer, Janssen, Amgen, Takeda, Daiichi Sankyo/UCB Japan
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Novartis, Pfizer, Roche, AstraZeneca, Boehringer Ingelheim, Bayer, Bristol Myers Squibb, Merck Serono, Daiichi Sankyo/UCB Japan
Pedro Rafael Martins de Marchi
Honoraria: Sanofi
Consulting or Advisory Role: Merck Sharp & Dohme, AstraZeneca, Bristol Myers Squibb, Roche, Bayer
Speakers' Bureau: Merck Sharp & Dohme, Bristol Myers Squibb, Roche, AstraZeneca, Novartis, Merck, Boehringer Ingelheim, Janssen, Takeda
Research Funding: AstraZeneca, Amgen, Merck Sharp & Dohme, Roche
Travel, Accommodations, Expenses: Merck Sharp & Dohme, AstraZeneca, Roche, Bristol Myers Squibb
Leandro Luongo Matos
Employment: Johnson & Johnson/Janssen (I)
Consulting or Advisory Role: MSD
Speakers' Bureau: MSD
Hisham Mehanna
Employment: Warwickshire Head and Neck Clinic
Leadership: Warwickshire Head and Neck Clinic, Warwickshire Head and Neck Clinic (I)
Stock and Other Ownership Interests: Warwickshire Head and Neck Clinic
Honoraria: AstraZeneca
Speakers' Bureau: MSD, Sanofi Pasteur, Merck
Research Funding: GlaxoSmithKline (Inst), MSD (Inst), Sanofi Pasteur (Inst), AstraZeneca (Inst)
William Nassib William Jr
Employment: Grupo Oncoclínicas
Honoraria: Roche/Genentech, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Merck, Lilly, Pfizer, Janssen, Takeda, Novartis, Daiichi Sankyo, Bayer, Regeneron, Sanofi/Aventis, Daiichi Sankyo/Astra Zeneca
Consulting or Advisory Role: AstraZeneca, Merck, Pfizer, Bristol Myers Squibb, Bayer, Roche, Daiichi Sankyo/Astra Zeneca
Speakers' Bureau: Boehringer Ingelheim
Research Funding: OSI Pharmaceuticals (Inst), Boehringer Ingelheim (Inst), Merck (Inst), Lilly (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Janssen (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Merck, Bristol Myers Squibb, Roche/Genentech, Daiichi Sankyo/Astra Zeneca
No other potential conflicts of interest were reported.
Aline Lauda Freitas Chaves
Consulting or Advisory Role: Merck Sharp & Dohme, Merck Serono, Knight Pharmaceuticals, Dr Reddy's, Danone
Speakers' Bureau: Merck Serono, Merck Sharp & Dohme, Knight Therapeutics, Dr Reddy's
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Merck Serono, Knight Pharmaceuticals, Dr Reddy's
Luiz Paulo Kowalski
Employment: University of São Paulo Medical School, A.C. Camargo Cancer Center
Research Funding: Ministry of Health, Brazil
Milena Perez Mak
Honoraria: Merck KGaA, Sanofi, Lilly, Bristol Myers Squibb, United Medical, Johnson & Johnson/Janssen
Travel, Accommodations, Expenses: Knight Pharmaceuticals
Dalvaro Oliveira de Castro Junior
Honoraria: Janssen Oncology, Merck Serono
Consulting or Advisory Role: Janssen Oncology
Speakers' Bureau: Janssen Oncology
Travel, Accommodations, Expenses: Bayer, Janssen Oncology
Thiago Bueno de Oliveira
Consulting or Advisory Role: MSD Oncology, AstraZeneca, Janssen Oncology, Knight Therapeutics, Lilly, Daiichi Sankyo/Astra Zeneca
Speakers' Bureau: MSD Oncology, BMS Brazil, AstraZeneca, Janssen Oncology, Merck Serono, Sanofi, Knight Therapeutics, Lilly, Daiichi Sankyo/Astra Zeneca, Roche/Genentech
Research Funding: MSD Oncology
Travel, Accommodations, Expenses: MSD Oncology, Janssen Oncology
Ligia Traldi Macedo
Stock and Other Ownership Interests: Bristol Myers Squibb (BMY)
Guilherme Harada
Honoraria: Lilly, Novartis, AstraZeneca, Merck Sharp Dohme, Bristol Myers Squibb, BeiGene, Daiichi Sankyo, Johnson & Johnson/Janssen, Takeda
Malu Viter da Rosa Barbosa
Employment: AstraZeneca
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: AstraZeneca, Merck
Travel, Accommodations, Expenses: AstraZeneca, Daiichi Sankyo/Lilly
Gilberto De Castro Junior
Honoraria: AstraZeneca, Pfizer, Merck Sharp & Dohme, Bristol Myers Squibb, Novartis, Roche, Amgen, Janssen, Merck Serono, Lilly, Takeda, Daiichi Sankyo/UCB Japan
Consulting or Advisory Role: Boehringer Ingelheim, Pfizer, Bayer, Roche, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Yuhan, Merck Serono, Janssen, Libbs, Sanofi, Novartis, Lilly, Takeda, Daiichi Sankyo/UCB Japan
Speakers' Bureau: AstraZeneca, Bayer, Novartis, Roche, Merck Serono, Bristol Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, Pfizer, Janssen, Amgen, Takeda, Daiichi Sankyo/UCB Japan
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Novartis, Pfizer, Roche, AstraZeneca, Boehringer Ingelheim, Bayer, Bristol Myers Squibb, Merck Serono, Daiichi Sankyo/UCB Japan
Pedro Rafael Martins de Marchi
Honoraria: Sanofi
Consulting or Advisory Role: Merck Sharp & Dohme, AstraZeneca, Bristol Myers Squibb, Roche, Bayer
Speakers' Bureau: Merck Sharp & Dohme, Bristol Myers Squibb, Roche, AstraZeneca, Novartis, Merck, Boehringer Ingelheim, Janssen, Takeda
Research Funding: AstraZeneca, Amgen, Merck Sharp & Dohme, Roche
Travel, Accommodations, Expenses: Merck Sharp & Dohme, AstraZeneca, Roche, Bristol Myers Squibb
Leandro Luongo Matos
Employment: Johnson & Johnson/Janssen (I)
Consulting or Advisory Role: MSD
Speakers' Bureau: MSD
Hisham Mehanna
Employment: Warwickshire Head and Neck Clinic
Leadership: Warwickshire Head and Neck Clinic, Warwickshire Head and Neck Clinic (I)
Stock and Other Ownership Interests: Warwickshire Head and Neck Clinic
Honoraria: AstraZeneca
Speakers' Bureau: MSD, Sanofi Pasteur, Merck
Research Funding: GlaxoSmithKline (Inst), MSD (Inst), Sanofi Pasteur (Inst), AstraZeneca (Inst)
William Nassib William Jr
Employment: Grupo Oncoclínicas
Honoraria: Roche/Genentech, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Merck, Lilly, Pfizer, Janssen, Takeda, Novartis, Daiichi Sankyo, Bayer, Regeneron, Sanofi/Aventis, Daiichi Sankyo/Astra Zeneca
Consulting or Advisory Role: AstraZeneca, Merck, Pfizer, Bristol Myers Squibb, Bayer, Roche, Daiichi Sankyo/Astra Zeneca
Speakers' Bureau: Boehringer Ingelheim
Research Funding: OSI Pharmaceuticals (Inst), Boehringer Ingelheim (Inst), Merck (Inst), Lilly (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Janssen (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Merck, Bristol Myers Squibb, Roche/Genentech, Daiichi Sankyo/Astra Zeneca
No other potential conflicts of interest were reported.
REFERENCES
1.
Yom SS, Harris J, Caudell J, et al.: Interim futility results of NRG-HN005, A randomized, phase II/III non-inferiority trial for non-smoking p16+ oropharyngeal cancer patients. Int J Radiat Oncol Biol Phys 120:S2-S3, 20242.
Smith JA, Mukherji SK, Frank SJ: De-escalation of adjuvant radiation dose in HPV-associated oropharyngeal cancer: A literature review. Oral Oncol 125:105590, 20233.
Huang EY, Wang YM, Chang SC, et al.: PET imaging in head and neck cancer: Current status, challenges, and future directions. Cancers 13:497, 20214.
Castaldi P, Leccisotti L, Bussu F, et al.: Role of (18)F-FDG PET-CT in head and neck squamous cell carcinoma. Acta Otorhinolaryngol Ital 33:1-8, 20135.
Dziegielewski PT, Teknos TN, Durmus K, et al.: Transoral robotic surgery for oropharyngeal cancer: Long-term quality of life and functional outcomes. JAMA Otolaryngol Head Neck Surg 139:1099-1108, 20136.
Deschuymer S, Nevens D, Duprez F, et al.: Randomized clinical trial on reduction of radiotherapy dose to the elective neck in head and neck squamous cell carcinoma: Results on the quality of life. Qual Life Res 30:117-127, 20217.
Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252-264, 20128.
Bhatia A, Burtness B: Human papillomavirus-associated oropharyngeal cancer: Defining risk groups and clinical trials. J Clin Oncol 33:3243-3250, 20159.
Rettig EM, Wentz A, Posner MR, et al.: Prognostic implication of persistent human papillomavirus type 16 DNA detection in oral rinses for human papillomavirus-related oropharyngeal carcinoma. JAMA Oncol 1:907-915, 201510.
Krupar R, Robold K, Gaag D, et al.: Immunologic and metabolic characteristics of HPV-negative and HPV-positive head and neck squamous cell carcinomas are strikingly different. Virchows Arch 465:299-312, 201411.
Balermpas P, Michel Y, Wagenblast J, et al.: Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer 110:501-509, 201412.
Vassilakopoulou M, Avgeris M, Velcheti V, et al.: Evaluation of PD-L1 expression and associated tumor-infiltrating lymphocytes in laryngeal squamous cell carcinoma. Clin Cancer Res 22:704-713, 201613.
Lin YM, Sung WW, Hsieh MJ, et al.: High PD-L1 expression correlates with metastasis and poor prognosis in oral squamous cell carcinoma. PLoS ONE 10:e0142656, 201514.
Stransky N, Egloff AM, Tward AD, et al.: The mutational landscape of head and neck squamous cell carcinoma. Science 333:1157-1160, 201115.
Bøje CR: Impact of comorbidity on treatment outcome in head and neck squamous cell carcinoma—A systematic review. Radiother Oncol 110:81-90, 201416.
Porter SR, Ukwas A: Cachexia and head and neck squamous cell carcinoma: A scoping review. Oral Dis 30:1746-1755, 202417.
Nascimento de Carvalho F, de Camargo Cancela M, Mesentier da Costa L, et al.: Disparities in stage at diagnosis of head and neck tumours in Brazil: A comprehensive analysis of hospital-based cancer registries. Lancet Reg Health Am 42:100986, 202518.
Hanna SA, Gouveia AG, Moraes FY, et al.: Lessons from the Brazilian radiotherapy expansion plan: A project database study. Lancet Reg Health Am 14:100333, 202219.
Brand NR, Qu LG, Chao A, et al.: Delays and barriers to cancer care in low- and middle-income countries: A systematic review. Oncologist 24:e1371-e1380, 201920.
Brasil. Lei nº 14.874, de 20 de março de 2024: Estabelece normas para pesquisa com seres humanos e institui o Sistema Nacional de Ética em Pesquisa com Seres Humanos. Diário Oficial da União. 2024. https://www.planalto.gov.br/ccivil_03/_ato2023-2026/2024/lei/l14874.htm21.
Rogers SN, Lowe D, Highet V, et al.: Patient characteristics and refusal to participate in a head and neck cancer intervention trial: Experience of two tertiary UK head and neck cancer centres. Ann R Coll Surg Engl 104:121-124, 202222.
Kaur G, Hutchison I, Mehanna H, et al.: Barriers to recruitment for surgical trials in head and neck oncology: A survey of trial investigators. BMJ Open 3:e002625, 201323.
Wiederholt PA, Connor NP, Hartig GK, et al.: Bridging gaps in multidisciplinary head and neck cancer care: Nursing coordination and case management. Int J Radiat Oncol Biol Phys 69:S88-S91, 2007 (suppl 2)24.
Dingman C, Hegedus PD, Likes C, et al.: A coordinated, multidisciplinary approach to caring for the patient with head and neck cancer. J Support Oncol 6:125-131, 200825.
Beeram M, Kennedy A, Hales N: Barriers to comprehensive multidisciplinary head and neck care in a community oncology practice. Am Soc Clin Oncol Educ Book 41:1-10, 202126.
Neal RD, Tharmanathan P, France B, et al.: Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer 112:S92-S107, 2015 (suppl 1)27.
Chang YL, Lin CY, Kang CJ, et al.: Association between multidisciplinary team care and the completion of treatment for oral squamous cell carcinoma: A cohort population-based study. Eur J Cancer Care 30:e13367, 202128.
Taberna M, Gil Moncayo F, Jané-Salas E, et al.: The multidisciplinary team (MDT) approach and quality of care. Front Oncol 10:85, 202029.
Prgomet D, Bišof V, Prstačić R, et al.: The multidisciplinary team (MDT) in the treatment of head and neck cancer—A single-institution experience. Acta Clin Croat 61:77-87, 2022 (suppl 4)30.
Taylor C, Atkins L, Richardson A, et al.: Measuring the quality of MDT working: An observational approach. BMC Cancer 12:202, 201231.
Licitra L, Keilholz U, Tahara M, et al.: Evaluation of the benefit and use of multidisciplinary teams in the treatment of head and neck cancer. Oral Oncol 59:73-79, 201632.
Wang YH, Kung PT, Tsai WC, et al.: Effects of multidisciplinary care on the survival of patients with oral cavity cancer in Taiwan. Oral Oncol 48:803-810, 201233.
Hughes C, Homer J, Bradley P, et al.: An evaluation of current services available for people diagnosed with head and neck cancer in the UK (2009–2010). Clin Oncol 24:e187-e192, 201234.
Townsend M, Kallogjeri D, Scott-Wittenborn N, et al.: Multidisciplinary clinic management of head and neck cancer. JAMA Otolaryngol Head Neck Surg 143:1213-1219, 201735.
Martelli-Junior H, Martelli DRB, Quirino IG, et al.: CNPq-supported medical researchers: A comparative study of research areas. Rev Assoc Med Bras 56:478-483, 201036.
Santos MR, Pinho DB: A atuação da FINEP no financiamento à inovação tecnológica no Brasil. Rev Brasde Inov 9:323-346, 201037.
Guedes MC, Costa LM: Fundos Estaduais para Apoio à Pesquisa e o financiamento da ciência no Brasil. Rev Bras Polit Cient Tecnol. 2014;7:45-6238.
Gössling G, Rebelatto TF, Villarreal-Garza C, et al.: Current scenario of clinical cancer research in Latin America and the Caribbean. Curr Oncol 30:653-662, 202339.
Oliveira TB, Ferreira da Silva FA, Motta T, et al.: A single-arm, multi-institutional, phase 2 study of a pembrolizumab-based organ preservation strategy for locally advanced larynx cancers: SMART-KEY (LACOG 0720) trial. J Clin Oncol 41, 2023 (suppl 16; abstr TPS6111)40.
Brazilian Group of Head and Neck Cancer: About GBCP. https://www.gbcp.org.br/sobre-o-gbcp41.
Peters S: How to become a successful researcher: Tips for early career researchers. Scand J Work Environ Health 40:432-434, 201442.
Collins KA, Brannan GD, Dogbey GY: Research dissemination: Guiding the novice researcher on the publication path. J Am Osteopath Assoc 115:324-330, 201543.
Estrada M, Hernandez PR, Schultz PW: A longitudinal study of how quality mentorship and research experience integrate underrepresented minorities into STEM careers. CBE Life Sci Educ 17:ar9, 2018
