INTRODUCTION
The overarching goal of precision oncology (PO) is to tailor medical care to the specific characteristics of each patient's cancer, allowing for more efficacious and less toxic treatments. Currently, PO optimizes treatment outcomes by leveraging comprehensive genomic profiling, matching this to genomically targeted therapies or immunotherapies. Beyond this, PO encompasses the continuum of transforming cancer care not only by offering treatments on the basis of molecular diagnostics or biomarkers but also through early detection and precision prevention., Recognizing the importance of tackling cancer at its early stages via precision prevention, the scope of the discussion below primarily emphasizes the implementation of PO in the recurrent/metastatic setting in solid tumors.
The merits and challenges of PO are well-documented in the literature, demonstrating not only its huge abilities to improve care but also the wide disparities that exist in access and the uneven take-up of this potential across the world.
On a global scale, the adoption and formulation of policies related to PO exhibit significant diversity. More affluent nations showcase notable progress in implementing PO practices, whereas in other regions, the uptake has been comparatively sluggish, except for some noteworthy exceptions. The pace of policy discourse is following distinct trajectories, influenced by the unique circumstances prevailing in individual countries or regions. The harmonization of diagnostic precision, targeted treatment, and the requisites of funding, infrastructure, and expertise remains an ongoing and evolving endeavor worldwide. Nevertheless, even within wealthier regions, advancements in PO often encounter impediments. The scope of progress is confined by the feasibility of actionable and druggable conditions. In addition, challenges arise from the fragmented nature of regulatory and health systems, characterized by siloed thinking. The uptake of innovation is also hindered by hesitancy, contributing to the complexities faced in the advancement of PO within these more affluent areas., However, the assessment of its merits cannot be precisely determined by gauging the average benefit across all patients. Its success can be remarkable under specific conditions, even when it is not universally accessible for all types of cancers and patients. The extraordinary outcomes in certain scenarios defy a simplistic evaluation on the basis of overall averages. Better information, dissemination of practices, standardization, and use of algorithmic approaches could improve the delivery of PO, with positive implications both for implementation and for policy.,
In this perspective, we present an overview of the current status of PO in various regions. We delineate the essential prerequisites for the successful integration of PO into health systems in the global landscape in local setting section and highlight the key challenges and barriers, including clinical, societal, and patient aspects. Finally, we provide a list of priorities for the broader success of PO, emphasizing the need for customization to local ecosystems. This involves adapting to local maturity levels in crucial domains—particularly governance, infrastructure, and financial aspects related to reimbursement, regulatory issues, health technology assessment (HTA), and value recognition.
BARRIERS, ROADBLOCKS, AND CHALLENGES TO IMPLEMENTATION
The standard flow envisaged is that an oncologist seeking useful patient data requests a genomic test of a recurrent or metastatic tumor. The request for a genetic test is sent to a certified laboratory either locally or centrally, which conducts the test using standardized procedures and returns a report with identified variants which can be added to the patient's electronic health record, besides actionable driver alterations like BRAF V600E or NTRK fusions and variants of unknown significance. On the basis of this profile, the physician seeks to find a matched therapy for the biomarker alteration in the tumor. Overcoming the major barrier in progress at the clinic level is dependent on helping physicians to order a test, to interpret molecular test results, and to recommend consequent care, as well as ensuring access to the relevant targeted agents. Below, we discuss the wide variation across different countries in terms of implementation of PO.
There are overarching challenges, but there are also specific barriers, summarized in Table 1, that include clinical challenges, infrastructure and resources, regulatory/ethical issues/data privacy and security, technological challenges, integration with health care systems/collaboration, data sharing, and—most importantly—financial and economic barriers.
TABLE 1
Barriers to Clinical Implementation of PO in the Real-World Setting
| Challenges | Issues |
|---|---|
| Clinical challenges | Physician/clinician training: Training health care professionals to interpret and apply genomic information in clinical settings. For example, What test to order? No treatment pathway, No trials Clinical decision support: Developing and integrating effective Clinical Decision Support Systems |
| Infrastructure and resources | Laboratory infrastructure: Establishing and maintaining advanced molecular diagnostic laboratories Data storage and management: Managing the vast amount of genomic and clinical data generated |
| Regulatory/ethical issues/data privacy and security | Regulatory approval: Navigating regulatory pathways for approval of precision therapies Ethical considerations: Addressing ethical concerns related to patient consent, data privacy, and potential misuse of genetic information Genomic data security and patient confidentiality: Implementing robust security measures to protect sensitive genomic information. Ensuring patient confidentiality |
| Technological challenges/integration with health care systems/collaboration and data sharing | Data integration: Difficulty in integrating diverse and large-scale genomic data into clinical workflows Analytical complexity: Dealing with the complexity of analyzing genomic, proteomic, and other omics data for individualized treatment decisions Interoperability: Facilitating interoperability between different health care systems and institutions International collaboration: Overcoming challenges related to data sharing and collaboration across borders EHR integration: Integrating genomic data into EHRs for seamless patient care Standardization: Establishing standards for data formats and interoperability |
| Financial and economic barriers | High costs: Covering the costs associated with genetic testing, personalized therapies, and data management Reimbursement models: Developing sustainable reimbursement models for PO |
| Patient-related challenges | Informed consent: Ensuring that patients understand the implications of genetic testing and personalized treatments Health literacy: Addressing disparities in health literacy that may affect patient understanding and participation |
| Awareness and education | Public awareness: Increasing awareness among the general public about the benefits and limitations of PO Clinician awareness: Ensuring that clinicians are informed and up-to-date on the latest developments |
Clinical
Lack of relevant health care provider education and patient awareness can be a major barrier, even leading to patients wanting to start treatment as soon as possible, rather than waiting for test results. Physicians need primary education in oncogenomics and other emerging omics technologies, so that they know the indications to test and treat as appropriate. A comprehensive approach that increases patient awareness, including of ongoing innovation, is crucial to overcome these barriers and advance the implementation of PO in routine clinical practice.
Infrastructure and Resources
Implementing PO, particularly within the context of Molecular Tumor Boards (MTBs) and Clinical Decision Support Systems (CDSS) for genotype-matched clinical trials, encounters multifaceted challenges related to infrastructure and resources. The complexities of data integration and interoperability are evident as molecular data, clinical records, and pertinent information arrive from diverse sources in varying formats, necessitating the integration and harmonization of heterogeneous data for effective decision making. Infrastructure requirements, including the need for high-performance computing and substantial storage capacity for genomic data, pose financial and logistical challenges. Expertise and training issues arise with the necessity of building and sustaining a skilled team incorporating molecular biologists, bioinformaticians, and clinical geneticists, demanding significant time and resources. Interdisciplinary collaboration among oncologists, geneticists, pathologists, and data scientists is essential but presents a notable challenge in establishing effective communication and workflows in many geographies. Standardization and quality control are critical, encompassing the need for accurate molecular data and standardized interpretation to prevent confusion among health care providers.
Regulatory/Ethical Issues
Regulatory and ethical considerations, such as ensuring data privacy, security, and obtaining approvals for new technologies, add further layers of complexity. The financial aspects of PO, involving costly molecular profiling and therapies, require health care institutions to allocate substantial resources. Integrating MTBs and CDSS seamlessly into existing clinical workflows is pivotal, yet resistance to change and disruptions to established workflows can impede successful integration. Ensuring patient access and diversity in genotype-matched clinical trials is a significant challenge, involving overcoming barriers related to geography, socioeconomic factors, and cultural considerations. Addressing these challenges demands a concerted effort involving health care providers, researchers, technology developers, policymakers, and regulatory bodies, emphasizing the continuous assessment and updating of strategies to keep abreast of advancements in genomics and technology.
Financial and Economic Barriers
Developing reliable novel tests and reproducibly demonstrating value to patients and health care systems are intrinsically difficult. Even when developed, tests linked to a specific treatment may not be used if the associated therapy is not widely authorized or reimbursed. A study by European Alliance for Personalised Medicine (EAPM) noted barriers at the level of health systems in terms of uncertain, selective, and slow reimbursement; inadequate infrastructure for data, biobanks, and research and development; and limited awareness of how precision medicine can be accessed and used. This issue is even more pronounced in low and middle income countries (LMICs) where infrastructure for cancer care, research, and data collection is lacking. Payers hesitate to accept and pay for novel tests—and it is consequently difficult for wide-scale use to emerge that can generate an evidence base to demonstrate the longer-term value of PO. In addition to lack of data showing clinical utility, technical issues of quality control, and an often-inadequate regulatory framework, there is no standalone business model for demonstrating value without also having companion therapy in development, a predicament compounded by a generalized risk aversion mindset among payers.
Data Infrastructure/Data Privacy/Security
Implementing PO introduces numerous challenges in data privacy and security because of the sensitive nature of genomic and health-related information. Genomic data's inherent identifiability poses a significant concern as it has the potential to reveal sensitive details about an individual's unique genetic makeup, necessitating protection against unauthorized reidentification. The collaboration required between health care institutions, research centers, and pharmaceutical companies for advancing PO introduces complexities in securely sharing genomic data while safeguarding patient privacy. Obtaining informed consent for the collection, storage, and sharing of genomic data is crucial, requiring efforts to ensure that patients fully comprehend the implications, given the complexity of genetic concepts. Cybersecurity threats, including the risk of data breaches, are heightened by the attractiveness of genomic data to cybercriminals, necessitating robust measures to prevent unauthorized access and data theft. The challenge of long-term data storage involves establishing secure solutions with appropriate retention policies. Interdisciplinary collaboration in PO mandates secure communication channels among clinicians, researchers, and bioinformaticians to maintain privacy. Regulatory compliance with health data protection regulations such as Health Insurance Portability and Accountability Act and General Data Protection Regulation is critical, demanding careful navigation in advancing research and clinical applications. The ethical use of genomic data, especially for secondary purposes like research or drug development, requires alignment with patients' original consent and ethical considerations. It is essential to ensure that health care professionals involved in PO are well-trained in data privacy and security practices, encompassing an understanding of risks, implementation of secure procedures, and patient education on data privacy. Addressing these challenges necessitates a comprehensive approach involving technology solutions, policy development, and ongoing education. Collaborative efforts among health care institutions, regulatory bodies, and cybersecurity experts are imperative to establish a robust and secure environment for the successful implementation of PO. This, in turn, demands infrastructure in terms of data storage; organizational, physical, digital, and research capacity; and biobanks, and it also demands sufficient oncology workforce and physician/cancer team knowledge. A framework providing this complex array of favorable factors against a background of chronically under-resourced health services is not easy to create and maintain. So, the potential for prevention, diagnosis, and treatment is not universally shared and exploited.
Addressing all these challenges requires collaboration among multistakeholder groups, including health care professionals, researchers, policymakers, health care industry, academic medical institutions, community centers, hospitals, regulatory authorities, governments, and patient advocacy groups. The lack of universal health coverage affects the feasibility of implementing PO in LMICs. The current paradigm in these settings may necessitate radically different approaches (Fig 1).
FIG 1
Barriers to globalizing precision oncology.
STRATEGIES TO ENHANCE IMPLEMENTATION
The pursuit of scientific innovations loses its purpose when it fails to translate into tangible benefits for patients in the real world. The successful introduction of precision medicine informed by advanced genomic testing depends on efforts from stakeholders across the health care ecosystem. Certain principal axes of action suggest themselves immediately. Two glaring examples that highlight this issue are the bottom-down approach used in Italy and the K-master program implemented in South Korea. In both instances, the gap between innovation and practical application underscores the challenges of ensuring that ground-breaking discoveries actually make a meaningful impact on patient care and outcomes. Shown in Table 2 are PO implementation steps in the bottom-up approach used in Italy and the K-Master PO program in South Korea.
TABLE 2
Examples of National PO Initiatives in South Korea and a Model for Strategy Development for PO Implementation From Italy
| K-MASTER South Korean initiative overview and findings |
| What is the K-MASTER? The K-MASTER enterprise embarked on a pioneering mission to compile and characterize the intricate genomic profiles of Korean patients grappling with advanced solid tumors. Leveraging well-established clinical NGS panels, the initiative meticulously captured a spectrum of major genomic aberrations, ranging from SNVs to CNAs and selected structural variations in genes pertinent to cancer |
| How? Since its inception in June 2017, K-MASTER has undertaken prospective clinical sequencing endeavors on a scale encompassing 4,028 Korean patients confronting advanced solid tumors. This concerted effort not only underscored the clinical utility of systematic prospective sequencing but also highlighted its significance in guiding patients toward ideally matched clinical trials for optimized therapeutic outcomes spanning various cancer types. Moreover, this nationwide, multi-institutional collaboration demonstrated the feasibility of such an approach in the real-world clinical setting, involving 55 cancer-treating hospitals and centers |
| Key outcomes and lessons learned: Through an integrated analytical framework, K-MASTER unraveled pivotal driver alterations and etiologies across 24 major cancer lineages among East Asian pan-cancer patients. This endeavor extended its scope beyond regional boundaries, probing the impact of ethnic ancestry on genomic diversity and clinical applicability using large-scale pan-cancer genomic cohorts. Notably, disparities emerged between Eastern and Western populations at both individual genomic event and pathway levels, underscoring the imperative of tailored approaches in PO initiatives. Moving forward, K-MASTER aims to delve deeper into the dynamic interplay between genomic aberrations and therapeutic responses across a diverse array of clinical trials, further amplifying the scope and efficacy of PO in the pursuit of improved patient care |
| Implementation of PO: A statement proposal endorsed by Italian Scientific Societies |
| Italian PO project: In Italy, the implementation of PO has historically followed an autonomous and top-down approach, resulting in inefficiencies and disparities in patient access across the country, mirroring challenges encountered in other Western nations. To address these issues, a collaborative method was tested, involving professionals, scientific societies, and government institutions, aiming to deliver PO innovations to patients through a bottom-up approach. This organizational research project scrutinized PO activities within five HCAs in one Italian region and compared observed challenges with three additional HCAs in other regions of Italy |
| Key outcome: Using validated multiple-step consensus methods, the project yielded 14 statements unanimously approved by the main scientific societies of oncology and pathology at the national level. These statements targeted critical issues hindering PO implementation in clinical practice. The strong professional consensus underlines the urgent need for prompt adoption of these statements within the national health care system, emphasizing the importance of synergistic collaboration among professionals, scientific societies, and health care institutions. It underscores the imperative of establishing uniform solutions for the implementation of innovation within the health care system, fostering equitable access and efficient delivery of PO services to patients nationwide |
We will discuss strategies to improve PO implementation in three major realms: stakeholder engagement, health system (re)organization, and finally generating an evidence base (Fig 2).
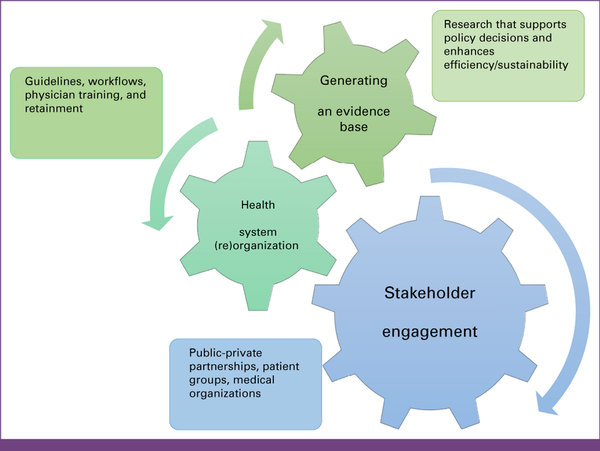
FIG 2
Strategies to improve precision oncology implementation via stakeholder engagement, health system (re)organization, and generating an evidence base.
STAKEHOLDER ENGAGEMENT (PUBLIC-PRIVATE PARTNERSHIPS, PATIENT GROUPS, AND MEDICAL ORGANIZATIONS)
Multistakeholder engagement, incorporating public-private partnerships, patient advocacy groups, and medical organizations, plays a pivotal role in advancing the global implementation of PO. Public-private partnerships are essential components of this collaborative effort, bringing together the resources, expertise, and innovation capabilities of both sectors. By fostering collaborations between government bodies, private companies, and research institutions, these partnerships facilitate the development and dissemination of cutting-edge technologies and treatments in PO. Patient advocacy groups bring a crucial perspective to the table, representing the interests and needs of individuals affected by cancer. Their involvement ensures that the implementation of PO aligns with patient values, preferences, and experiences. This inclusivity contributes to the development of patient-centered approaches, enhancing the overall effectiveness and acceptance of PO solutions.
Medical organizations, including academic research institutions, hospitals, and professional societies, play a role in driving the adoption of PO at a global scale. They contribute by conducting research, disseminating best practices, and providing training and education to health care professionals. The collective expertise of these organizations helps establish standardized guidelines and protocols, fostering a consistent and high-quality implementation of PO across diverse health care settings.
In summary, multistakeholder engagement is a synergistic force that combines the strengths of public-private partnerships, the patient perspective from advocacy groups, and the expertise of medical organizations to enhance global PO implementation. This collaborative approach not only accelerates scientific advancements but also ensures that PO benefits reach a broad spectrum of patients while respecting individual values and preferences.
HEALTH SYSTEM (RE)ORGANIZATION (GUIDELINES, WORKFLOWS, AND PHYSICIAN TRAINING AND RETAINMENT)
To effectively implement PO within a health system, a strategic (re)organization is imperative, involving the development of comprehensive guidelines, streamlined workflows, and targeted physician training and retainment initiatives. Robust guidelines should be established to standardize protocols, encourage interdisciplinary collaboration, and ensure regular updates to incorporate the latest PO advancements. Workflows need to be carefully designed to seamlessly integrate PO into existing clinical pathways, focusing on key decision points and efficient data management. Patient engagement should be prioritized within workflows, fostering informed decision making through effective communication and consent processes.
Physician training plays a pivotal role, necessitating specialized PO-focused programs. Continuous education, practical training through simulations and case studies, and the incorporation of the latest genomic advancements into training curricula are essential components. Simultaneously, physician retainment efforts should recognize and reward professionals actively engaged in PO, providing avenues for career advancement and maintaining a supportive work environment.
In addition, technology integration is crucial, requiring investments in robust information technology infrastructure to support the storage, retrieval, and analysis of large-scale genomic data. Decision support systems should be implemented to assist health care providers in interpreting complex genomic information and making evidence-based treatment decisions.
By addressing these facets comprehensively, a health system can create an environment conducive to successful PO implementation. This approach not only enhances patient outcomes but also contributes to the advancement of personalized oncology, consolidating the health system's position at the forefront of cutting-edge health care practices.
Realizing the full potential of PO necessitates comprehensive efforts, including specialized personnel training; the establishment of robust infrastructure in molecular biology, clinical research, computational biology, and bioinformatics; and the validation of biomarkers in diverse populations and the creation of regional biobanks. To address the dynamic challenges faced by next-generation sequencing (NGS)—such as the lack of standardized procedures, issues in quality management, sequencing workflows, data handling, and analysis, alongside affordability and accessibility concerns—a more agile regulatory framework adapted to local conditions is crucial. This framework should also provide intellectual property protections to ensure quality and streamlined oversight.
Currently, the field of NGS faces disparities in reimbursement for tests, hindering uniform adoption. Encouraging the integration of NGS into health care systems, a key driver for global accessibility to personalized medicine, relies on health care professionals and decision makers evaluating the maturity of NGS practices in their respective countries.
Efforts should extend to educating health care professionals, decision makers, and the general public about precision diagnostics and therapies, emphasizing their merits, current limitations, and future potential. Advocating for a globally acceptable list of minimal clinically actionable alterations for NGS testing in specific diseases, followed by a tiered approach for potential alterations, akin to OncoKB actionable tiers, would standardize NGS testing worldwide and facilitate universal public funding.
GENERATING AN EVIDENCE BASE (RESEARCH THAT SUPPORTS POLICY DECISIONS AND ENHANCES EFFICIENCY/SUSTAINABILITY)
Demonstration of Value
To counter the indifference—even antipathy—of payers to PO, greater efforts are urgently needed to show the immediate utility of its correctly conducted use. The key is to show that it can be—and is—effective for patients in the short term and to develop more compelling studies of how over time, the capabilities of PO in prevention and early detection can reduce long-term health care expenditure, permit better treatment choices, and thus avoid waste of resources.
Every year, Europe spends more than one trillion euros on health care. Roughly one fifth of this amount is being spent on drugs, and half or more in treating patients who will not respond to the drug they receive, at a cost of €100 billion or more per year. Available estimates suggest that only between one quarter (oncology) and one half (many other areas of medicine) of patients respond positively to prescribed drugs. Despite the potential of PO for radical improvements in health and care, governments, shackled by the constant fight to meet ever-growing demands on public resources, have largely overlooked the longer-term advantages of reduced or delayed morbidity and mortality and the elimination of wasteful or inappropriate therapy. Health care and life expectancy continue to differ widely across countries and regions, a situation that will not change unless motivated governance is brought to bear. It is important to first acknowledge the complexity of estimating the proportion of patients with advanced cancer who benefit from PO applications. One approach to estimating the benefit of PO applications is to examine specific tumor types where such treatments have demonstrated efficacy. For instance, in certain subtypes of non–small cell lung cancer, breast cancer, or melanoma, targeted therapies have shown significant improvements in outcomes for a subset of patients with specific genetic mutations or biomarkers. While objective numbers may not be readily available for all tumor types, efforts to extrapolate from available data can provide valuable insights into the impact of PO on patient outcomes.
Enhanced evidence holds the potential to guide health care authorities in critical funding decisions. Molecular tumor boards emerge as valuable tools, offering insights into molecular reports and facilitating the implementation of tailored therapies. The EAPM asserts that successful precision medicine implementation hinges on comprehensive national plans, alignment across HTA and regulatory realms, and robust infrastructure encompassing data storage, digital health, research, and biobanks. Furthermore, sustaining a proficient workforce, spanning clinicians, laboratory medicine experts, and scientists, is imperative. Collaboration among health professionals and patient advocates; strengthened connections between regulators, payers, and public health authorities; and secure data sharing adhering to robust patient privacy laws and ethical guidelines are indispensable. Standard operating procedures ensure quality control. In the United States, proposed solutions involve potential federal-level funding models independent of pharmaceutical companies, coupled with more streamlined data sharing. ASCO recommends software applications to link patient data systematically, facilitating queries on genomic knowledge bases.
According to the study by Horgan et al, sharing information on the response to targeted drugs among patients with rare mutations or complex mutational patterns is crucial for advancing PO. Fragmented health informatics systems pose challenges to comprehensive real-world analysis of new treatments and accurate estimates of the impact of evolving cancer technologies on health systems. The European Commission emphasizes that a digital health agenda, integral to advancing cancer research and real-world applications, necessitates proficient bioinformatic, statistical, and advanced data analytics skills and frameworks.
BRIDGING THE DIVIDE BETWEEN HIGH-INCOME COUNTRIES AND LMICs FOR PO
As has been discussed, the landscape of cancer research and PO is markedly imbalanced, heavily favoring high-income countries while neglecting the unique challenges faced by LMICs. This disparity underscores the urgent need to recalibrate the PO focus of cancer research to be more inclusive and relevant to LMICs.
Addressing the gaps in cancer research within LMICs is imperative to foster region-specific advancements. It demands a fundamental re-evaluation of existing approaches to confront the pressing issues in cancer care within these regions. This shift in perspective is essential for achieving meaningful progress and improving outcomes for individuals affected by cancer in LMICs.
To bridge the divide between high-income and lower-middle–income countries in PO, several potential solutions can be explored. These solutions aim to improve access to anticancer drugs, enhance coordination of cancer care, and increase the availability of specialized cancer centers.
One key step is to ensure that essential oncology drugs are included in the national essential medicine lists of lower-middle–income countries. This inclusion would prioritize these drugs and facilitate their availability and affordability. In addition, improving price transparency in the pharmaceutical industry can help lower-middle–income countries negotiate fair prices for oncology drugs, reducing the financial burden on patients and health care systems. Implementing a pooled drug procurement system, where countries collectively negotiate drug prices and purchase medications in bulk, can further drive down costs and improve access to oncology drugs. This approach allows for greater purchasing power and can benefit LMICs by ensuring a stable supply of affordable medications. Increasing the availability of dedicated cancer centers in lower-middle–income countries is another crucial step. These centers should be equipped with state-of-the-art technologies and staffed by trained health care professionals. By centralizing cancer care, patients can benefit from comprehensive services, including molecular profiling, genetic testing, and targeted therapies. Simplifying the drug reimbursement process can also facilitate access to PO. Streamlining administrative procedures and reducing bureaucratic hurdles can expedite the approval and reimbursement of oncology drugs, allowing patients to receive timely and appropriate treatment. Furthermore, increasing the number of clinical trials in lower-middle–income countries can provide patients with access to innovative treatments and contribute to the advancement of PO. Collaborations between high-income and lower-middle–income countries and international research partnerships can help expand clinical trial opportunities and ensure that the benefits of PO are accessible to all. Improving the coordination of cancer care is vital to optimize treatment outcomes. This involves establishing effective referral systems, enhancing communication between health care providers, and promoting multidisciplinary approaches to cancer management. By fostering collaboration and knowledge exchange among health care professionals, lower-middle–income countries can improve the quality and efficiency of cancer care delivery. In conclusion, bridging the divide between high-income and lower-middle–income countries in terms of access to PO requires comprehensive solutions. These solutions include adding anticancer drugs to national essential medicine lists, improving price transparency, implementing pooled drug procurement systems, and increasing the availability of dedicated cancer centers. Simplifying the drug reimbursement process, increasing the number of clinical trials, and improving the coordination of cancer care are also crucial steps. By implementing these measures, we can strive for more equitable access to PO and reduce the disparities in cancer outcomes between countries.
The overarching milestone for initiating PO programs should include establishing basic diagnostic and treatment infrastructure, ensuring availability and affordability of standard cancer care, training health care professionals in genomics and precision medicine, and finally developing or adopting guidelines for the implementation of PO. It is important to prioritize basic health care needs before embarking on PO initiatives, particularly in low-income settings. It is very important that PO programs use value frameworks such as the ASCO Value Framework and the European Society for Medical Oncology Magnitude of Clinical Benefit Scale for evaluating and incorporating PO interventions. These frameworks can help countries assess the cost-effectiveness and clinical benefits of PO interventions.
DISCUSSION
In conclusion, unlocking the full potential of PO requires a paradigm-shifting journey. This transformative process involves not only a profound restructuring of clinical research and clinical care infrastructure but also the establishment of a dynamic and adaptable system capable of navigating the rapid advancements in cancer research. Recognizing that innovation does not journey seamlessly to the patient's bedside within an ideal timeframe, it becomes crucial for the oncology community to take proactive measures. This involves not only cultivating fresh capabilities in technology, medicine, and diagnostics but also fostering innovative and unconventional approaches to thinking and delivering care to patients. This comprehensive overhaul is essential to integrate PO effectively, bridging the gap from laboratory breakthroughs to clinical application. The overarching objective is to transcend the limitations inherent in specialized academic domains, predominantly concentrated in affluent nations. We need to think globally and act locally. This ensures a more inclusive, equitable, and accessible future for patients with cancer globally. The aspiration is to shape a global landscape where the benefits of precision medicine extend to all, painting a brighter outlook for individuals grappling with the challenges of cancer on a global scale.
AUTHOR CONTRIBUTIONS
Conception and design: Denis Horgan, Marcel Tanner, David Thomas, Surbhi Grover, Rodrigo Dienstmann, Woong-Yang Park, Brigette Ma, Marc Van den Bulcke, John Longshore, Vivek Subbiah
Administrative support: Marcel Tanner, Charu Aggarwal, Naureen Starling, Vivek Subbiah
Provision of study materials or patients: Rodrigo Dienstmann, Woong-Yang Park, Lillian L. Siu, Brigette Ma, Umberto Malapelle
Collection and assembly of data: Denis Horgan, David Thomas, Lina Basel-Salmon, Rodrigo Dienstmann, Hadi Mohamad Abu Rasheed, Brigette Ma, Rocío Ortiz-López, Silvia Castillo Taucher, Naureen Starling, Vivek Subbiah
Data analysis and interpretation: Denis Horgan, Marcel Tanner, Charu Aggarwal, David Thomas, Surbhi Grover, Rodrigo Dienstmann, Tira Jing Ying Tan, Lillian L. Siu, Brigette Ma, Andrea Ferris, Naureen Starling, Umberto Malapelle, John Longshore, Hugo Alberto Barrera Saldaña, Vivek Subbiah
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Marcel Tanner
Stock and Other Ownership Interests: Novartis, Roche, Nestle Health Science (I)
Charu Aggarwal
Consulting or Advisory Role: AstraZeneca, Daiichi Sankyo/AstraZeneca, Sanofi/Regeneron, Pfizer, Boehringer Ingelheim, Takeda, Arcus Biosciences, Gilead Sciences, Novocure, AbbVie
Speakers' Bureau: AstraZeneca (I)
Research Funding: Merck Sharp & Dohme (Inst), AstraZeneca/MedImmune (Inst), Daiichi Sankyo/AstraZeneca (Inst), Loxo@Lilly (Inst), Candel Therapeutics (Inst)
David Thomas
Employment: Australian Unity, Omico
Honoraria: Roche
Research Funding: Pfizer, Amgen, AstraZeneca, Elevation Oncology, Roche, Bayer, Microba (Inst), Seagen (Inst), Sun Pharma (Inst), Lilly (Inst), George Clinical (Inst), InterVenn Biosciences
Travel, Accommodations, Expenses: Amgen
Surbhi Grover
Consulting or Advisory Role: GenesisCare
Rodrigo Dienstmann
Employment: Oncoclínicas
Stock and Other Ownership Interests: Trialing Health
Consulting or Advisory Role: Roche, Foundation Medicine, AstraZeneca
Speakers' Bureau: Roche, Ipsen, Sanofi, MSD Oncology, Servier, Amgen, Libbs, AstraZeneca, Lilly, GlaxoSmithKline, Janssen Oncology, Takeda, Gilead Sciences, Pfizer
Research Funding: Merck, Novartis (Inst), Daiichi Sankyo/AstraZeneca (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), Merck Serono (Inst)
Tira Jing Ying Tan
Honoraria: AstraZeneca, MSD Oncology
Consulting or Advisory Role: AstraZeneca, MSD Oncology, Everest Medicine, Daiichi Sankyo/AstraZeneca, Gilead Sciences
Research Funding: Bayer (Inst), Novartis (Inst), AstraZeneca (Inst), Seagen (Inst), Roche/Genentech (Inst), Daiichi Sankyo/AstraZeneca (Inst), Sanofi (Inst), Lilly (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Pfizer
Woong-Yang Park
Employment: Geninus
Leadership: Geninus
Stock and Other Ownership Interests: Geninus
Lillian L. Siu
Leadership: Treadwell Therapeutics (I)
Stock and Other Ownership Interests: Agios (I)
Consulting or Advisory Role: Merck, Roche, Voronoi Health Analytics, GlaxoSmithKline, Seagen, Arvinas, Navire, Relay Therapeutics, Daiichi Sankyo/UCB Japan, Amgen, Pangea, Tubulis GmbH, Medicenna, LTZ Therapeutics, Marengo Therapeutics, AstraZeneca, Nerviano Medical Sciences, Incyte, Gilead Sciences
Research Funding: Bristol Myers Squibb (Inst), Genentech/Roche (Inst), GlaxoSmithKline (Inst), Merck (Inst), Novartis (Inst), Pfizer (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), Bayer (Inst), Amgen (Inst), Astellas Pharma (Inst), AbbVie (Inst), EMD Serono (Inst), 23andMe (Inst), Daiichi Sankyo/UCB Japan (Inst), Gilead Sciences (Inst), Marengo Therapeutics (Inst), Incyte (Inst), LegoChem Biosciences (Inst), Loxo/Lilly (Inst), Medicenna (Inst), Takara Bio (Inst)
Brigette Ma
Honoraria: Taiho Pharmaceutical, Merck Serono, Daiichi Sankyo, AstraZeneca, MSD, Y-Biologics, TopAlliance BioSciences Inc
Consulting or Advisory Role: Merck Sharp & Dohme, Y-Biologics, Viracta Therapeutics, Alentis Therapeutics, Pierre Fabre, AstraZeneca, Bristol Myers Squibb
Research Funding: Merck Serono
Travel, Accommodations, Expenses: Merck Serono
Andrea Ferris
Stock and Other Ownership Interests: JNJ
Travel, Accommodations, Expenses: Amgen
Naureen Starling
Honoraria: Merck Serono, Servier, Pierre Fabre, MSD Oncology, Seagen, GlaxoSmithKline, Merck, BMS, AstraZeneca, Daiichi Sankyo, Natera, Astellas Pharma, Tempus
Consulting or Advisory Role: MSD Oncology, GlaxoSmithKline, Gilead Sciences, Seagen, Janssen, Takeda, Moderna Therapeutics, Bristol Myers Squibb, AstraZeneca
Research Funding: Gilead Sciences (Inst)
Travel, Accommodations, Expenses: MSD Oncology, Guardant Health, Servier, GlaxoSmithKline, Takeda, Bristol Myers Squibb
Uncompensated Relationships: Guardant Health
Umberto Malapelle
Employment: Roche (I)
Consulting or Advisory Role: Boehringer Ingelheim, Roche, MSD, Amgen, Thermo Fisher Scientific, Lilly, Diaceutics, GlaxoSmithKline, Merck, AstraZeneca
Speakers' Bureau: Boehringer Ingelheim, AstraZeneca, Roche, MSD, Amgen, Merck, Thermo Fisher Scientific, Lilly, Diaceutics, GlaxoSmithKline
John Longshore
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Hugo Alberto Barrera Saldaña
Research Funding: Laboratorios Columbia SA (México)
Vivek Subbiah
Consulting or Advisory Role: Loxo/Lilly, Relay Therapeutics (Inst), Pfizer (Inst), Roche (Inst), Bayer (Inst), Incyte (Inst), Novartis (Inst), Pheon Therapeutics (Inst), AbbVie (Inst), Illumina, AADi, Foundation Medicine
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), NanoCarrier (Inst), Northwest Biotherapeutics (Inst), Genentech/Roche (Inst), Berg Pharma (Inst), Bayer (Inst), Incyte (Inst), Fujifilm (Inst), PharmaMar (Inst), D3 Oncology Solutions (Inst), Pfizer (Inst), Amgen (Inst), AbbVie (Inst), Multivir (Inst), Blueprint Medicines (Inst), Loxo (Inst), Vegenics (Inst), Takeda (Inst), Alfasigma (Inst), Agensys (Inst), Idera (Inst), Boston Biomedical (Inst), Inhibrx (Inst), Exelixis (Inst), Turning Point Therapeutics (Inst), Relay Therapeutics (Inst)
Other Relationship: Medscape, Clinical Care Options
No other potential conflicts of interest were reported.
REFERENCES
1.
Moyers JT, Subbiah V: Think globally, act locally: Globalizing precision oncology. Cancer Discov 12:886-888, 20222.
Malone ER, Oliva M, Sabatini PJB, et al.: Molecular profiling for precision cancer therapies. Genome Med 12:8, 20203.
Yang J, Nittala MR, Velazquez AE, et al.: An overview of the use of precision population medicine in cancer care: First of a series. Cureus 15:e37889, 20234.
Baird AM, Westphalen CB, Blum S, et al.: How can we deliver on the promise of precision medicine in oncology and beyond? A practical roadmap for action. Health Sci Rep 6:e1349, 20235.
Knott T, Creeden J, Horbach B, et al.: Stakeholders' expectations of precision medicine: A qualitative study to identify areas of (mis)alignment. Health Sci Rep 6:e1428, 20236.
Fountzilas E, Tsimberidou AM, Vo HH, et al.: Clinical trial design in the era of precision medicine. Genome Med 14:101, 20227.
Kruk ME, Gage AD, Arsenault C, et al.: High-quality health systems in the sustainable development goals era: Time for a revolution. Lancet Glob Health 6:e1196-e1252, 20188.
Abernethy A, Adams L, Barrett M, et al.: The promise of digital health: Then, now, and the future. NAM Perspect 6, 20229.
Horgan D, Borisch B, Richer E, et al.: Propelling health care into the twenties. Biomed Hub 5:15-67, 202010.
Krzyszczyk P, Acevedo A, Davidoff EJ, et al.: The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci) 6:79-100, 201811.
Nedungadi P, Iyer A, Gutjahr G, et al.: Data-driven methods for advancing precision oncology. Curr Pharmacol Rep 4:145-156, 201812.
Chen Y: Health technology assessment and economic evaluation: Is it applicable for the traditional medicine? Integr Med Res 11:100756, 202213.
Martinez-Martin N, Magnus D: Privacy and ethical challenges in next-generation sequencing. Expert Rev Precis Med Drug Dev 4:95-104, 201914.
Whitcomb DC: Barriers and research priorities for implementing precision medicine. Pancreas 48:1246-1249, 201915.
Tamborero D, Dienstmann R, Rachid MH, et al.: The Molecular Tumor Board Portal supports clinical decisions and automated reporting for precision oncology. Nat Cancer 3:251-261, 202216.
Horgan D, Baker M, Riegman P, et al.: Personalised medicine—Bringing innovation to the healthcare system. Biomed Hub 2:16-21, 2017 (suppl 1)17.
Pereno A, Eriksson D: A multi-stakeholder perspective on sustainable healthcare: From 2030 onwards. Futures 122:102605, 202018.
OECD: Public health genomics in Korea, 2020. https://www.oecd-ilibrary.org/sites/3b1ee34f-en/index.html?itemId=/content/component/3b1ee34f-en19.
Fundytus A, Sengar M, Lombe D, et al.: Access to cancer medicines deemed essential by oncologists in 82 countries: An international, cross-sectional survey. Lancet Oncol 22:1367-1377, 202120.
Warner JL, Prasad I, Bennett M, et al.: SMART cancer navigator: A framework for implementing ASCO workshop recommendations to enable precision cancer medicine. JCO Precis Oncol 10.1200/PO.17.0029221.
Horgan D, Curigliano G, Riess O, et al.: Identifying the steps required to effectively implement next-generation sequencing in oncology at a national level in Europe. J Pers Med 12:72, 202222.
European Union: Commission staff working document impact assessment report accompanying the document proposal for a regulation of the European Parliament and of the Council on the European Health Data Space, 2022. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52022SC013123.
Pramesh CS, Badwe RA, Bhoo-Pathy N, et al.: Priorities for cancer research in low- and middle-income countries: A global perspective. Nat Med 28:649-657, 2022
