INTRODUCTION
Respiratory syncytial virus (RSV) infection is a major cause of disease and death in young children globally []. In the United States, RSV infection is the leading cause of infant hospitalization [, ].
Preterm infants or those with comorbidities like chronic lung disease (CLD) or hemodynamically significant congenital heart disease (HS-CHD) experience higher rates of RSV-related hospitalization throughout their first year of life (FYOL) []. Recommendations for administering RSV pre-exposure prophylaxis (ie, palivizumab) reflect heightened risk in these infant subgroups []. However, full-term infants without comorbidities account for the majority [] of infants with medically attended (MA) RSV (up to 75% overall and 70% of hospitalizations over several recent RSV seasons in the United States []) during their first RSV season.
Much like influenza, RSV transmission follows seasonal outbreak patterns in which cases typically accumulate during the winter respiratory disease season. Rates in the northern hemisphere spike between fall and early spring, before subsiding again over the summer months []. Both the timing and magnitude of seasonal outbreaks vary geographically []. Month of birth determines the age at which infants pass through their first RSV season: an infant born at the onset of their first RSV season will spend their earliest months of life exposed to circulating RSV, whereas an infant born just after RSV season offset will experience heightened exposure at an older age [, ]. This timing of exposure is clinically significant because infants’ risk of severe RSV-related complications peaks in their first weeks of life and wanes as they age [, , ]. Designing effective population-level prevention strategies requires a detailed understanding of the interplay between RSV dynamics, disease severity, and healthcare utilization [], as well as clinically and epidemiologically relevant demographic patterns.
In this analysis of three large insurance claims databases, we estimated the risk of MA RSV lower respiratory tract infection (LRTI) among infants in the United States during their first RSV season, stratified by birth month and comorbidity status. We also compared the risk among infants during their first RSV reason to that through their FYOL.
METHODS
We have provided detailed descriptions of the creation of the target population and birth cohorts and the classification of comorbidity groups in a prior publication [], with relevant details reiterated here.
Main Analysis
We estimated the risk of MA RSV LRTI and classified RSV episodes (see case definition below) according to the highest level of care received, defined in least-to-greatest intensity as outpatient, emergency department (ED), or inpatient hospitalization. We recorded the first RSV event during infants’ first RSV season and described the occurrences of these outcomes by birth month and comorbidity group.
Birth Cohorts
We built three retrospective cohorts of infants born in the United States between July 1, 2016 and February 29, 2020 in each of three deidentified insurance claims databases: MarketScan Commercial® Multi-State Claims and Encounters (CCAE), MarketScan Multi-State® Medicaid Database (MDCD), and Optum’s de-identified Clinformatics® Data Mart Database (CDM). Infants were eligible for inclusion if they were born during this time window and were discharged alive from their birth hospitalization prior to the end of their first Census division-specific RSV season (Supplementary Methods). Analyses of first-season RSV occurrence include a small number of infants who were at risk for a short period of time, or not at all, because they were born near the end of the season. Nonetheless, these infants were part of the birth cohort(s) of interest and remain in the denominator for the first-season analyses.
RSV Case Definition
We used two definitions of the RSV index diagnosis that triggered the start of an MA RSV LRTI episode. Lack of systematic laboratory testing for RSV among children necessitated that we rely on diagnostic code-based proxies to assign these definitions to the index diagnosis. The specific definition used International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes B974, J121, J205, and J210, all of which name RSV explicitly. Code B974, when identified in the outpatient or ED setting, must have occurred within ±5 days of another qualifying respiratory diagnosis (Supplementary Table 1). The sensitive definition included all codes used for the specific definition plus 2 codes for unspecified bronchiolitis (J218, J219).
The specific definition assumes that all unspecified bronchiolitis is unrelated to RSV, while the sensitive definition assumes all unspecified bronchiolitis is RSV-related. Given the extreme assumptions of each definition, we consider their usage in tandem to provide informal bounds on estimates of RSV risk. (Approximately 60% of unspecified bronchiolitis diagnoses among children may be RSV-related [].)
For both RSV index diagnosis definitions, we classified RSV events by the highest level of care (Supplementary Table 2) attached to a sensitive RSV diagnosis in the 0–7 days following the index diagnosis date (day 0). This procedure encodes the assumption that sensitive diagnoses occurring shortly after a specific RSV diagnosis are related to that specific diagnosis. See [] and Supplementary Table 1 for additional information on how we classified RSV diagnoses. The highest level of care can be an indicator for the severity of an MA RSV LRTI event. We focused on the first RSV event because second infections during the same season are rare.
Birth Month and Comorbidity Groups
We assigned each infant a birth month based on their date of birth (CCAE, MDCD) or their birth hospitalization admission date (CDM). We classified infants as belonging to one of three mutually exclusive comorbidity groups defined by gestational age and select comorbidities: (1) healthy term, (2) palivizumab-eligible, and (3) other comorbidities (but not palivizumab-eligible) [].
An infant met the criteria for group A if they lacked ICD-10-CM or diagnosis-related group (DRG) codes indicating preterm birth and had no relevant comorbidities.
An infant in group B met any of the following criteria: (1) gestational age < 29 weeks; (2) gestational age 29–31 weeks, plus CLD; (3) gestational age ≥ 29 weeks, plus HS-CHD; or (4) DRG-indicated preterm birth with a missing gestational age but with CLD or HS-CHD (or both).
An infant met the criteria for group C if they met any of the following criteria: (1) a gestational age between 29 and 31 weeks but without CLD or HS-CHD; (2) a gestational age between 32 and 36 weeks but without HS-CHD; (3) DRG-indicated preterm with an unknown gestational age and neither CLD nor HS-CHD; and (4) a gestational age of at least 37 weeks with relevant comorbid conditions but not HS-CHD. Supplementary Table 4 lists the ICD-10-CM codes used to classify CLD and HS-CHD. For gestational age classification criteria, see Supplementary Table 3.
Secondary Analysis
In our secondary analysis, we compared the risk of MA RSV LRTI during infants’ first RSV season to that of their FYOL. We truncated the birth cohorts for both follow-up periods on February 28, 2019, in order to allow infants the chance to accrue a full year of follow-up in the FYOL analysis. For these infants, we recorded the first RSV event occurring from birth hospitalization discharge through the 365th day of life. We assigned infants to (potentially) updated comorbidity group and covariate categories, based on diagnoses through the FYOL.
Statistical Analysis
We conducted all statistical analyses in R [].
Follow-up
Infants were followed from the date of birth to hospitalization discharge. In the main analysis, we followed infants until they had an RSV event, disenrolled from insurance, or reached the end of their first RSV season (administrative censoring), whichever occurred first. In the secondary analysis, we followed infants until they had an RSV event, disenrolled from insurance, or reached the end of their FYOL, whichever occurred first.
We described loss of insurance eligibility by birth year, sex, census division, comorbidity group, low birth weight, gestational age, plan type, MA RSV LRTI pre-first RSV season, HS-CHD, CLD, any chronic condition (including CHD or CLD; Supplementary Table 4). We treated all variables as categorical, using the levels depicted in Table 1.
Inverse Probability Weights
We considered loss of insurance eligibility a source of potentially informative loss to follow-up (LTFU) and estimated stabilized inverse probability of censoring weights (IPCW) to minimize selection bias in all estimates of RSV risk []. Weight denominators represented the probability of an infant’s maintaining insurance eligibility through a given follow-up period, conditional on covariates assumed to influence both loss of insurance eligibility and RSV risk. We estimated weight numerators as the probability of an infant’s maintaining insurance eligibility through the end of a given follow-up period, conditional on the stratification variables (see Supplementary Methods). We described the distributions of sample characteristics by LTFU and calculated standardized mean differences to contrast censored infants with those included in the analytic sample (Table 1, Supplementary Figure 1).
Outcome Estimates
We fit saturated marginal structural logistic regression models (R survey package []) to estimate the risk of MA RSV LRTI within each stratum of interest (Supplementary Methods). We present risks (ie, percentages) and 95% confidence intervals.
RESULTS
Sample Characteristics
For simplicity, we report analytic sample characteristics in the main analysis under the sensitive RSV index diagnosis (Table 1, Supplementary Figure 1). Because each alternative RSV definition, birth window, and follow-up period implies a different analytic sample, we summarize the differences between the analytic samples in Supplementary Figures 2–4.
We identified 580,975; 948,882; and 430,036 infants eligible for inclusion in the CCAE, MDCD, and CDM databases, respectively. Of these, 90%, 95%, and 65% were observed through the end of the follow-up period and retained in the analytic sample. Loss to follow-up varied by birth year, birth month, and the presence of a preseason MA RSV LRTI event.
Across insurance claims databases, approximately 80% of infants were classified as healthy term infants, 3% as palivizumab-eligible, and the rest as having other comorbidities. The analytic samples were also similar with respect to female sex (48.4–48.8%), low birth weight (4.6–6.6%), CLD (0.4–0.7%), HS-CHD (2.2–2.4%), and any chronic condition (7.8–10.5%). Infants were least likely to be born April through June.
Comparing the commercial databases (CCAE, CDM), the analytic samples differed in the distributions of Census division and insurance plan type. The three most common Census divisions in the CCAE database were South Atlantic (20.2%), East North Central (18.5%), and Mid Atlantic (16.1%), while in the CDM they were South Atlantic (20.1%), West South Central (16.1%), and East North Central (15.4%). The three most common payer types in CCAE were EPO/PPO (52.3%), CDHP/HDHP (22.1%), and HMO (11.0%), while in the CDM they were POS/POS with capitation (49.1%), CDHP/HDP (30.2%), and EPO/PPO (10.5%).
At the time of their initial first-season MA RSV LRTI event (main analysis follow-up period) under the specific RSV definition, infants had a median age of 19 weeks in the CCAE (IQR: 11–26), 17 in the MDCD (IQR: 10–25), and 17 in the CDM (IQR: 10–25). Infants in comorbidity group A experienced their first RSV event at 18 (IQR: 11–26), 17 (IQR: 10–24), and 17 (IQR: 9–24) weeks in the CCAE, MDCD, and CDM, respectively; infants in comorbidity group B experienced their first RSV event at 21 (IQR: 14–29), 21 (IQR: 14–29), and 20 (IQR: 13–26) weeks; and infants in comorbidity group C experienced their first RSV event at 19 (IQR: 12–27), 18 (IQR: 10–26), and 17 (IQR: 11–25) weeks.
Under the sensitive RSV definition, infants at their initial first-season MA RSV LRTI event had a median age of 20 weeks in the CCAE (IQR: 14–27), 19 in the MDCD (IQR: 12–26), and 19 in the CDM (IQR: 12–26). Infants in comorbidity group A experienced their first RSV event at 20 (IQR: 14–27), 19 (IQR: 12–26), and 19 (IQR: 12–26) weeks in the CCAE, MDCD, and CDM, respectively; infants in comorbidity group B experienced their first RSV event at22 (IQR: 14–30), 21 (IQR: 13–29), and 21 (IQR: 14–27) weeks; and infants in comorbidity group C experienced their first RSV event at 21 (IQR: 14–28), 19 (IQR: 12–27), and 20 (IQR: 13–26) weeks.
Risk of MA RSV LRTI by Birth Month
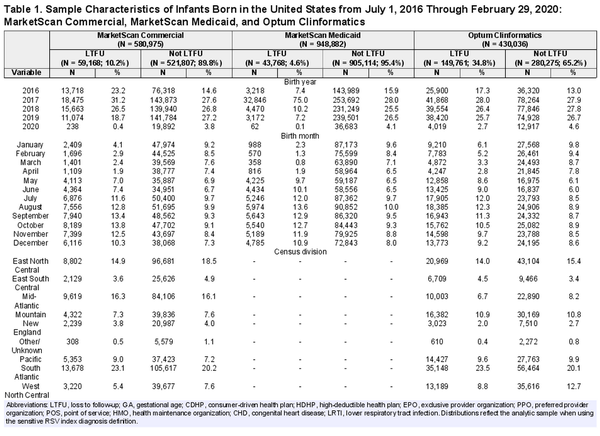
Infants born primarily in the summer months tended to have the highest risk of MA RSV LRTI during their first RSV season (Figure 1). Under the specific RSV definition, this overall risk peaked among July births in CCAE (6%), September births (10%) in MDCD, and June births in CDM (7%). Under the sensitive RSV definition, June births were at the highest risk in CCAE and CDM (19–20%) and July in MDCD (26%). Regardless of database and RSV definition, infants born in March were at the lowest risk of MA RSV LRTI during their first RSV season (0.3–1.9%). In commercial claims, MA RSV LRTI events with an outpatient case as their highest level of care drove changes in overall RSV risk by birth month. In Medicaid, however, outpatient- and ED-coded RSV episodes drove overall risk.
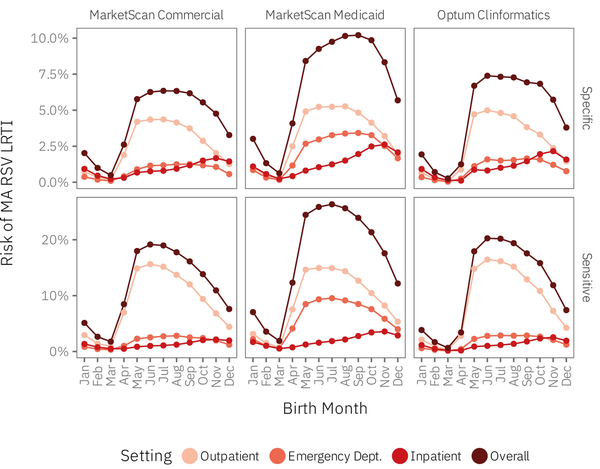
Figure 1
Risk of MA RSV LRTI among infants born in the United States from July 1, 2016 through February 29, 2020 during their first RSV season, by index diagnosis definition, highest level of care, insurance claims database, and calendar month of birth. Note the differing y-axis scales by index diagnosis definition. The data underlying this figure appear in Supplementary Tables 5–7 within the “All comorbidity groups” row group. These tables include 95% confidence limits, suppressed here for readability.
Infants born during their first RSV season had the highest risk of having an MA RSV LRTI event involving hospitalization, a risk that peaked among November births across claims databases (2–4%). Infants born in March or April consistently experienced the lowest risk of RSV-related hospitalization (0.1–0.5%). The risk of inpatient MA RSV LRTI increased progressively for May through November births.
Stratifying estimates by comorbidity group yielded similar results (Figure 2; Supplementary Tables 6–7). The most notable differences concerned palivizumab-eligible infants (comorbidity group B) in the CCAE and MDCD databases. March births in comorbidity group B within these datasets did not experience a drop in the overall risk of MA RSV LRTI of the same magnitude as healthy term infants (comorbidity group A) or those with other comorbidities (comorbidity group C). The RSV risk among comorbidity group B infants reached its nadir at 2.3–2.9% (specific) and 8.2–11.2% (sensitive) versus 0.34–0.40% (specific) and 1.1–1.3% (sensitive) for infants in comorbidity groups A and C. Finally, under the specific RSV definition, comorbidity group C infants in the MDCD database were at highest risk of an MA RSV LRTI event if they were born in October, the latest such peak.
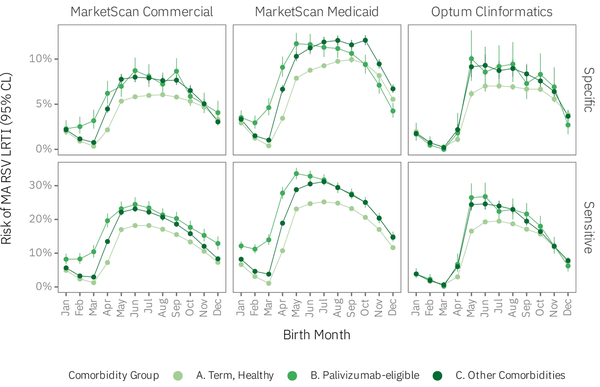
Figure 2
Overall risk of MA RSV LRTI among infants born in the United States from July 1, 2016 through February 29, 2020 during their first RSV season, by index diagnosis definition, insurance claims database, calendar month of birth, and comorbidity group. Note that the y-axis scales differ by index RSV diagnosis definition. The data underlying this figure appear in Supplementary Tables 5–7, within the “All Settings” column. Abbreviations: A, healthy term infants; B, palivizumab-eligible; C, other comorbidities.
Follow-up Through FYOL
Following infants through their FYOL yielded additional MA RSV LRTI events (Figure 3) under both RSV definitions, primarily among infants born during the first half of the year. Whereas infants born roughly during the summer months had the highest risk of a first-season MA RSV LRTI, infants born throughout the year experienced comparable risk of an MA RSV LRTI through their FYOL. The majority of these additional MA RSV LRTI events involved the outpatient or ED setting as the highest level of care, suggested by only a modest change in the risk of RSV hospitalization under the alternative follow-up duration.
Figure 3
Risk of MA RSV LRTI overall and RSV-related inpatient hospitalization among infants born from July 1, 2016 through February 28, 2019 during their first RSV season and through their FYOL, by birth month, RSV definition, and follow-up duration. Note that the y-axis scale for the overall row differs from that of the inpatient row.
DISCUSSION
We found that the overall risk for MA RSV LRTI during infants’ first RSV season peaked among infants born May through September, while the risk of RSV-related inpatient hospitalization was visibly higher among infants born October through December (Figure 1). This result was consistent across the three insurance claims databases (CCAE, MDCD, CDM), though the risk of RSV, both overall and inpatient, proved higher among Medicaid infants than commercial infants [].
Our findings regarding birth month align with studies among European infants and children [, ]. These studies, focusing primarily on RSV hospitalizations, found that bronchiolitis incidence [] and RSV-related LRTI incidence and hospitalization [, , ] peaked among infants born in summer and fall. Jourdain et al. [] developed a risk prediction model for infants and considered all infants born in October and November to be high-risk, regardless of comorbidity status, and considered those born in September and December high-risk if premature or in the case of multiparity.
Studies from the United States also generally agree with our findings. Infants born from November through January have the highest risk of RSV-related hospitalization [, ], regardless of comorbidity profile [] and varying by chronological age at infection []. Another study, which included unspecified bronchiolitis in its RSV definition, estimated birth month-specific hospitalization risks in the National Inpatient Sample that closely mirror our own results under the sensitive RSV definition []. Finally, a prospective cohort of preterm infants (32–35 weeks gestational age) exhibited similar RSV outpatient and hospitalization risk but observed less variation in RSV risk across birth months than we did [, ]. May births had among the highest outpatient RSV risks but a nonmonotonic decay in risk thereafter; RSV hospitalization risk exhibited an almost monotonic increase from May through February. Of note, our studies did not focus on the same target population, which may be an explanation for the difference in estimated RSV dynamics. We also relied on claims data to detect RSV diagnoses, while Simões et al. [, ] employed systematic laboratory testing, suggesting potential under-ascertainment of RSV in our analysis.
Using diagnosis codes to identify RSV in insurance claims, as we have done in the absence of routinely collected laboratory data, may result in under-ascertainment and/or misclassification of outcomes. One study focusing on children younger than 5 in Germany during the RSV season compared “specific” and “sensitive” ICD-10 code sets similar to ours []. The authors found that RSV-specific codes identified only 6% of laboratory-confirmed RSV infections, while augmenting RSV-specific codes with general respiratory illness codes increased the sensitivity to 44% []. RSV-specific codes had 99.8% specificity, compared to 90–91% for the augmented code sets. If these results generalize approximately to US infants, we would expect our results to underestimate the true population risk of RSV, which we already assumed for our “specific” RSV diagnosis definition.
Our results add additional context to the literature by including RSV events involving three healthcare settings: outpatient, ED, and inpatient. By the time infants reach their first RSV season, those born during the summer months are already up to a few months old. While their older age upon entering the RSV season protects against severe RSV-related complications [, , ], they remain at the highest risk of having MA RSV LRTI, usually resulting in outpatient or ED care. Consequently, infants born in the summer months, being older, would be more likely to appear in the outpatient or ED setting, while infants born during the RSV season would be younger, at higher risk for complications, and more likely to be hospitalized.
When we stratified results by comorbidity group, we found that palivizumab-eligible infants were at higher overall risk of MA RSV LRTI and RSV-related hospitalization. While a similar general pattern of RSV risk by birth month appeared for all comorbidity groups, palivizumab-eligible infants born January through April had a visibly higher risk of RSV than healthy term infants and infants with other comorbidities under both the “specific” and “sensitive” index diagnosis definitions within the CCAE and MDCD but not in CDM. While we did not stratify our results by Census division, geographic variation in the timing of the RSV season could alter which birth months produce infants at the highest risk for RSV. The same could be said for RSV severity (episodes involving the ED or hospitalization). Our analysis took partial account of geographic differences in RSV circulation and testing practice by linking infants to their Census division and considering the RSV season start and end dates specific to these divisions [].
We also found that infants who do not acquire RSV during their first season remain at risk of MA RSV LRTI through their FYOL. Whereas infants born during the first half of the year are at substantially lower risk of having a first-season MA RSV LRTI, these same infants’ first-year risk is comparable to that of infants born during the rest of the year (Figure 3). This observation suggests that infants who are not infected during their first RSV season commonly acquire RSV during their second RSV season, and this additional risk typically involves the outpatient and/or ED settings rather than inpatient hospitalizations.
To reduce outpatient and ED use overall, infants born throughout the year may benefit from RSV prevention. Nonetheless, targeted RSV prevention efforts (eg, non-pharmaceutical interventions [] and long-acting monoclonal antibodies [, ]) might consider that RSV severity varies by birth month. To reduce RSV-related hospitalizations, preventing RSV infection in palivizumab-eligible infants, as well as those with other comorbidities, may prove most efficient. Newly available preventive measures such as nirsevimab and maternal RSV vaccination target wider populations of infants [, ]. Trials have demonstrated that nirsevimab reduces MA RSV LRTI and RSV-related hospitalization in both preterm and healthy term infants [, ]. Current guidelines in the United States recommend that nirsevimab be administered shortly before RSV season onset to infants up to 8 months old or, for those at enhanced risk of severe RSV, up to 8–19 months old []. Our results suggest these guidelines protect infants in the period(s) during which they are at the highest risk of MA RSV LRTI. In our data, we saw that infants born in the first few months of the year were at the lowest risk of experiencing a first-season MA RSV LRTI event but that many would go on to experience such an event later in their FYOL. For infants born at the tail end of an RSV season who do not receive nirsevimab shortly after birth, a dose administered prior to the onset of their second season could reduce the incidence of outpatient and ED events.
Early observational data from Luxembourg—where 84% of neonates received a dose of nirsevimab in 2023 and where RSV became a notifiable disease—seem to show population-level impact on RSV-related hospitalizations among children during the 2023–2024 RSV season (through epidemiologic week 52) when compared to the 2022–2023 season []. While encouraging, these results should be interpreted with caution given that the SARS-CoV-2 pandemic disrupted the customary seasonal patterns of RSV circulation [, ]. Long-term observational data based on laboratory-confirmed RSV infections should study changes in MA RSV LRTI incidence that might be associated with nirsevimab or maternal RSV vaccine uptake while accounting for a potential, possibly gradual reversion of RSV transmission to its historic dynamics. Comparing MA RSV LRTI risk by birth month to that of pre-pandemic birth cohorts (such as ours) could reveal changes to which infant subgroups are at the highest risk through their FYOL and, consequently, lead to refined recommendations for optimal timing of interventions. When possible, researchers should also characterize the spectrum of MA RSV LRTI events across the outpatient, ED, and inpatient settings, in order to provide a fuller picture of how healthcare utilization may change and to suggest opportunities for more efficient targeting of pre-exposure prophylaxis. Disentangling the respective effects of each preventive intervention would require individual-level data on nirsevimab administration and linkage to the mother’s medical or prescribing records to ascertain maternal vaccination status.
Limitations
Our study inherits some of the limitations of our prior work []. First, our specific and sensitive RSV definitions may capture different respiratory infections because we used ICD-10-CM codes to identify RSV and other respiratory diagnoses in the absence of definitive laboratory diagnoses. Second, our “palivizumab-eligible” classification relies on ICD-10-CM and DRG codes for gestational age, pregnancy term, and/or qualifying comorbidities, and so, only approximates such eligibility, leading to the potential for misclassification of palivizumab eligibility. Third, we were unable to verify in claims whether infants eligible for palivizumab actually received the indicated prophylaxis. Stratifying “palivizumab-eligible” infants by receipt of palivizumab could have provided valuable information about whether receiving palivizumab alters the relationship between birth month and MA RSV LRTI risk/severity. Fourth, the SARS-CoV-2 pandemic disrupted RSV transmission dynamics worldwide [, ], leading us to end follow-up on February 29, 2020, well before the end of a standard RSV season, in order to restrict inference to customary seasonal epidemics. Fifth, it is difficult to say whether some minor observed differences between databases are genuine (eg, due to differences in patient populations or data recording practices) or due to imperfect harmonization between our analyses. Sixth, we did not account for selecting the IPCW denominator model penalties via cross-validation in our CIs. See Supplementary Discussion for details on select limitations.
CONCLUSIONS
The risk of first-season MA RSV LRTI peaks for infants born from May through September. Infants born during the last 2–3 months of the year are at the highest risk of RSV-related hospitalization, as they enter their first RSV season quite young. However, infants’ risk of MA RSV LRTI is not confined to their first season. Through the FYOL the overall risk of MA RSV LRTI is comparable across birth months, with the additional cases progressing to care at the outpatient or ED settings. This additional RSV risk underscores the need to document MA RSV LRTI beyond infants’ first RSV season, to guide prioritization and timing of targeted preventive interventions.
References
- 1. Thomas E, Mattila J-M, Lehtinen P, Vuorinen T, Waris M, Heikkinen T. Burden of respiratory syncytial virus infection during the first year of life. J Infect Dis2020; 223:811–7.
- 2. Arriola CS, Kim L, Langley G, et al. Estimated burden of community-onset respiratory syncytial virus–associated hospitalizations among children aged <2 years in the United States, 2014–15. J Pediatric Infect Dis Soc2019; 9:587–95.
- 3. Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet2010; 375:1545–55.
- 4. Goldstein E, Finelli L, O’Halloran A, et al. Hospitalizations associated with respiratory syncytial virus (RSV) and influenza in children, including children diagnosed with asthma. Epidemiology2019; 30:918–26.
- 5. Hansen CL, Chaves SS, Demont C, Viboud C. Mortality associated with influenza and respiratory syncytial virus in the US, 1999-2018. JAMA Netw Open2022; 5:e220527.
- 6. Reichert H, Suh M, Jiang X, et al. Mortality associated with respiratory syncytial virus, bronchiolitis, and influenza among infants in the united states: A birth cohort study from 1999 to 2018. J Infect Dis2022; 226:S246–54.
- 7. Bylsma LC, Suh M, Movva N, Fryzek JP, Nelson CB. Mortality among US infants and children under 5 years of age with respiratory syncytial virus and bronchiolitis: A systematic literature review. J Infect Dis2022; 226:S267–81.
- 8. Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr2003; 143:127–32.
- 9. Suh M, Movva N, Jiang X, et al. Respiratory syncytial virus is the leading cause of United States infant hospitalizations, 2009-2019: A study of the National (Nationwide) Inpatient Sample. J Infect Dis2022; 226:S154–63.
- 10. McLaurin KK, Farr AM, Wade SW, Diakun DR, Stewart DL. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol2016; 36:990–6.
- 11. Brady MT, Byington CL, Davies HD, et al. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics2014; 134:415–20.
- 12. Movva N, Suh M, Reichert H, et al. Respiratory syncytial virus during the COVID-19 pandemic compared to historic levels: A retrospective cohort study of a health system. J Infect Dis2022; 226:S175–83.
- 13. Gantenberg JR, van Aalst R, Zimmerman N, et al. Medically attended illness due to respiratory syncytial virus (RSV) infection among infants born in the United States between 2016 and 2020. J Infect Dis2022; 226:S164–74.
- 14. Midgley CM, Haynes AK, Baumgardner JL, et al. Determining the seasonality of respiratory syncytial virus in the United States: The impact of increased molecular testing. J Infect Dis2017; 216:345–55.
- 15. Rose EB, Wheatley A, Langley G, Gerber S, Haynes A. Respiratory syncytial virus seasonality - United States, 2014-2017. MMWR Morb Mortal Wkly Rep2018; 67:71–6.
- 16. Moriyama M, Hugentobler WJ, Iwasaki A. Seasonality of respiratory viral infections. Ann Rev Virol2020; 7:83–101.
- 17. Centers for Disease Control and Prevention. The National Respiratory and Enteric Virus Surveillance System (NREVSS). Respiratory syncytial virus surveillance. 2021 (https://archive.cdc.gov/www_cdc_gov/surveillance/nrevss/rsv/index.html)
- 18. Demont C, Petrica N, Bardoulat I, et al. Economic and disease burden of RSV-associated hospitalizations in young children in France, from 2010 through 2018. BMC Infect Dis2021; 21:730.
- 19. Kieffer A, Beuvelet M, Sardesai A, et al. Expected impact of universal immunization with nirsevimab against RSV-related outcomes and costs among all US infants in their first RSV season: A static model. J Infect Dis2022; 226:S282–92.
- 20. Tong S, Amand C, Kieffer A, Kyaw MH. Incidence of respiratory syncytial virus related health care utilization in the United States. J Global Health2020; 10:020422.
- 21. Rha B, Curns AT, Lively JY, et al. Respiratory syncytial virus-associated hospitalizations among young children: 2015-2016. Pediatrics2020; 146:e20193611.
- 22. Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus–associated hospitalizations among children less than 24 months of age. Pediatrics2013; 132:e341–8.
- 23. Lloyd PC, May L, Hoffman D, Riegelman R, Simonsen L. The effect of birth month on the risk of respiratory syncytial virus hospitalization in the first year of life in the United States. Pediatr Infect Dis J2014; 33:e135–40.
- 24. Suh M, Movva N, Jiang X, et al. Respiratory syncytial virus burden and healthcare utilization in united states infants <1 year of age: Study of nationally representative databases, 2011-2019. J Infect Dis2022; 226:S184–94.
- 25. Kim L, Rha B, Abramson JS, et al. Identifying gaps in respiratory syncytial virus disease epidemiology in the United States prior to the introduction of vaccines. Clin Infect Dis2017; 65:1020–5.
- 26. Turi KN, Wu P, Escobar GJ, et al. Prevalence of infant bronchiolitis-coded healthcare encounters attributable to RSV; respiratory syncytial virus. Health Sci Rep2018; 1:e91.
- 27. R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2023 (https://www.R-project.org/)
- 28. Howe CJ, Cole SR, Lau B, Napravnik S, Eron JJ. Selection bias due to loss to follow up in cohort studies. Epidemiology2016; 27:91–7.
- 29. Lumley T. Analysis of complex survey samples. J Stat Softw2004; 9:1–19.
- 30. Barbieri E, Cavagnis S, Scamarcia A, et al. Assessing the burden of bronchiolitis and lower respiratory tract infections in children ≤24 months of age in Italy, 2012–2019. Front Pediatr2012; 11:1143735.
- 31. Birkhaug IM, Inchley CS, Aamodt G, Ånestad G, Nystad W, Nakstad B. Infectious burden of respiratory syncytial virus in relation to time of birth modifies the risk of lower respiratory tract infection in infancy. Pediatr Infect Dis J2013; 32:e235–41.
- 32. Jourdain M, Benchaib M, Ploin D, et al. Identifying the target population for primary respiratory syncytial virus two-step prevention in infants: Normative outcome of hospitalisation assessment for newborns (NOHAN). Vaccines2022; 10:729.
- 33. Li Y, Batinović E, Milić P, Markić J. The role of birth month in the burden of hospitalisations for acute lower respiratory infections due to respiratory syncytial virus in young children in Croatia. PLoS One2022; 17:e0273962.
- 34. Boyce TG, Mellen BG, Mitchel EF, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr2000; 137:865–70.
- 35. Simões EAF, Anderson EJ, Wu X, Ambrose CS. Effects of chronologic age and young child exposure on respiratory syncytial virus disease among US preterm infants born at 32 to 35 weeks gestation. PLoS One2016; 11:e0166226.
- 36. Simões EAF, Anderson EJ, Wu X, Ambrose CS. Correction: Effects of chronologic age and young child exposure on respiratory syncytial virus disease among US preterm infants born at 32 to 35 weeks gestation. PLoS One2016; 11:e0168882.
- 37. Cai W, Tolksdorf K, Hirve S, et al. Evaluation of using ICD-10 code data for respiratory syncytial virus surveillance. Influenza Other Respir Viruses2019; 14:630–7.
- 38. Hamid S, Winn A, Parikh R, et al. Seasonality of respiratory syncytial virus – United States, 2017–2023. MMWR Morb Mortal Wkly Rep2023; 72:355–61 (https://www.cdc.gov/mmwr/volumes/72/wr/mm7214a1.htm). (Accessed March 4, 2024).
- 39. Drysdale SB, Cathie K, Flamein F, et al. Nirsevimab for prevention of hospitalizations due to RSV in infants. N Engl J Med2023; 389:2425–35. (Accessed January 30, 2024).
- 40. Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med2022; 386:837–46.
- 41. Jones JM, Fleming-Dutra KE, Prill MM, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: Recommendations of the advisory committee on immunization practices—United States, 2023. MMWR Morb Mortal Wkly Rep2023; 72:920–5. (https://www.cdc.gov/mmwr/volumes/72/wr/mm7234a4.htm) (Accessed February 2, 2024).
- 42. Horgan R, Hughes BL, Waller J, Hage Diab Y, Saade G. Understanding new recommendations for respiratory syncytial virus prevention in pregnancy. Obstetrics Gynecol2024; 143:484–90.
- 43. Ernst C, Bejko D, Gaasch L, et al. Impact of nirsevimab prophylaxis on paediatric respiratory syncytial virus (RSV)-related hospitalisations during the initial 2023/24 season in Luxembourg. Euro Surveill2024; 29:2400033. (https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2024.29.4.2400033) (Accessed January 30, 2024).
- 44. Garg I, Shekhar R, Sheikh AB, Pal S. Impact of COVID-19 on the changing patterns of respiratory syncytial virus infections. Infect Dis Rep2022; 14:558–68.
- 45. Britton PN, Hu N, Saravanos G, et al. COVID-19 public health measures and respiratory syncytial virus. Lancet Child Adolesc Health2020; 4:e42–3.
- 46. Mattia GD, Nenna R, Mancino E, et al. During the COVID-19 pandemic where has respiratory syncytial virus gone? Pediatr Pulmonol2021; 56:3106–9.