Introduction
Benzodiazepines (neuroscience-based nomenclature (NbN): positive allosteric modulators (GABA-A receptor, benzodiazepine site)) are a treatment for anxiety disorders (; ) and insomnia (; ) but are no longer considered the first-line treatment for either indication. However, benzodiazepine use remains widespread (; ; ), especially in older adults (; ; ). In recent data from Ontario, the prevalence of benzodiazepine use in community-dwelling older adults was 11% (), with 60% using low doses (50% or less than the World Health Organization defined daily dose (DDD)), 25% using moderate doses (51–100% of the DDD) and 15% using high doses (greater than the DDD).
Regular benzodiazepine use over prolonged periods is controversial due to concerns of tolerance (leading to progressive elevation of dose), dependence, risk of difficult withdrawal and other adverse events (). While older adults are susceptible to anxiety disorders and sleep problems, the potential for adverse consequences associated with the use of benzodiazepines appears to be greater than in younger people, particularly in terms of increased risk of falls, fractures (; ), cognitive impairment (; ) and mortality (). Older adults are more likely to adopt a chronic pattern of use (; ; ; ; ), with one Canadian study reporting a mean duration of use of 75.5 days in new benzodiazepine users aged >66 years (). Other studies have suggested that the duration of use may be even longer, especially in older adults with comorbid dementia where a mean duration of 2 years has been reported ().
The issues of whether benzodiazepines should be prescribed to older adults and if so whether treatment should be restricted to a maximum duration have been addressed by numerous national and international guidelines and position statements. However, the controversy (; ) is unresolved, and the guidelines have adopted contrasting positions. Some guidelines (; ) take the view that benzodiazepines should be avoided entirely in older adults. A second group, comprising both guidelines for adults and those specific to older adults (; ; ; ; ), states that short-term benzodiazepine use may be acceptable for some indications. ‘Short-term use’ is often not defined but at least one guideline suggests a maximum duration of around 4 weeks. A further group of guidelines (; ; ; ) acknowledge that time-limited use is preferable, but allow for long-term use in circumstances where the benefits outweigh the risks.
Various approaches have been used to characterize benzodiazepine adverse outcomes in both short- and long-term use. Randomized controlled trials have demonstrated the efficacy of certain anxiolytic benzodiazepines for anxiety disorders (; ); however, these trials have less utility for examining rarer adverse outcomes or outcomes that emerge after prolonged periods of exposure beyond the duration of the trial. In particular, randomized trials examining outcomes of long-term benzodiazepine use are uncommon; those that exist, such as a 3-year study of clonazepam versus paroxetine in panic disorder (), have a weaker design than the more numerous short-term trials. We are not aware of any randomized trials that specifically compare chronic and intermittent benzodiazepine use.
An alternative approach to characterizing benzodiazepine outcomes is the use of observational methods such as cohort studies. Most previous observational studies have not been derived from population-based data; rather, they have been derived from clinical settings, which affect their generalizability. A few recent population-based studies have focussed on a single outcome in relation to benzodiazepine exposure, such as those reporting the association of benzodiazepine use with hip fractures (), poor outcome after total hip replacement in arthritis () and Alzheimer’s disease (). A further study of this type found no association between benzodiazepine use and dementia/cognitive decline ().
Confounding by indication, whereby confounders are associated with both selection of treatment (such as a drug, dose level or indeed pattern of prescribing) and outcomes of interest, is a common problem in observational studies (; ). Propensity score matching () may be used to reduce the impact of this phenomenon. However, the existing studies described above did not employ propensity score matching, instead controlling only for a more limited number of covariates. They may therefore be more susceptible to the effects of measured and unmeasured confounding, especially where characteristics present at baseline may feasibly be associated with both the choice of drug or prescribing pattern and adverse outcomes.
In this study, our aim was to characterize the risks associated with chronic versus intermittent benzodiazepine use in older adults after a new benzodiazepine prescription, with a follow-up period of up to 360 days using population-based administrative health care data. To achieve this, we compared a cohort of chronic benzodiazepine users with a propensity score matched cohort of intermittent benzodiazepine users. The primary outcome was falls resulting in hospital or emergency department (ED) visits, while the secondary outcomes included hip and wrist fractures, hospital/ED visits, long-term care (LTC) admission and mortality. We hypothesized that the risk of adverse outcomes is increased in chronic benzodiazepine users relative to intermittent users, and that this elevated risk would persist after adjustment for benzodiazepine dose.
Methods
The study was approved by the Research Ethics Board at the Centre for Addiction and Mental Health, Toronto.
Data sources
This retrospective population-based cohort study used linked administrative health care databases held by ICES (formerly known as the Institute for Clinical Evaluative Sciences), located in Ontario, Canada. The following databases are linked by unique, encrypted identifiers for all individuals eligible for provincial health insurance: (1) The Canadian Institute for Health Information Discharge Abstract database (DAD) and Same Day Surgery (SDS) database for information on hospitalizations and associated diagnoses; (2) The National Ambulatory Care Reporting System metadata (NACRS) for information on hospital and community-based ambulatory care; (3) The Ontario Health Insurance Plan (OHIP) physician billing database providing information on physician billing claims and associated diagnoses; (4) The Ontario Mental Health Reporting System (OMHRS) for mental health-related admissions and associated diagnoses; (5) The Ontario Drug Benefit (ODB) database which contains details on prescription medications dispensed to patients >65 years; (6) The Registered Persons Database with demographic information on Ontario residents (RPDB); and (7) The Ontario Cancer Registry (OCR) and Activity Level Reporting (ALR) databases to identify patients with a diagnosis or treatment of cancer.
Inclusion/exclusion criteria
The benzodiazepine-exposed cohort consisted of (1) community-dwelling adults resident in Ontario, (2) aged 66 years or older, (3) with a first prescription for a benzodiazepine (index date) between January 2002 and December 2013. A 12-month look-back period was used to confirm the incident benzodiazepine prescription. Exclusion criteria were (1) LTC facility residence from 1 year before the index date to 180 days after index to focus on a community-dwelling population, (2) individuals receiving palliative care from 1 year before the index date to 180 days after index or a cancer diagnosis prior to 180 days after index, to exclude incident benzodiazepine use at end of life or for palliative care; and (3) documented diagnosis of drug abuse or specific indications for benzodiazepine use such as epilepsy or alcohol withdrawal in 5 years before the index date to 180 days after index, to focus on a population with likely incident benzodiazepine use for anxiety or insomnia. We also excluded individuals who died or were admitted to hospital in the first 180 days after the index date as we would have incomplete information to categorize their drug exposure.
Exposure
To measure the pattern of benzodiazepine use, we used the ‘days’ supply’ field, which captures the estimated number of days covered by the prescription in the ODB database. We divided the cohort into ‘chronic’ and ‘intermittent’ benzodiazepine users based on their pattern of benzodiazepine prescriptions received in the 180-day period after the first prescription (exposure period). Extrapolating from , who described marked variations in definitions of ‘long term’ or ‘chronic use’, we defined chronic use as a supply of 120 or more days within the 180-day exposure period. Intermittent use was thus defined as less than a 120-day supply prescribed in the 180-day exposure period. We calculated the mean daily benzodiazepine dose using lorazepam dose equivalents (LoDEq) using a table derived from over the exposure period, dividing the total dose dispensed by days’ supply.
Propensity matching
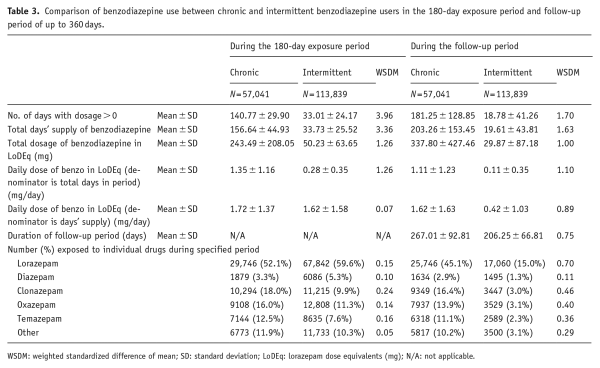
The propensity score matching technique () addresses confounding by indication by matching subjects on a combined score generated from variables that may be linked to the likelihood that an individual will receive a certain exposure, in this case the pattern of benzodiazepine prescribing. Propensity scores for chronic versus intermittent benzodiazepine prescribing were estimated using 24 covariates ascertained on cohort entry, across six domains as listed in Table 1.
One-to-two matching was conducted between the chronic and intermittent benzodiazepine users using nearest-neighbour matching on the basis of age (±1 year), sex, and propensity score within a calliper ±0.20 standard deviations of the score. Any chronic user who could not be matched to two intermittent users but could be matched to one was accepted along with their one matched intermittent user. There was no subsequent replacement. A C-statistic was calculated to evaluate the goodness of fit. Standardized differences were used to compare the prevalence of characteristics between the two groups, with an absolute standardized difference of >10% signifying a meaningful imbalance ().
Outcomes
After the 180-day exposure period used to define cohort membership, individuals were followed either until the outcome of interest occurred, or to the end of the follow-up period at 360 days. Individuals were censored at the point at which they ceased to meet the inclusion criterion for their cohort examining overlapping 180-day periods at 90-day intervals. For example, chronic users were censored if they had two consecutive 180-day periods with less than 120 days’ supply prescribed. Intermittent users were censored if either they had a single 180-day period in which they received 120 days’ supply or more, or a single 180-day period for which they received no benzodiazepine supply. Individuals from either cohort were also censored at the point they met any of the criteria in exclusions (a) to (e) above, that is, receiving palliative care, LTC residence, cancer diagnosis, drug abuse, epilepsy, alcohol withdrawal, or death.
We measured the following endpoints during follow up: (1) fall-related ED or hospital visits (primary outcome), (2) fragility fractures (i.e. hip and wrist fractures), (3) hospitalizations or ED visits for any reason, (4) LTC admission and (5) death (see Supplementary Table 1 for detailed outcome definitions). Fall-related emergency and hospital visits were selected as the primary outcome given that falls are a major cause of mortality, morbidity and disability in older adults () and have a large impact on health care costs (). The association between medication exposure and falls is well-established () and medication-related falls are potentially preventable adverse events.
Data analyses
We estimated relative risk for each outcome during follow up for the propensity score matched chronic users against intermittent users by hazard ratios (HRs) calculated from Cox regression models. The proportional hazard assumption was tested using martingale residuals for each primary and secondary outcome.
Analyses were (1) unadjusted and (2) adjusted for mean daily benzodiazepine usage (in LoDEq) during the follow-up period. We did not adjust for sex or age group at entry (patients were matched on these variables), but instead undertook stratified analyses by sex and age group (i.e. 66–75, 76–85 and ≥ 86 years at entry).
Results
In total, we identified 57,072 chronic benzodiazepine users for matching in a 1:2 ratio to a pool of 312,468 intermittent benzodiazepine users. In the matching process, 56,798 chronic users were matched to two intermittent users, and a further 243 could be matched to only one intermittent user, for a total of 113,989 intermittent users included. This left 31 chronic users (0.05%) who could not be matched to an intermittent user were therefore not included in the analysis. The demographic characteristics of the two cohorts were similar and are presented in Table 2. The two groups were well-matched on all variables used in calculating propensity scores (C-statistic for goodness of fit = 0.603) with weighted standard difference of means between 0.00 and 0.03 in every case (Table 2).
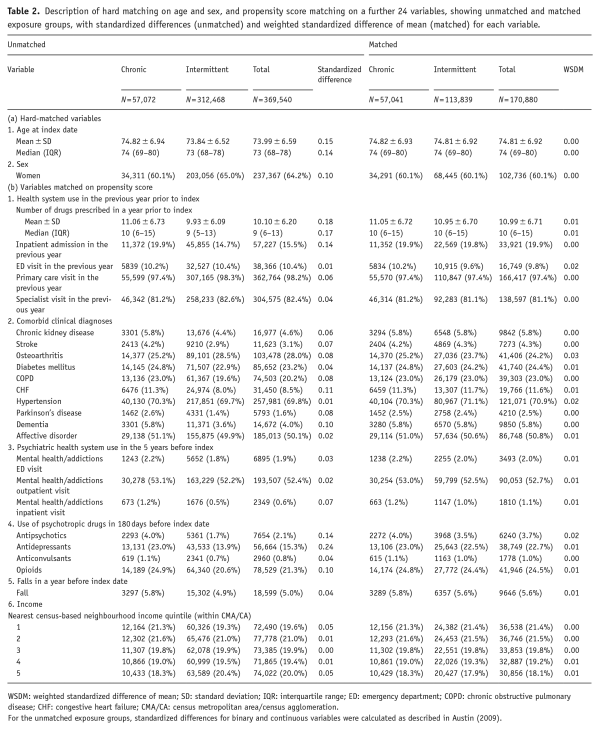
Table 2. Description of hard matching on age and sex, and propensity score matching on a further 24 variables, showing unmatched and matched exposure groups, with standardized differences (unmatched) and weighted standardized difference of mean (matched) for each variable.
Exposure period
During the 180-day exposure period (Table 3) used to define cohort membership, total benzodiazepine quantity prescribed in LoDEq was greater in the chronic user group (243.5 ± 208.1 mg vs 50.2 ± 63.7 mg, weighted standardized difference of mean = 1.3). Chronic users had benzodiazepines supplied for 140.8 ± 29.9 days compared with 33.0 ± 24.2 days in intermittent users (Table 3). The mean daily benzodiazepine dose (total prescribed divided by days supplied) was similar between the two groups (1.7 ± 1.4 mg LoDEq vs 1.6 ± 1.6 mg LoDEq, weighted standardized difference of mean = 0.07). In both cohorts, lorazepam was the most frequently prescribed benzodiazepine, with 52.1% of chronic users and 59.6% of intermittent users being prescribed this drug.
Follow-up period
For the primary outcome of hospitalization/ED visit due to falls, there were 14.9 million days of follow up examined in the chronic user group and 23.1 million days in the intermittent user group. Days of follow up were similar in the secondary analyses, although the exact numbers varied depending on the occurrence of the outcome events. For the primary outcome of hospitalization/ED visit due to falls, the mean number of days of follow up per person (Table 3) was higher in the chronic group (267.0 ± 92.8 days) than in the intermittent group (206.3 ± 66.8 days, weighted standardized difference of mean = 0.75). Chronic users were prescribed substantially greater daily benzodiazepine doses (in LoDEq) than were intermittent users in this period (1.1 ± 1.2 mg/day vs 0.11 ± 0.35 mg/day, weighted standardized difference of mean = 1.10). Unlike in the exposure period, the mean difference in daily doses considering only days for which benzodiazepines were actually prescribed in the follow-up period was substantially greater in the chronic users (1.6 ± 1.6 mg/day vs 0.42 ± 1.0 mg/day, weighted standardized difference of mean = 0.89).
In both cohorts, individuals were censored prior to reaching the maximum possible follow up of 360 days most commonly for their pattern of benzodiazepine prescription ceasing to meet the criteria for either chronic or intermittent use. In the chronic cohort, 10.1% received no further benzodiazepines in the follow-up period and were subject to censoring at the earliest opportunity, after 180 days. In the intermittent group, 74.3% were subject to censoring at this time-point because no further benzodiazepines being prescribed.
Outcomes
The primary outcome of hospitalization/ED visits due to falls occurred during the follow-up period in 2606 (4.6%) chronic users and 3592 (3.2%) intermittent users, representing a HR of 1.13 (95% CI: 1.08 to 1.19; p < 0.0001) (Table 4). When stratifying by sex (Table 4), the excess risk associated with chronic use was observed in both men (HR = 1.18, 95% CI: 1.08 to 1.29; p < 0.001) and women (HR = 1.12, 95% CI: 1.05 to 1.19; p < 0.001). When stratifying by age group (Supplementary Table 2), increased risk in chronic users was observed in both the groups aged 66–75 years (HR = 1.10, 95% CI: 1.02 to 1.20; p = 0.020) and 76–85 years (HR = 1.18, 95% CI: 1.09 to 1.28; p < 0.0001), but not in the oldest group aged 86 years or more (HR = 1.08, 95% CI: 0.96 to 1.22; p = 0.200).
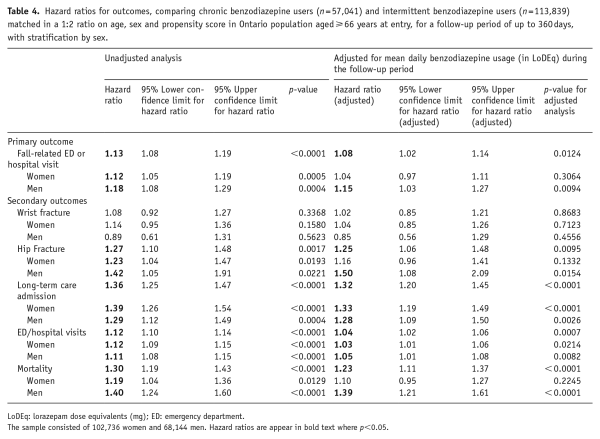
Table 4. Hazard ratios for outcomes, comparing chronic benzodiazepine users (n = 57,041) and intermittent benzodiazepine users (n = 113,839) matched in a 1:2 ratio on age, sex and propensity score in Ontario population aged ≥ 66 years at entry, for a follow-up period of up to 360 days, with stratification by sex.
For most of the secondary outcomes, with the one exception of wrist fractures, chronic benzodiazepine use again conferred significantly greater risk than intermittent use. Chronic users were at elevated risk for hip fracture (HR = 1.27, 95% CI: 1.10 to 1.48; p = 0.002), LTC admission (HR = 1.36, 95% CI: 1.25 to 1.47; p < 0.0001), hospital/ED visits for any reason (HR = 1.12, 95% CI: 1.10 to 1.14; p < 0.0001) and mortality (HR = 1.30, 95% CI: 1.19 to 1.43; p < 0.0001). There was no significant effect of sex in the stratified analyses. Stratification by age yielded a difference between groups only for hip fractures whereby people aged 76–85 years at entry were the only age-band at a significantly excess risk (Supplementary Table 2).
The proportional hazard assumption held for all primary and secondary outcomes.
Adjustment for benzodiazepine dose
Adjustment for mean benzodiazepine dose per day during follow up resulted in only small reductions in the HRs for chronic versus intermittent usage for each of the outcomes (Table 4). Once again, chronic benzodiazepine use was associated with significantly greater risk of the primary outcome of hospital/ED visits due to falls (HR = 1.08, 95% CI: 1.02 to 1.14; p = 0.012) than was intermittent use in the observation period. As before, there were associations with four of the secondary outcomes (hip fractures (HR = 1.25, 95% CI: 1.06 to 1.48; p = 0.001), LTC admission (HR = 1.32, 95% CI: 1.20 to 1.45; p < 0.0001), hospital/ED visits for any reason (HR = 1.04, 95% CI: 1.02 to 1.06; p < 0.001) and mortality (HR = 1.23, 95% CI: 1.11 to 1.37; p < 0.0001)).
HRs were generally smaller in analyses stratified by age and sex. A recurrent finding in these analyses was that the risk conferred by chronic benzodiazepine use was markedly stronger in men than in women despite women accounting for more than 60% of the sample. For the primary outcome of hospital/ED visits due to falls, chronic benzodiazepine use was associated with a HR of 1.15 in men (95% CI: = 1.03 to 1.27; p < 0.001) but there was no evidence of association in women (HR = 1.04, 95% CI: 0.97 to 1.11; p = 0.306). Similarly, chronic benzodiazepine use conferred excess risk in men of secondary outcomes for hip fracture (HR = 1.50, 95% CI: 1.08 to 2.09; p = 0.015) and mortality (HR = 1.39, 95% CI: 1.21 to 1.61; p < 0.0001) while there was no significant association for these outcomes in women. Only for LTC admission (men: HR = 1.28, 95% CI: 1.09 to 1.59; p = 0.003; women: HR = 1.33, 1.19 to 1.49; p < 0.0001) and hospital/ED visits for any reason (men: HR = 1.05, 95% CI: 1.01 to 1.08; p = 0.008; women: HR = 1.03, 95% CI: 1.01 to 1.06; p = 0.021) was chronic benzodiazepine use associated with excess risk in both sexes.
Stratified analyses by age group in dose-adjusted analyses yielded less consistent results (Supplementary Table 2). For the primary endpoint of hospital/ED visits due to falls, there were no associations with chronic benzodiazepine use in any of the three age groups (for 65–75 years: HR = 1.01, 95% CI: 0.92 to 1.10; p = 0.914; for 76–85 years: HR = 1.08, 95% CI: 0.98 to 1.18; p = 0.127; and for >86 years: HR = 0.98, 95% CI = 0.85 to 1.12; p = 0.766). However, for secondary endpoints, there was a greater risk in chronic compared with intermittent users aged 66–75 years of LTC admission, hospital/ED visits and mortality, and a greater risk in chronic users aged 76–85 years of hip fracture and LTC admission only.
Discussion
A pattern of chronic benzodiazepine use in the 180 days after incident use, compared to intermittent benzodiazepine use, was associated with significantly greater risk for the primary outcome of hospital/ED visits due to falls, and for the secondary outcomes, hip fractures, hospitalizations/ED visits, LTC admission and death, but not for wrist fractures. The excess risk held true once the models were adjusted for mean daily benzodiazepine dose. This latter finding is important because we found that the mean daily benzodiazepines during the follow-up period was several-fold higher in the chronic use group. The fact that the significantly increased risk remained after adjustment for mean daily dose suggests that the pattern of chronic use of benzodiazepines carries risks relative to intermittent use which are independent of its association with greater drug exposure, assuming that the propensity score matching was able to encompass variables that explain any baseline difference between the groups.
These findings raise the question as to whether there might be a neurobiological explanation for excess risk of adverse outcomes in chronic relative to intermittent users. Benzodiazepine use over time is associated with biological changes in key chemical systems with which benzodiazepines interact. Suggested mechanisms () include downregulation in the number of GABA-A benzodiazepine receptors with prolonged use, uncoupling of receptor binding and the subsequent impact on GABA, regulation of protein degradation, internalization and synthesis, which might result in switching of specific subunits in the GABA-A benzodiazepine receptor over time (; ) and effects on mRNA synthesis or degradation. Any of these changes may be associated with increased propensity for adverse effects. It is possible that such changes occur more readily when benzodiazepine exposure is chronic and regular, than when exposure is intermittent and sporadic. Drug accumulation may be a factor contributing to adverse outcomes associated with chronic benzodiazepine use. Older adults are at risk of experiencing benzodiazepine accumulation if age-related pharmacokinetic changes which prolong drug half-life, such as increased volume of distribution, co-occur with reduced clearance of hepatically metabolized long-acting benzodiazepines including diazepam () and nitrazepam. Accumulation is more likely to occur with chronic rather than intermittent drug use.
A second interesting finding of this study is that chronic benzodiazepine use appears to confer markedly greater risk of adverse outcomes relative to intermittent use in men than is the case in women. This was true for the primary endpoint of hospital/ED visits due to falls and also for three of the five secondary outcomes (hip fractures, hospital/ED visits for any reason, and mortality). Register-based studies (; ; ) report consistently that the population prevalence of benzodiazepine prescribing is 1.5 to 2 times higher in women than in men. There are also systematic differences between men and women who are prescribed benzodiazepines which have been previously described () and are observed in our cohort, namely, more chronic health issues including vascular disease in men, and more mental health problems and osteoporosis in women. Sex-specific effects in psychopharmacological systems such as GABA-mediated neurotransmission are receiving increased attention (). One neuroimaging study has reported that women had greater GABA-A benzodiazepine receptor binding availability than men, a measure that was associated with nicotine craving and pain sensitivity (). Genetic polymorphisms relating to GABA-A receptor subunit expression and the phenotype of substance use disorders have been described with associations in women only (). In animal models, literature has developed on sex differences in the expression of certain GABA-A benzodiazepine receptor subunits in specific brain areas such as somatosensory thalamus and cortex in control rats (). We are unable to offer a biological link between these observations and the present finding that in older adults chronic compared with intermittent benzodiazepine use carries excess risk of certain outcomes in men only. However, evidence on sex-specific differences in receptor availability or subunit expression and distribution makes it plausible that changes occurring with chronic benzodiazepine use might exacerbate the impact on the GABA-benzodiazepine system, and thereby on adverse outcomes, in one sex preferentially. Future studies can explore the question of sex differences in risks associated with benzodiazepine use by developing cohorts matched on the propensity of both receiving a benzodiazepine and being a male.
Strengths of the study are that it has a large, population-based sample that provides adequate numbers, including supporting the stratified analyses for sex described above. Propensity score matching based on 24 variables in six distinct categories to reduce risk of confounding by indication is a further strength. The study has some potential weaknesses which need to be acknowledged. Most fundamentally, as this is a comparison of two patterns of benzodiazepine use, it cannot elucidate the risks of benzodiazepines versus no treatment and therefore does not allow us to judge the merit of guidelines which advises avoiding benzodiazepines entirely in older adults. In those analyses where we do not find a difference between chronic and intermittent use, we are unable to draw a comparison to no treatment, that is, to say whether these two prescribing patterns are equally safe or equally dangerous compared to no benzodiazepine. While we have accounted for many potential confounding variables through hard matching for age group and sex, and made use of a robust propensity score matching approach, the possibility of residual confounding remains as propensity score matching can only be undertaken on observable variables. The ODB database includes all available benzodiazepines but does not allow the possibility to examine the use of the related class of Z-drugs. In Ontario, only zopiclone is commonly prescribed, but is not reimbursed by the ODB so our analyses cannot take account of this ‘benzodiazepine-like’ drug. A further issue is the criteria we have used to define ‘chronic’ and ‘intermittent’ users. In selecting a cut-off of benzodiazepine prescriptions for at least 120 days during the 180-day exposure period, we believe that we chose a threshold which took account of previous work () and has face-validity for chronic use, but we cannot be certain that this cut-off is underpinned biologically in terms of differentiating individuals who are likely to have neurochemical changes resulting from their benzodiazepine use from those who likely will not. Note, however, that the two groups had very different mean exposure to benzodiazepines in the exposure period (140 vs 33 days out of 180 days) so appear to be two fairly distinct cohorts. We also incorporated rules for removing or ‘censoring’ individuals over time when they ceased to meet the criteria for ‘chronic’ or ‘intermittent’ use, but again the extent to which these definitions map on to the biological profiles is open to debate.
It must also be recognized that many individuals who entered the cohort by fulfilling our criteria for chronic or intermittent use were censored before the end of the follow-up period due to changes in their pattern of usage over the course of the study. This applies especially in the intermittent user group where almost three quarters of individuals had no further benzodiazepines in the first 180 days of the follow-up period and were therefore censored at the earliest opportunity. Although censoring individuals reduces power, the methodology has the strength of being naturalistic – it reflects the reality that people often change their benzodiazepine use as time progresses, with some eliminating them entirely. The methodology employed ensured that those who continue to use benzodiazepines with the same pattern that they did in the exposure period will contribute more exposure time in the follow-up period than those who changed their usage pattern. Overall, with the greater rate of censoring in the intermittent user group partially offsetting the initial 1:2 matching, the final ratio of person-days for the observation period was 1:1.55. Nevertheless, there may be a limitation in terms of ascertainment bias if adverse outcomes could be observed more frequently by virtue of proportionally more chronic users than intermittent users remaining in the cohorts as time progresses. However, we believe that this limitation is likely to be small and its impact is outweighed by the need to ensure that exposure patterns continue to reflect actual medication use in a population subject to marked changes in use of benzodiazepines over time. Finally, ODB registers provide data on filled/dispensed benzodiazepine prescriptions which is a proxy for benzodiazepine exposure – therefore, some people may have obtained prescribed medications but not actually taken them. It is possible that differences between chronic and intermittent users in taking their medications after filling prescriptions could be a source of bias.
In illustrating excess risks for chronic use over intermittent use, we have captured an issue with benzodiazepines, which cannot be revealed by studies that simply undertake meta-analysis of side effects in short-term trials. A good example of such a study is the recent meta-analysis of , which compared the side effect burden of benzodiazepines in panic disorder treatment trials of up to 12 weeks duration to that of SSRIs, concluding that incidence of adverse effects was slightly lower in people randomized to benzodiazepines. While such a meta-analysis is informative, it cannot address the issue of excess harms of benzodiazepines with chronic use over the longer periods we have examined in this study.
While this study illustrates that chronic benzodiazepine use carries enhanced risks of most of the adverse outcomes relative to intermittent use, especially in older men, it must be recognized that the excess risk described is merely one factor to be taken into consideration when deciding whether ongoing benzodiazepine use is acceptable or unacceptable for a given patient who has been taking these drugs for long periods and is deriving benefit, especially where there has been no evidence of dose escalation or tolerance to the therapeutic effect. Clearly, when discussing the way forward with patients in this situation, the excess risks we have enumerated here must be considered against the risks of withdrawal and rebound of symptoms of their original disorder which may occur if established benzodiazepine treatment is reduced in dose and/or frequency, as well as the potential adverse effects of other medications that might be introduced to allow this to happen.
We call for further research to attempt to replicate these results in other large drug prescription databases. These findings should inform future editions of guidelines, which advise on benzodiazepine prescription, especially if replicated in further studies. In addition, there is potential for further analyses in this and other datasets to examine whether individual benzodiazepines or sub-groups based on pharmacological characteristics differ in terms of excess risk in older adults with chronic relative to intermittent use. This might include comparisons of short-acting versus long-acting benzodiazepines, and of drugs eliminated by conjugation versus those subject to extensive liver metabolism given the increased risk of accumulation with some hepatically metabolized drugs.
Our study provides clear-cut evidence that there are significant excess risks associated with chronic benzodiazepine use compared to intermittent use. Importantly, our study design does not permit us to speculate about the safety or risks of prescribing benzodiazepines compared to no treatment. The excess risks quantified here can be used to help inform decision-making by older adults and clinicians about whether short- or long-term benzodiazepine use is a reasonable option for symptom management, weighing up the risks and benefits on a case-by-case basis, as is advocated by some current guidelines (; ; ; ).
Declaration of conflicting interests The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The study was funded through a grant from the University of Toronto Department of Psychiatry Excellence Funds.
Simon JC Davies
https://orcid.org/0000-0003-0095-5993
Andrea Iaboni
https://orcid.org/0000-0003-4268-6832
Supplemental material Supplemental material for this article is available online.
References
- American Geriatrics Society (2019) Beers Criteria Update Expert Panel (2019) American Geriatrics Society 2019 Updated AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatric Society 67: 674–694.
- Andrews G, Bell C, Boyce P, et al. (2018) Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of panic disorder, social anxiety disorder and generalised anxiety disorder. Australian & New Zealand Journal of Psychiatry 52: 1109–1172.
- Austin PC (2009) Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Communications in Statistics – Simulation and Computation 38: 1228–1234.
- Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behavioral Research 46: 399–424.
- Baldwin DS, Aitchison K, Bateson A, et al. (2013) Benzodiazepines: Risks and benefits: A reconsideration. Joint Report from a Working Group drawn from the Royal College of Psychiatrists Psychopharmacology Special Interest Group and the British Association for Psychopharmacology. Journal of Psychopharmacology 27: 967–971.
- Baldwin DS, Anderson IM, Nutt DJ, et al. (2014) Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: A revision of the 2005 guidelines from the British Association for Psychopharmacology. Journal of Psychopharmacology 28: 403–439.
- Bandelow B, Lichte T, Rudolf S, et al. (2015) The German guidelines for the treatment of anxiety disorders. European Archives of Psychiatry and Neurological Sciences 265: 363–373.
- Bandelow B, Zohar J, Hollander E, et al. (2008) World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders, first revision. World Journal of Biological Psychiatry 9: 248–312.
- Bartlett G, Abrahamowicz M, Tamblyn R, et al. (2004) Longitudinal patterns of new Benzodiazepine use in the elderly. Pharmacoepidemiology and Drug Safety 13: 669–682.
- Bateson AN (2002) Basic Pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawal. Current Pharmaceutical Design 8: 5–21.
- Beziz D, Colas S, Collin C, et al. (2016) Association between exposure to benzodiazepines and related drugs and survivorship of total hip replacement in arthritis: A Population-Based Cohort Study of 246,940 patients. PLoS ONE 11: e0155783.
- Billioti de Gage S, Moride Y, Ducruet T, et al. (2014) Benzodiazepine use and risk of Alzheimer’s disease: Case-control study. British Medical Journal 349: g5205.
- Bolea-Alamanac B, Bailey SJ, Lovick TA, et al. (2018) Female psychopharmacology matters! Towards a sex-specific psychopharmacology. Journal of Psychopharmacology 32: 125–133.
- Buscemi N, Vandermeer B, Friesen C, et al. (2007) The efficacy and safety of drug treatments for chronic insomnia in adults: A meta-analysis of RCTs. Journal of General Internal Medicine 22: 1335–1350.
- Canadian Psychiatric Association (2006) Clinical practice guidelines. Management of anxiety disorders. Canadian Journal of Psychiatry 51(8 Suppl. 2): 9S–91S.
- Conn DK, Hogan DB, Amdam L, et al. (2020) Canadian guidelines on benzodiazepine receptor agonist use disorder among older adults title. Canadian Geriatrics Journal 23: 116–122.
- Crowe SF, Stranks EK (2018) The residual medium and long-term cognitive effects of benzodiazepine use: An updated meta-analysis. Archives of Clinical Neuropsychology 33: 901–911.
- Cumming RG, Le Couteur DG (2003) Benzodiazepines and risk of hip fractures in older people: A review of the evidence. CNS Drugs 17: 825–837.
- Davies SJ, Jacob B, Rudoler D, et al. (2018) Benzodiazepine prescription in Ontario residents aged 65 and over: A population-based study from 1998 to 2013. Therapeutic Advances in Psychopharmacology 8: 99–114.
- Esterlis I, McKee SA, Kirk K, et al. (2013) Sex-specific differences in GABAA-benzodiazepine receptor availability: Relationship with sensitivity to pain and tobacco smoking craving. Addiction Biology 18: 370–378.
- Fang SY, Chen CY, Chang IS, et al. (2009) Predictors of the incidence and discontinuation of long-term use of benzodiazepines: A population-based study. Drug and Alcohol Dependence 104: 140–146.
- Florence CS, Bergen G, Atherly A, et al. (2018) The medical costs of fatal falls and fall injuries among older adults. Journal of the American Geriatrics Society 66: 693–698.
- Foy A, O’Connell D, Henry D, et al. (1995) Benzodiazepine use as a cause of cognitive impairment in elderly hospital inpatients. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 50: M99–106.
- Gray SL, Dublin S, Yu O, et al. (2016) Benzodiazepine use and risk of incident dementia or cognitive decline: Prospective population based study. British Medical Journal 352: i90.
- Greenblatt DJ, Harmatz JS, Shader RI (2020) Diazepam in the elderly: Looking back, ahead, and at the evidence. Journal of Clinical Psychopharmacology 40: 215–219.
- Hood SD, Davies SJC (2019) Anxiety disorder treatment guidelines: Little by little a consensus emerges. Australian & New Zealand Journal of Psychiatry 53: 80–84.
- Huerta C, Abbing-Karahagopian V, Requena G, et al. (2016) Exposure to benzodiazepines (anxiolytics, hypnotics and related drugs) in seven European electronic healthcare databases: A cross-national descriptive study from the PROTECT-EU project. Pharmacoepidemiology and Drug Safety 25(Suppl. 1): 56–65.
- Iaboni A, Bronskill SE, Reynolds KB, et al. (2016) Changing pattern of sedative use in older adults: A population-based cohort study. Drugs and Aging 33: 523–533.
- Joffe MM (2000) Confounding by indication: The case of calcium channel blockers. Pharmacoepidemiology and Drug Safety 9: 37–41.
- Katzman MA, Bleau P, Blier P, et al. (2014) Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry 14(Suppl 1): S1.
- Kurko TA, Saastamoinen LK, Tähkäpää S, et al. (2015) Long-term use of benzodiazepines: Definitions, prevalence and usage patterns – a systematic review of register-based studies. European Psychiatry 30: 1037–1047.
- Lader M (2011) Benzodiazepines revisited – will we ever learn? Addiction 106: 2086–2109.
- Leipzig RM, Cumming RG, Tinetti ME (1999) Drugs and falls in older people: A systematic review and meta-analysis: I. Psychotropic Drugs. Journal of the American Geriatrics Society 47: 30–39.
- Li H, Huguenard JR, Fisher RS (2007) Gender and age differences in expression of GABAA receptor subunits in rat somatosensory thalamus and cortex in an absence epilepsy model. Neurobiology of Disease 25: 623–630.
- Lin SK, Chen CK, Ball D, et al. (2003) Gender-specific contribution of the GABA(A) subunit genes on 5q33 in methamphetamine use disorder. The Pharmacogenomics Journal 3: 349–355.
- Lorenz-Guertin JM, Bambino MJ, Das S, et al. (2019) Diazepam accelerates GABAAR synaptic exchange and alters intracellular trafficking. Frontiers in Cellular Neuroscience 13: 163.
- McMahon AD (2003) Approaches to combat with confounding by indication in observational studies of intended drug effects. Pharmacoepidemiology and Drug Safety 12: 551–558.
- Morgan SG, Weymann D, Pratt B, et al. (2016) Sex differences in the risk of receiving potentially inappropriate prescriptions among older adults. Age and Ageing 45: 535–542.
- Nardi AE, Freire RC, Mochcovitch MD, et al. (2012) A randomized, naturalistic, parallel-group study for the long-term treatment of panic disorder with clonazepam or paroxetine. Journal of Clinical Psychopharmacology 32: 120–126.
- National Institute for Health and Clinical Excellence (2011) Generalised anxiety disorder and panic disorder (with or without agoraphobia) in adults. Clinical Guideline 113. London: National Institute for Health and Clinical Excellence.
- Nguyen T-L, Collins GS, Spence J, et al. (2017) Double-adjustment in propensity score matching analysis: Choosing a threshold for considering residual imbalance. BMC Medical Research Methodology 17: 78.
- Olfson M, King M, Schoenbaum M (2015) Benzodiazepine use in the United States. JAMA Psychiatry 72: 136–142.
- Poly TN, Islam MM, Yang HC, et al. (2020) Association between benzodiazepines use and risk of hip fracture in the elderly people: A meta-analysis of observational studies. Joint Bone Spine 87(3): 241–249.
- Quagliato LA, Cosci F, Shader RI, et al. (2019) International Task Force on Benzodiazepines. Selective serotonin reuptake inhibitors and benzodiazepines in panic disorder: A meta-analysis of common side effects in acute treatment. Journal of Psychopharmacology 33: 1340–1351.
- Requena G, Huerta C, Gardarsdottir H, et al. (2016) Hip/femur fractures associated with the use of benzodiazepines (anxiolytics, hypnotics and related drugs): A methodological approach to assess consistencies across databases from the PROTECT-EU project. Pharmacoepidemiology and Drug Safety 25 (Suppl. 1): 66–78.
- Saarelainen L, Tolppanen AM, Koponen M, et al. (2018) Risk of death associated with new benzodiazepine use among persons with Alzheimer disease: A matched cohort study. International Journal of Geriatric Psychiatry 33: 583–590.
- Taipale H, Koponen M, Tanskanen A, et al. (2015) Long-term use of benzodiazepines and related drugs among community-dwelling individuals with and without Alzheimer’s disease. International Clinical Psychopharmacology 30: 202–208.
- Takeshima N, Ogawa Y, Hayasaka Y, et al. (2016) Continuation and discontinuation of benzodiazepine prescriptions: A cohort study based on a large claims database in Japan. Psychiatry Research 237: 201–207.
- Tanay VM, Greenshaw A, Baker G, et al. (2001) Common effects of chronically administered antipanic drugs on brainstem GABAA receptor subunit gene expression. Molecular Psychiatry 6: 404–412.
- Virani A, Bezchlibnyk-Butler K, Jeffries JJ, et al. (eds) (2012) Clinical Handbook of Psychotropic Drugs (19th rev edn). Göttingen: Hogrefe Publishing.
- Wilson S, Anderson K, Baldwin D, et al. (2019) British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: An update. Journal of Psychopharmacology 33: 923–947.