Introduction: Depression and loss of brain plasticity
New neurobiological conceptualizations of depression are finally generating fresh leads for novel therapeutics with antidepressant action (; ; ; ). Prior theory first published more than 60 years ago initially oversimplified depression as a unitary disorder due to a hypothesized “monoamine deficiency,” especially serotonin and/or norepinephrine deficiencies (reviewed in ). Although the evidence for serotonin deficiency in depression has been recently criticized () as well as supported (), essentially all drugs with antidepressant action until now have in fact acted to boost the availability of monoamines, especially serotonin (reviewed in ).
Modern research is moving beyond the monoamine hypothesis to emphasize as well that inefficient information processing in various brain circuits hypothetically lead to the symptoms of depression as downstream consequences of genetic vulnerabilities and stress, possibly triggering loss of brain plasticity accompanied by central neuroinflammatory processes in critical brain circuits (; ; ; ). According to this notion, depression results when factors such as childhood adversity, chronic stress, obesity, disruption of the microbiome, and chronic inflammatory medical illnesses trigger activation of microglia which in turn release proinflammatory mediators, leading to oxidative stress, hypothalamic pituitary adrenal axis dysfunction, and loss of critical growth factors with consequential poor synaptic maintenance, synaptic loss, dendritic arborization loss, and potentially irreversible neuronal loss (; ; ; ; ).
Rapid onset “plastogens”—agents that induce central nervous system plasticity
A new theory of antidepressant action is a corollary to the idea that brain plasticity is lost in depression and states that successful treatment of depression occurs when growth factors that induce brain plasticity are synthesized and released (; ; ; ). Drugs with antidepressant action may mediate their effectiveness by acting as “plastogens,” namely agents that induce new synapse formation (Table 1) (; ; ; ).
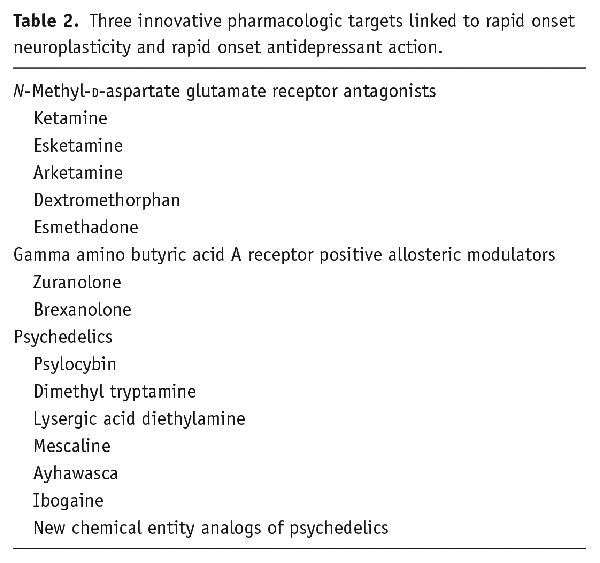
Rapid onset neuroplasticity associated with rapid onset antidepressant response is thus the driving force behind therapeutic innovation for novel antidepressant treatments (; ; ; ). In fact, essentially all drugs with antidepressant action are plastogens and share a final common pathway of inducing brain plasticity. This includes not only the well-known monoaminergic drugs with antidepressant actions that act with a delay to generate growth factors, plasticity as well as clinical antidepressant actions (; ; ; ; ), but also by three groups of novel agents that induce the rapid synthesis and release of growth factors and induce axonal sprouting and immediate synaptic arborization within minutes to hours and with rapid onset antidepressant clinical actions (reviewed below) (; ; ) . These new agents may also be effective in patients for whom the classical monoaminergic drugs for depression are not effective (; ; ). The three classes of novel compounds with emerging evidence for rapid onset antidepressant effects associated with rapid onset plasticity that act at novel pharmacologic targets include (1) the N-methyl-D-aspartate (NMDA) receptor antagonists, (2) the gamma amino butyric acid (GABA) A receptor positive allosteric modulators (PAMs), and (3) psychedelics that target serotonin 2A/2C receptors (Table 2).
All these new agents are plastogens with evidence of inducing rapid onset brain plasticity. Some of these agents could be considered “psychoplastogens” that also induce rapid onset mental status changes such as dissociation by ketamine/esketamine (; ) and hallucinations/psychotomimetic experiences by psychedelics (; ; ) during the time when the rapid onset of plasticity and rapid onset antidepressant effects are presumably occurring (Table 1). This has led to the proposal that in fact the strength of the subjective experience of these agents, especially the psychedelics, is linked to their therapeutic efficacy (; ; ). However, oral NMDA antagonists such as dextromethorphan (; ) and esmethadone as well as the GABA A PAMs (; ) do not induce such mental status changes yet are rapid onset plasticity inducers with rapid acting antidepressant actions and can be considered “neuroplastogens” (Table 1). Such observations have also led to the counterproposal that rather than an important element to the therapeutic outcome, psychotomimetic experiences are instead undesirable behavioral toxicities that should ideally be factored out of future molecules targeting the same receptors, especially serotonin receptors targeted by the psychedelics (; ). Some have even reminded us that the psychotomimetic experience of psychedelics was once exploited as a form of “dark persuasion” by the U.S. government as a potential “truth serum” for confessions, and for potential “brainwashing,” warning us to be careful of the potential for “dark neuroplasticity” of unwanted adverse experiences as well as therapeutic experiences if psychotomimetic states are required for efficacy ().
Alongside these more modern targets of pharmacology, there is a longstanding history of electroconvulsive therapy (ECT), referred to as repeated electroconvulsive seizure (rECS) in animal models, which is noted to have similar actions in terms of hippocampal neuroplasticity as conventional SSRi treatments (). Furthermore, the effect of rECS was proposed to have an effect of larger impact on the affective rather than cognitive function alongside fluoxetine specifically, making the two fairly analogous in terms of overall treatment goals and measured outcomes ().
Glutamate-targeted therapeutics; NMDA antagonists are both neuroplastogens and psychoplastogens
The first agents acting at a novel non-monoaminergic target to demonstrate rapid onset of antidepressant action associated with rapid onset of neuroplasticity were the NMDA antagonists. After administration of subanesthetic yet often dissociation-inducing intravenous infusions of the NMDA antagonist ketamine, there are now well documented and highly replicated observations of rapid onset antidepressant actions in patients who failed prior antidepressant monoaminergic treatments (; ; ; ; ; ; ; ; ). These findings, followed by formal regulatory approval of intranasal esketamine, have ignited an NMDA “gold rush” of compounds targeting this receptor (; ; ; ; ; ; ). Now available is also the oral NMDA antagonist dextromethorphan combination with the monoamine antidepressant bupropion for chronic daily treatment (; ). In clinical testing is esmethadone (the NMDA preferring and mu opiate non-preferring enantiomer of racemic methadone; ; ; ; ; ) and several other versions of ketamine, esketamine, arketamine, dextromethorphan combined with quinidine, and more, are in development (; ; ; ; ). All have rapid onset synaptogenesis and release of various growth factors, including mammalian target of rapamycin, brain derived neurotrophic factor (BDNF), vascular endothelial growth factor and others (; ; ; ; ; ; ). Which growth factor might be the most important, where plasticity is critical to occur in brain circuits, and how this leads to rapid recovery from depression remain topics of vigorous investigation (Table 3). Presumably, the induction of synaptic protein synthesis and synapse formation restores synaptic function as a leading idea of how NMDA antagonist induced neuroplasticity leads to antidepressant action (; ; ; ; ; ; Zanos, 2018). It is not immediately clear what role, if any, that altered mental status changes such as dissociation play in the therapeutic efficacy of NMDA antagonists for depression.
Two hypotheses potentially explain the NMDA antagonist mechanism of rapid onset antidepressant effects. One posits that it is the downstream release of a burst of glutamate by NMDA antagonism that leads in turn to stimulation of another glutamate receptor, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid is what provokes the synthesis and release of growth factors to cause synaptogenesis (Krystal, 2019; reviewed in ). Another hypothesis suggests that NMDA blockade of the NMDA receptor subtype C and D receptors (those not blocked by magnesium at rest and thus most sensitive to blockade by open channel NMDA antagonists) that induces the synthesis and release of growth factors such as BDNF (; ). In either case, the final common pathway is hypothesized to be induction of neuroplasticity via synaptogenesis from release of growth factors.
GABA targeted therapeutics; GABA A PAMs acting at neurosteroid sites are neuroplastogens
The GABAergic system is a relatively new target for an antidepressant, as this neurotransmitter system has been classically targeted by anxiolytics, hypnotics and anticonvulsants, especially by benzodiazepines. Benzodiazepines have long been known as PAMs of a subset of GABA A receptors, namely those containing gamma subunits that mediate phasic inhibition at GABA synapses (reviewed in ). However, it is now known that a new set of GABA A targets is emerging for antidepressant action, namely the neurosteroid PAM site (). The actions of neurosteroids not only target the familiar GABA A receptors where benzodiazepines act, but also at an entirely different set of GABA A receptors as well (reviewed in ; ). That is, benzodiazepines as well as neurosteroids are PAMs at those GABA A receptors that are synaptic, benzodiazepine sensitive, contain gamma subunits, and mediate phasic inhibition at GABA synapses (). Neurosteroids but not benzodiazepines, are PAMs at additional GABA A receptors: namely, those that are extra synaptic, benzodiazepine insensitive, contain delta subunits and mediate tonic inhibition at GABA synapses (). Since benzodiazepines do not have particularly effective antidepressant actions, it is these extrasynaptic GABA A receptors (neurosteroid sensitive and benzodiazepine insensitive) that are thought to be the targets for rapid acting antidepressant effects (; ). The neuroplastic actions of neurosteroids trigger changes in tonic inhibition at GABA synapses, down regulation of GABA A receptors, secretion of growth factors such as BDNF and axonal sprouting, with presumed synaptogenesis (; ; ; ; ; ; ). Preclinical research also shows that BDNF is released by neuroactive steroids (; ; ; ; ) and presumably rapid onset neuroplasticity is a mediator of antidepressant action of these GABA A PAMs as well.
One neurosteroid GABA A PAM brexanolone is already approved as a 60-h intravenous infusion for the treatment of post-partum depression (; ). This particular type of depression is linked to rapid reduction in the high neurosteroid levels of pregnancy immediately following delivery of a baby. It appears that restoring the high neurosteroid levels normally associated with pregnancy for a few hours when depression occurs soon after giving birth can not only be immediately effective but durable without the need for further antidepressant treatment, perhaps by allowing more time for receptors to reset to normal lower levels of neurosteroids of women who are not pregnant (; ).
Clinical trials of an oral neurosteroid zuranolone in non-post-partum major depressive disorder also show rapid onset antidepressant efficacy (). Much more needs to be done to document this phenomenon in neurosteroid-induced antidepressant action, including why the antidepressant actions after 2 weeks of oral administration and then drug discontinuation are durable in some depressed patients and not in other cases () (Table 3). Mental status changes other than sedation are not generally associated with the neurosteroid GABA A PAMs ().
Psychedelics targeting serotonin: Psychoplastogens and potential neuroplastogens?
While psychedelics have been used in various cultures for centuries, and a brief era of interest in their potential therapeutic actions occurred in the 1950s and 1960s, regulations restricted their therapeutic and research use thereafter (). However, new understanding of the potential benefits of this class is emerging (; ; ; ; ). Psychedelics by definition cause psychotomimetic and hallucinatory experiences and are now known to induce neuroplasticity and synaptogenesis very quickly, in the same time course as the hallucinatory experiences and rapid antidepressant action (; ; ; ; ). A great debate exists in the field as to whether one wants a psychoplastogen or a neuroplastogen acting at serotonin receptors (Table 3). Those in favor of mental status changes believe that this is part of the therapeutic experience and without it, psychedelics would be ineffective (; ; ). This leads to discussions about how to regulate the patient’s experience during hallucinatory states following dosing of a psychedelic. On the other hand, groups such as are working to remove the psychotomimetic experience and preserve rapid neuroplasticity and antidepressant effects possibly by altering the manner in which psychedelics engage serotonin receptors, especially 5HT2A and 5HT2C receptors. Ideas include developing new agonists for these receptors with biased agonism for neuroplasticity without psychotomimetic actions, possibly by exploiting different signal transduction pathways, heterodimerization of serotonin receptors with Trk B receptors, theoretically resulting in a neuroplastic rather than a psychoplastic signal result (). Time will tell whether the best psychedelic derived antidepressants will prove to be psychoplastogens or neuroplastogens.
Summary
Finally, after more than 60 years of monoamine drugs for depression that take weeks to act and result in symptomatic remission in only a subset of patients, the field has now exploded with promising new agents for depression. Rapid onset of brain plasticity triggered at three novel pharmacologic targets is driving innovation for rapid onset antidepressant actions and for patients who fail to respond to classical monoaminergic agents for depression. Many questions remain to be answered but advances in treatment for depression are proceeding at a fast pace, and much of the research is aimed at unlocking the therapeutic mechanisms linked to neuroplasticity.
Declaration of conflicting interests The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: neither Dr. Cooper nor Dr. Seigler has anything to disclose.
Declaration of conflicting interests Dr. Stahl has served as a consultant to Acadia, Alkermes, Allergan, AbbVie, Axsome, Clearview, Eisai Pharmaceuticals, Gedeon Richter, Intra-Cellular Therapies, Karuna, Levo Therapeutics, Lundbeck, Merck, Neurocrine, Otsuka, Relmada, Sage Therapeutics, Sunovion, Supernus, Taliaz, Teva, Tris Pharma, and VistaGen; he holds options in Genomind, Lipidio, and Delix; he has served on speakers bureaus for Acadia, Lundbeck, Otsuka, Perrigo, Servier, Sunovion, and Teva; and he has received research and/or grant support from Acadia, Allergan/AbbVie, Avanir, Braeburn Pharmaceuticals, Eisai, Eli Lilly, Harmony Biosciences, Indivior, Intra-Cellular Therapies, Ironshore, Neurocrine, Otsuka, Pear Therapeutics, Sage, Shire Sunovion, Supernus, and Torrent.
Funding The author(s) received no financial support for the research, authorship, and/or publication of this article.
Takesha Cooper
https://orcid.org/0000-0001-8850-3136
Michael David Seigler
https://orcid.org/0000-0001-8634-0572
References
- Almeida FB, Nin MS, Barros HMT (2020) The role of allopregnanolone in depressive-like behaviors: Focus on neurotrophic proteins. Neurobiol Stress 12: 100218. DOI:
- Bader V, Winklhofer KF (2020). Mitochondria at the interface between neurodegeneration and neuroinflammation. Semin Cell Dev Biol 99: 163–171. DOI:
- Bauer ME, Teixeira AL (2021) Neuroinflammation in mood disorders: Role of regulatory immune cells. Neuroimmunomodulation 28: 99–107. DOI:
- Benatti C, Blom JM, Rigillo G, et al. (2016). Disease-induced neuroinflammation and depression. CNS Neurol Disord Drug Targets 15: 414–433. DOI:
- Bettini E, Stahl SM, De Martin S, et al. (2022) Pharmacological comparative characterization of REL-1017 (Esmethadone-HCl) and other NMDAR channel blockers in human heterodimeric N-methyl-d-aspartate receptors. Pharmaceuticals 15: 882–883. DOI:
- Björkholm C, Monteggia LM (2016) BDNF – A key transducer of antidepressant effects. Neuropharmacology 102: 72–79. DOI:
- Capuco A, Urits I, Hasoon J, et al. (2020) Current perspectives on gut microbiome dysbiosis and depression. Adv Ther 37: 1328–1346. DOI:
- Carlezon WA, Duman RS, Nestler EJ (2005) The many faces of CREB. Trends Neurosci 28: 436–445. DOI: PMID 15982754. S2CID 6480593.
- Cramer SC, Sur M, Dobkin BH, et al. (2011) Harnessing neuroplasticity for clinical applications. Brain 134, 1591–1609. DOI:
- De Martin S, Gabbia D, Folli F, et al. (2021) REL-1017 (esmethadone) increases circulating BDNF levels in healthy subjects of a phase 1 clinical study. Front Pharmacol 12: 671859. DOI:
- Dimsdale JE (2021) Dark Persuasion: A History of Brainwashing From Pavlov to Social Media. New Haven, CT: Yale University Press.
- Duman CH, Duman RS (2015) Spine synapse remodeling in the pathophysiology and treatment of depression. Neurosci Lett 601: 20–29.
- Erritzoe E, Godlewska BR, Rizzo G, et al. (2022) Brain serotonin release is reduced in patients with depression: A [11C]-Cimbi-36 positron emission tomography study with D-amphetamine challenge. Biol Psychiatry. Epub ahead of print 29 October 2022. DOI:
- Fava M, Stahl S, Pani L, et al. (2022) REL-1017 (esmethadone) as adjunctive treatment in patients with major depressive disorder: A phase 2a randomized double-blind trial. Am J Psychiatry 179: 122–131. DOI:
- Fogaça MV, Fukumoto K, Franklin T, et al. (2019) N-Methyl-D-aspartate receptor antagonist D-methadone produces rapid, mTORC1-dependent antidepressant effects. Neuropsychopharmacology 44: 2230–2238. DOI:
- Frieder A, Fersh M, Hainline R, et al. (2019) Pharmacotherapy of postpartum depression: Current approaches and novel drug development. CNS Drugs 33: 265–282. DOI:
- Gerhard DM, Wohleb ES, Duman RS (2016) Emerging treatment mechanisms for depression: Focus on glutamate and synaptic plasticity. Drug Discov Today 21: 454–464. DOI:
- Gonda X, Dome P, Neill JC, et al. (2021) Novel antidepressant drugs: Beyond monoamine targets. CNS Spectr 28: 1–10. DOI:
- Goodwin GM, Aaronson ST, Alvarez O, et al. (2022) Single dose psilocybin for a treatment-resistant episode of major depression. N Engl J Med 387L18l: 1637–1648.
- Gunduz-Bruce H, Silber C, Kau L, et al. (2019) Trial of SAGE-217 in patients with major depressive disorder. N Engl J Med 381: 903–911. DOI:
- Hare BD, Duman RS (2020) Prefrontal cortex circuits in depression and anxiety: Contribution of discrete neuronal populations and target regions. Mol Psychiatry 25: 2742–2758. DOI:
- Henter ID, Park LT, Zarate CA Jr (2021) Novel glutamatergic modulators for the treatment of mood disorders: Current status. CNS Drugs 35: 527–543. DOI:
- Hong J, Vernon D, Kunovac J, et al. (2022) Emerging drugs for the treatment of major depressive disorder. Expert Opin Emerg Drugs 27: 1–13. DOI:
- Krystal JH, Abdallah CG, Sanacora G, et al. (2019) Ketamine: A paradigm shift for depression research and treatment. Neuron 101: 774–778. DOI:
- Ly C, Greb AC, Cameron LP, et al. (2018) Psychedelics promote structural and functional neural plasticity. Cell Rep 23: 3170–3182. DOI:
- McIntyre RS, Subramaniapillai M, Lee Y, et al. (2019) Efficacy of adjunctive infliximab vs placebo in the treatment of adults with bipolar I/II depression: A randomized clinical trial. JAMA Psychiatry 76: 783–790. DOI:
- Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. (2018) Brexanolone injection in post-partum depression: Two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 392: 1058–1070. DOI:
- Molero P, Ramos-Quiroga JA, Martin-Santos R, et al. (2018) Antidepressant efficacy and tolerability of ketamine and esketamine: A critical review. CNS Drugs 32: 411–420. DOI:
- Moncrieff J, Cooper RE, Stockmann T, et al. (2022) The serotonin theory of depression: A systematic umbrella review of the evidence. Mol Psychiatry 27. DOI:
- Niciu MJ, Ionescu DF, Richards EM, et al. (2014) Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm 12: 907–924. DOI:
- Nielson EM, Guss J (2018) The influence of therapists’ first-hand experience with psychedelics on psychedelic-assisted psychotherapy research and therapist training. J Psychedelic Stud 2: 64–73. DOI:
- Nin MS, Martinez LA, Pibiri F, et al. (2011) Neurosteroids reduce social isolation-induced behavioral deficits: A proposed link with neurosteroid-mediated upregulation of BDNF expression. Front Endocrinol 2: 73. DOI:
- Olsen D (2020) The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci 4: 563–567.
- Pallotto M, Deprez F (2014) Regulation of adult neurogenesis by GABAergic transmission: Signaling beyond GABAA-receptors. Front Cell Neurosci 8: 166. DOI:
- Pittenger C, Duman RS (2008) Stress, depression and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology 33: 88–109.
- Price RB, Duman R (2020) Neuroplasticity in cognitive and psychological mechanisms of depression: An integrative model. Mol Psychiatry 25: 530–543. DOI:
- Reddy HM, Poole JS, Maguire GA, et al. (2020) New medications for neuropsychiatric disorders. Psychiatr Clin North Am 43: 399–413. DOI:
- Reiff CM, Richman EE, Nemeroff CB, et al. (2020) Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry 177: 391–410. DOI:
- Shimizu H, Ishizuka Y, Yamazaie H, et al. (2015) Alloprengnanolone increases mature excitatory synapses along dendrites via proteinkinase A signalling. Neuroscience 305: 139–145.
- Singh B, Henneberger C, Betances D, et al. (2006) Altered balance of glutamatergic/GABAergic synaptic input and associated changes in dendrite morphology after BDNF expression in BDNF deficient hippocampal neurons. J Neurosci 26: 7189–7200.
- Stahl S (2019) Dextromethorphan/bupropion: A novel oral NMDA (N-methyl-D-aspartate) receptor antagonist with multimodal activity. CNS Spectr 24: 461–466. DOI:
- Stahl SM (2021) Stahl’s essential psychopharmacology. In: Mood Disorders, 5th edn, chapter 6. New York, NY: Cambridge University Press, pp 244–282.
- Stahl SM, DeMartin S, Mattarei A, et al. (2022) Esmethadone (REL-1017) and other uncompetitive NMDAR channel blockers may Improve mood disorders via modulation of synaptic kinase-mediated signaling. Int J Mol Sci 23: 12196. DOI:
- Stewart CA, Reid IC (2000) Repeated ECS and fluoxetine administration have equivalent effects on hippocampal synaptic plasticity. Psychopharmacology 148: 217–223. DOI:
- Tabuteau H, Jones A, Anderson A, et al. (2022) Effect of AXS-05 (dextromethorphan-bupropion) in major depressive disorder: A randomized double-blind controlled trial. Am J Psychiatry 179: 490–499. DOI:
- Troubat R, Barone P, Leman S, et al. (2021) Neuroinflammation and depression: A review. Eur J Neurosci 53: 151–171. DOI:
- Vashchinkina E, Manner AK, Vekovisheva O, et al. (2014) Neurosteroid agonist at GABA A receptor induces persistent neuroplasticity in VTA dopamine neurons. Neuropsychopharmacol 39: 727–737.
- Wang M (2011) Neurosteroids GABA-A receptor function. Front Endocrinol 2: DOI:
- Zanos P, Gould TD (2018) Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23: 801–811. DOI:
- Zhang JC, Yao W, Hashimoto K (2022) Arketamine, a new rapid-acting antidepressant: A historical review and future directions. Neuropharmacology 218: 109219. DOI: