Introduction
Eating disorders (EDs), including Anorexia Nervosa (AN), Bulimia Nervosa (BN), and Binge Eating Disorder (BED)—the worldwide lifetime prevalence of which has been estimated to be approximately 4%–5%—have been defined as psychiatric disorders characterized by disturbances in eating behaviors, and related preoccupations around body shape, weight, and eating (). The widely known approach to the pathogenesis of EDs is the interplay of environmental factors with genetic predisposition (; ; ; ; ). Although the exact mechanism underpinning this interplay is still unclear, a widely accepted assumption of environmental effects on neurobiological processes is the epigenetic modification of key regulatory genes (). Dynamic epigenetic processes help fine-tune the gene expression repertoire in response to environmental stimuli (). For example, higher hippocampal serotonin (5-hydroxytryptamine; (5-HT)) 1A receptor expression was reported in individuals with posttraumatic stress disorder relative to controls (reviewed by ), and among unmedicated participants with a current depressive episode, who were exposed to childhood adversity (). In addition, recent life stress was associated with elevated 5-HT1A receptor levels in the amygdala, insula, and orbital cortex of depressed individuals in another cohort (). Similarly, heightened 5-HT1A receptor density in the fronto-temporo-parietal brain regions was observed in individuals with EDs (not only in actively ill but also in weight-restored individuals) relative to healthy controls (reviewed by ; ). In other words, environmental stressors might disrupt developmental trajectories and outcomes by interfering with the neurobiological framework (), changing receptors’ expression, which, in turn, can increase individual vulnerability to psychiatric disorders, including EDs.
Over the years, many neurobiological systems have been linked to EDs (e.g., ; ), with 5-HT possibly as the most commonly investigated system (e.g., ; ). Alterations in central 5-HT system functioning are thought to contribute to dysregulation in eating behaviors as well as to related cognitive and emotional correlates of EDs, including perfectionism, impulsivity, and emotional reactivity (). Such alterations may occur at various levels, including (but not limited to) the functioning of multiple receptors (e.g., 5-HT1A, 5-HT2A), the serotonin transporter (5-HTT/SLC6A4), enzyme involved in amine neurotransmitters’ metabolism (monoamine oxidase A; (MAOA)), and rate-limiting enzymes for brain 5-HT synthesis (tryptophan hydroxylase 1 and 2; (TPH1, TPH2)) (). It is important to highlight the multiple layers of serotonergic neurotransmission control, with 5-HT1A autoreceptors localized on the cell body attenuating the firing rate of the serotonergic neurons, and the 5-HT1B autoreceptors at the axon terminals blocking the synaptic 5-HT release. These receptors can also act as heteroreceptors, controlling other neurotransmitters’ release in different brain regions (; ). Therefore, their multiple roles should be taken into account when interpreting the consequences of their pharmacological stimulation/inhibition via systemic administration of agonist or antagonist drugs. Moreover, the functions of 5-HT1A receptors can change throughout developmental phases. Therefore, we should consider both their spatial and temporal roles when interpreting experimental results ().
In addition to 5-HT, research has supported the role of oxytocin (OT) in the pathophysiology of EDs (e.g., ; ; ; ). Like 5-HT, OT has been shown to play a significant role in regulating affect, cognitive control, metabolism, stress, and social behaviors (e.g. ; ), all important correlates of risk or maintenance factors for disordered eating behaviors. Also, OT has been found to stimulate the release of 5-HT and modulate its availability in the primates’ central nervous system (). Hence, the therapeutic effects of central oxytocinergic and serotonergic stimulation might shed light on the neurobiological mechanisms in eating patterns, namely the regulation of caloric intake, metabolism, and weight (for review, see ; ; ).
The current paper aims to review specifically the role of 5-HT and OT, particularly their interactions, in the (risk for) disordered eating. It is clear that EDs have multifactorial causes and involve various neurobiological (e.g., metabolic, immunological, neurodevelopmental) substrates (), which is why alterations in 5-HT and OT would not necessarily fully explain the variance in ED risk for a given individual. That being said, synthesizing results of research conducted in animals and humans on 5-HT and OT and their interactions is relevant, considering that these two biological processes are among the most widely studied neurobiological factors in relation to eating behaviors and EDs. In spite of extensive animal work on maladaptive eating behaviors, translational studies in humans remain scarce. While this limits the applicability of existing research evidence in the context of EDs, animal models including (but not limited to) an activity-based anorexia model have generated certain key symptoms in individuals with AN (), hence ensuring their relevance in the current review. Furthermore, in light of the view that EDs have neurodevelopmental origins (Connan et al., 2003; ), we discuss the role of the central 5-HT and OT in EDs from a neurodevelopmental perspective. Next, we review studies assessing the impact of serotonergic intervention on brain OT and vice versa, those examining the effect of OT on brain 5-HT. We conclude by synthesizing findings from animal models and human studies and describe a developmental biopsychosocial pathway relevant to EDs that includes the role of 5-HT-OT interactions in the brain. Avenues for future research are also discussed.
Brain serotonin and disordered eating
Development of the 5-HT system
Serotonin is a molecule derived from an amino acid called tryptophan, synthesized mainly in the neuroendocrine cells of the stomach and the intestines by TPH1 and decarboxylase enzymes. Behaving as a paracrine messenger in the periphery, 5-HT is also released into the blood, where platelets take it up and release it upon their activation (see review by ). It acts as a neurotransmitter in the enteric nervous system, and in the central nervous system where TPH2 isoform is predominant (reviewed by Pratelli and Pasqualetti, 2020). For the current review, we will mainly focus on brain 5-HT. The central serotonergic neurons are located in the raphe nuclei, and project to almost all brain regions, most importantly to the prefrontal and cingulate cortical regions, the limbic system, and the hypothalamus (). Serotonergic pathways’ development in the brain, including synthesis, innervation, and fiber density, has been reviewed extensively (e.g., ; ). Research findings indicate that the 5-HT neurons are one of the earliest to appear in the developing living organism, emerging as early as the first month of the gestational period. Moreover, each component of the central 5-HT system (e.g., receptors, enzymes) has a unique role in brain development, including neurogenesis, neuronal differentiation, axon myelination, synaptic remodeling, pruning, and maintenance (; ).
Alterations in the central 5-HT system, especially early in life, may lead to long-lasting changes in neurotransmission and brain structure. Indeed, findings of pharmacological studies and genetic (“knock-out” and “knock-in”) animal models that experimentally induced 5-HT depletion during prenatal and early postnatal periods showed associations between persistent reductions in 5-HT concentration and the volume of adult rats’ brain regions, including frontal cortex, hippocampus, thalamus, and raphe nuclei (e.g., ; ; ).
Early environmental adverse factors (such as chronic stress during pregnancy or in infancy) could also alter brain serotonergic activity. For instance, adolescent and adult offspring of rodent and primate mothers subjected to stress during pregnancy—in the form of restraint stress, exposure to nicotine, or altered diet (ethanol-based, high-fat, or protein-deprived)—displayed an increase in TPH2, 5-HTT, and 5-HT2A receptors’ availability in the raphe, hippocampal, and frontal cortical regions (e.g., ; ; ; ; ; ; ). A decrease in 5-HT1A receptor availability in the cerebral cortex and brainstem has also been reported in adult male rats after perinatal nicotine exposure (). In addition, exposure to early-life social stress in the form of parental deprivation (peer-reared condition) in rhesus monkeys has been associated with a decrease in 5-HT1A receptor density and reduced 5-HTT availability in the hypothalamus, hippocampus, and raphe (; ). However, inconsistent results have been reported regarding the 5-HT concentration in the prefrontal cortex in adult male offspring exposed to postnatal maternal separation, with some findings showing an increase () and others indicating a decrease ().
Possible detrimental effects of prenatal adversity on central 5-HT functioning have been observed in human studies. However, studies are mostly correlational. For instance, 5- to 12-year-old children with fetal alcohol syndrome exhibited lowered 5-HTT availability in the prefrontal cortex relative to control children (). In addition, exposure to perinatal adversity, including in utero exposure to nicotine or experiencing asphyxia at birth, has also been linked to lowered 5-HT synthesis capacity in the hippocampus and the orbitofrontal cortex in healthy adults (). Overall, the evidence highlights the importance of 5-HT for the developing brain and the susceptibility of the 5-HT system to early adverse environmental experiences.
The developmental role of central 5-HT: Relevance for EDs
Various research groups examined rodent eating behaviors, measuring different serotonergic components in the brain and using paradigms that include food restriction (acute restraint or starvation) and activity-based models (for review, see ). For instance, a decrease in 5-HTT availability in the frontal cortex has been observed in young adult rats experiencing up to 2 weeks of food restriction, relative to freely fed controls (). Research using the food restriction models showed that pharmacological stimulation of the 5-HT1A receptor in the nucleus accumbens (NAcc) was associated with a dose-dependent reduction in food intake in food-restricted rats (). Later on, reported only transiently inhibited feeding in food-deprived mice after stimulation of 5-HT1A and 5-HT1B receptors, when the drugs were injected in the ventral tegmental area (VTA). Stimulation of 5-HT1B and 5-HT2C receptors in the raphe and hypothalamus was associated with hypophagia in food-deprived mice (reviewed by ). Similarly, 5-HT4 receptor stimulation in the NAcc has been shown to induce hypophagia in food-restricted rats (, ). The (in)activity of 5-HT3,5,6 receptors has not been consistently linked to changes in food intake and body weight (reviewed by , ). The preceding findings suggest that brain 5-HT effects on eating behaviors depend on the brain area stimulated, the dose administered, and the type of receptors targeted (). Nonetheless, while increased central 5-HT level/activity may contribute to hypophagia, decreased central 5-HT levels/activity may contribute to hyperphagia ().
Opposite effects of serotonergic drugs have been shown in activity-based anorexia models in which rodents have restricted food access along with access to a running wheel (). Activity-based symptoms of anorexia were intensified following a primarily peripheral increase of 5-HT (e.g., via subcutaneous or intraperitoneal administration of 5-HT releasing fenfluramine) while the same effect would be observed following a central 5-HT reduction (e.g., via an irreversible TPH enzyme inhibitor parachlorophenylalanine administration; reviewed by ).
Furthermore, the modulatory role of substance use such as nicotine in both brain 5-HT functioning and food intake has been studied. A 3-day-long food deprivation (compared to ad libitum feeding regime) in rats was associated with a decrease in serotonergic neurons in the raphe, which, in turn, was linked to an increased desire for food (). Co-administration of intraperitoneal nicotine to these food-deprived rats counteracted this effect by increasing the number of serotonergic cells in the raphe, which, in turn, was linked to a suppressed food desire (). Such findings are relevant for AN, an ED characterized by food restriction and, at least in a subgroup of individuals, a heightened rate of smoking and nicotine dependence (). Indeed, alterations in the brain 5-HT levels might influence our susceptibility to such reinforcers as tobacco and food (), increasing vulnerability to various disorders, including EDs.
In living humans, 5-HT effects on mood, cognition, and food intake can be studied reliably in the laboratory with acute tryptophan depletion—a method used to experimentally lower 5-HT levels by administering an amino acid mixture that includes all essential amino acids except tryptophan, thereby temporarily reducing 5-HT synthesis (). Research has shown that acute tryptophan depletion leads to increases in depressive symptoms, anxiety, irritability, desire to binge, food intake, and body image-related fears in individuals currently diagnosed with or recovered from BN, compared to healthy controls (e.g., ; ; ; ). In individuals diagnosed with AN, one study found that, relative to controls, acute tryptophan depletion led to decreased anxiety among currently symptomatic and recovered women (), while another study did not find any significant change in either mood or anxiety among individuals recovered from AN (see ). Further research is necessary to clarify the link between 5-HT neurotransmission and psychological functioning in individuals recovered from AN.
No acute tryptophan depletion studies so far have been conducted in individuals with BED. In the general population, an ad libitum consumption of high-carbohydrate (vs high-protein or mixed) meals has been associated with an increased tryptophan to other large neutral amino acid ratio (; ). Blood tryptophan level has been negatively associated with the desire to binge, regardless of the meal type (). It could be argued that, unlike healthy individuals in whom tryptophan availability and food intake work together to regulate appetite and eating patterns, individuals with (or susceptible to) BN present a disturbed relation between tryptophan, 5-HT, and food intake. On one hand, lowered tryptophan levels (induced by dietary restriction) can trigger binge-eating episodes, thereby perpetuating a cycle of bingeing and purging. On the other hand, food intake in individuals with AN may contribute to alterations (most likely, increase) in 5-HT levels and to dysphoric mood. This, in turn, increases the likelihood of these individuals re-engaging in fasting to reduce distress (). The preceding would perpetuate a reciprocal relation between 5-HT disturbances, impaired psychological functioning, and maladaptive (restrictive) eating behaviors despite self-starvation.
Human research on neurobiological correlates of EDs has used brain imaging techniques of single-photon emission computed tomography (SPECT) and positron emission tomography (PET) combined with various radioligands to characterize the functioning of the brain serotonergic components in vivo. Although PET studies are usually not feasible in research on a large-scale basis and are primarily correlational, PET is, to date, the most direct method to study neurotransmitters in the living human brain (). Considering the clinical presentation of EDs involving a complex interplay between motivational processes, cognitions, and affect, PET studies provide insight into neurochemical correlates of ED symptoms that animal models cannot address. Indeed, findings of PET neuroimaging research comparing the functioning of elements in the brain serotonergic system of healthy adults and those with AN and BN showed heightened 5-HT1A receptor activity (represented by an increase in [11C]WAY100635 or [18F]MPPF ligand’s binding potential) in the clinical groups (for review, see e.g., ). In addition, lowered central 5-HTT activity (indicated by reduced [11C]DASB, [11C]MADAM, or [123I]nor-beta-CIT ligands’ binding potential) in the hypothalamus, NAcc, and raphe was observed in individuals diagnosed with AN, BED, and in those diagnosed with or recovered from BN (vs healthy controls) (e.g., ; ; ; ; ).
Overall, animal and human research supported the role of the 5-HT system in disordered eating. Alterations in the functioning of central 5-HT components, including a decrease in 5-HTT in the raphe and an increase in 5-HT1A receptors’ activity in the frontal brain regions and the hypothalamus, have been observed in hypophagic food-deprived animals as well as in individuals with EDs.
Brain OT and disordered eating
Development of the OT system
OT is a neuropeptide mainly produced by hypothalamic neurons projecting to the pituitary gland and other parts of the central nervous system (; ) to influence various behaviors including eating and social behaviors (; ; ). The major part of OT production occurs in the paraventricular and supraoptic nuclei shortly after birth (; ; ), with OT receptor (OXTR) emerging as early as the first day after birth in rats (; ) and potentially within the first postnatal weeks in primates ().
OT operates through its receptor, expressed in the amygdala, hippocampus, NAcc, and hypothalamus (; ; ). OT signaling is involved in hypothalamic development by governing the formation of new blood vessels (). Animal research indicated that in addition to modulating brain activity (; ), OT affects the maturation of various brain regions (e.g., amygdala, hippocampus, hypothalamus, neocortex) across the lifespan, with higher contribution for subcortical regional development within two postnatal weeks and for neocortical development during the third postnatal week (; ). Hence, alterations occurring in the brain OT system, particularly early in life, might be followed by persistent modifications in the central OT pathway and brain development. For instance, findings from knockout animal studies showed that OT depletion was associated with persistent reductions of neuronal activity in the adult rodents’ brain regions, including hypothalamus and amygdala (; ; ).
Early environmental adverse factors could also alter OT levels in the brain. Research evidence stems primarily from animal studies in which rodents have been exposed to prenatal stress in the form of exposure to ethanol (via a liquid diet) or exposure to nicotine (via an osmotic minipump) and to early postnatal stress in the form of maternal separation or deprivation. For instance, relative to non-stressed controls, adult rats born to dams subjected to stress during pregnancy presented impaired social behaviors and diminished OT levels in the brain, particularly in the hypothalamus and VTA (e.g., ; ). The social impairments were reversed via central OT administration into the amygdala (). Consistent with the preceding finding, mice exposed to high (relative to low) levels of maternal and peer interactions from birth to weaning displayed greater OXTR availability in the amygdala (; ) and more affiliative (less aggressive) behaviors ().
Overall, early-life environmental conditions influence brain OT and social behaviors later in life. Essentially, OT is involved in various prosocial behaviors such as sharing resources, including food, and bonding with others, particularly mates, family, and peers (e.g., ; ; ). OT effects on these behaviors depend on whether the environment increases or hinders closeness to others (; ). Impairments in social bonding—mainly characterized by lowered parental sensitivity to the offspring and by offspring’s insecure attachment early in life and their subsequent relationship difficulties with mates—are central in many interpersonal models of EDs (). More precisely, inadequate childhood attachment plays a key role in developing maladaptive interpersonal skills, including fear of being perceived negatively by others and avoidance of expressing emotions in front of others, resulting in poor social support. In turn, poor social support could perpetuate disordered eating ().
Social attachment and disordered eating: Implication of brain OT
Enduring attachment, particularly the early-life bond between the child and the primary caregiver, is crucial for the quality of interpersonal relationships and general well-being. Impaired social attachment has been discussed as a risk factor for developing EDs (; ). Social attachment has been argued to enhance the functioning of an internal working model—a framework featuring various mental representations allowing the understanding of the self, others, and the world—contributing to social cognition, emotion processing, and behavior regulation (; ). In line with the preceding, individuals with a personal or parental history of EDs were more likely to have experienced insecure attachment, poor parent child interaction, in particular during feeding, as well as to have difficulties recognizing other people’s emotions and regulating one’s emotions and behaviors, relative to healthy individuals without any history of EDs (; ).
OT is perhaps the most commonly investigated neuroendocrine modulator of social attachment and eating behaviors. Released primarily during breastfeeding, OT is essential for mother–infant attachment as it increases parental sensitivity toward the child and positive parent–child interactions (). The impairment of mother–infant bonding may affect attachment style, with possible consequences for the future development of relationships (). Furthermore, attachment anxiety has been associated with a greater risk of impaired self-identity (), with a possible tendency to employ body weight as a basis of self-definition (). In addition, insecure early-life attachment has been associated with psychological inflexibility in adulthood (), the central components of which have been posited to be such maladaptive coping strategies as cognitive fusion, defined as a tendency to get deeply entangled in one’s thoughts, and experiential avoidance, characterized by unwillingness to acknowledge unwanted inner experiences (). In turn, impaired self-identity, more ruminative thoughts about eating, shape, and weight, and attempted avoidance of related negative thoughts and emotions have been associated with ED symptoms (; ). Accordingly, nurturing familial context and secure parent–infant attachment may play a protective role by promoting body image satisfaction and positive relationships across the lifespan, potentially employing the internalization of stable working models from early in life ().
OT has been involved in various aspects of social attachment and affiliation, including the formation of social bonds and prosocial behaviors (; ). The effect of endogenous peripheral OT concentration in social attachment remains however unclear, with some studies reporting a positive association between (blood or plasma) OT and social attachment anxiety and avoidance (e.g., ; ), and other studies reporting no association between plasma OT levels and attachment dimensions (e.g., ; ). A recent meta-analysis on the relationship between OT and attachment showed that endogenous OT levels, measured peripherally in blood or saliva, were not associated with attachment dimensions (). However, exogenous OT administered intranasally was shown to reduce insecure attachment-related behaviors in adults, especially those related to attachment avoidance ().
In addition to its involvement in social attachment, OT has been linked to eating behaviors. Its primary effect is to reduce food intake (). Heightened OT levels have been argued to represent a state variable in the context of dietary restriction and socioemotional impairments (). In line with the preceding, a small-scale study showed heightened postprandial serum OT levels in individuals with AN (relative to healthy controls) that were positively associated with symptoms of AN and heightened anxiety (). However, overnight serum OT levels are the lowest in individuals with AN, higher in normal-weight healthy individuals, and the highest in individuals who are overweight or obese (). One hypothesis attempting to explain the possibly contradictory OT pattern in AN suggests that low fasting OT levels represent a trait variable or a response to chronic undernutrition, gradually contributing to the pathogenesis of AN (). This idea is supported by animal research showing that acute fasting was associated with a transient decrease in hypothalamic OT-containing neuronal activity, which was reversible by subsequent food consumption, while chronic fasting-related change remained persistent (). In addition, lowered fasting OT levels (in the brain and blood) were observed in individuals with current AN (; ; ).
Altered OT system functioning may further exacerbate problems in (socio)emotional regulation (), contributing to the development of maladaptive eating behaviors. For example, individuals with dysfunctional eating behaviors may tend to eat alone and avoid social meals. Maladaptive eating behaviors might constitute an attempt to regulate emotions and cope with stress, a link not devoid of oxytocinergic underpinnings. While low (plasma) OT level might reflect a downregulated hormone level due to chronic fasting/undernutrition in the context of AN (), heightened levels of OT were observed in individuals with AN following food intake ().
Early-life environmental influences on OT levels in the rodents’ brain—as seen in the previous section—appear to convene in such brain areas as hypothalamus, amygdala, and hippocampus (; ; ; ; ), which might constitute a neural basis through which OT operates to regulate eating behaviors in humans as well.
The developmental role of central OT: Relevance for EDs
Cumulative evidence of animal studies, involving a change in the brain OT levels via pharmacological manipulations, highlights hypophagic properties of OT (for review, see ; ). For instance, a single OT administration along the brainstem or in the hypothalamus in food-deprived adult male rats has been associated with a decrease in hunger, time spent eating, weight, and food consumption, including sucrose intake (e.g., ; ; ; ; ). In addition, OT administration into the NAcc and VTA has been associated with reduced sucrose intake (), while the administration of OXTR antagonists (e.g., L-368,899) into the VTA has been associated with an increase in sugar consumption in rodents (; ).
Most experimental studies in humans used a double-blind placebo-controlled crossover design involving an intranasal OT administration to individuals with a current diagnosis of an ED and their healthy counterparts. The intranasal administration of OT—via potentially increased central OXTR activity—has been suggested to improve stress reactivity, socioemotional functioning, and (eventually) maladaptive eating behavior in individuals with EDs (). However, results of human studies of intranasal OT effect on eating behaviors and eating-related cognitions are mixed. In one study, women with AN (relative to healthy controls) showed a decrease in attentional bias to food- and body-related images following 10 consecutive intranasal OT treatments, while there was no change in caloric consumption in either group (). In another study, women with BN and BED (relative to healthy controls) displayed increased attentional bias to food images and (sad) emotion recognition (; ). However, there was no OT-related change in binge-eating behavior after a double-dose intranasal OT treatment (). Furthermore, the results of a meta-analysis showed that a single dose of intranasal OT had not been linked to a change in emotion recognition and attentional bias in individuals with AN (). However, exogenous OT has been associated with lowered caloric consumption in healthy individuals but not in individuals with EDs (). The preceding findings indicate the irreproducibility of the intranasally administered OT-related outcomes in humans as well as the importance of taking the OT dose and specific ED diagnosis into account while studying experimentally the potential effect of OT in individuals with EDs. That being said, peripheral administration of OT has been associated with decreased food intake in rats and humans who underwent fasting (, ; ; ). The hypophagic property has also been reported in rats following a central administration of OT (; ; ), which prompts the need to investigate brain OT levels in vivo in humans. While researchers have developed and tested various PET radioligands to map OXTR in vivo (; ), a reliable OT ligand has yet to be established.
Functional magnetic resonance imaging (fMRI) studies combined with intranasal OT administration have shown some insight into the OT-related effects on regional brain function in individuals with EDs. Although methodologies of studies are highly heterogeneous, reviews and meta-analyses of fMRI studies generally conclude OT-related decreased activation in response to food images in various brain areas, including amygdala, VTA, insula, and prefrontal, orbitofrontal, and anterior cingulate cortices among individuals diagnosed with or recovering from AN, relative to healthy controls (). In addition, reduced medial prefrontal and posterior cingulate cortical activity in response to social stimuli has been found in individuals with a history of AN carrying a common OXTR gene variant (rs2254298 A allele) (), which was previously associated with more severe ED symptoms (e.g., eating obsessions and appearance concerns) among individuals with AN by the same research group (). Such altered corticolimbic functioning might underlie some of the maladaptive cognitions and affect regulation processes typically occurring in EDs, including body concerns, fears of being judged by others, as well as lowered motivation and pleasure experienced from food (). These maladaptive emotional-cognitive processes may overturn hypothalamic signaling of hunger (; ) and/or promote restrictive eating and purging behaviors.
The preceding findings highlight the role of brain regions such as the amygdala, insula, and frontal cortical regions as potential neural bases through which OT operates to regulate eating behaviors and associated emotions and cognitions. Considering that 5-HT is released within corticolimbic brain regions (), both OT and 5-HT might operate within a common neural network to manage similar behaviors, emotions, and cognitions. In the next section, the effect of 5-HT alterations in the central nervous system on subsequent OT functioning, and vice versa, is discussed.
Bidirectional relation between serotonin and OT
Effect of 5-HT intervention on central OT
One of the earliest types of evidence about the interplay of the 5-HT and OT systems in the brain came from electrophysiological experiments. An increase in the firing rate of hypothalamic OT-containing neurons was observed following electrical stimulation of serotonergic dorsal raphe neurons in adult male albinos (). Using pharmacological treatments, found that 5,7-dihydroxytryptamine-induced central 5-HT depletion was associated with reduced OT concentration in the pituitary gland, located below the hypothalamus. Intraperitoneal administration of the 5-HT releaser (and 5-HT2 receptor agonist) d-fenfluramine stimulated OT-expressing cells in the rat’s hypothalamus, which was blocked by pre-treatment of selective reuptake inhibitor fluoxetine (). The same research group also found that subcutaneous administration of the 5-HT2 receptor agonist 2,5-dimethoxy-4-iodoamphetamine stimulated OT-expressing cells in the rat’s hypothalamus, which was blocked by pre-treatment of MDL100,907, a 5-HT2A receptor antagonist ().
In addition to the impact of 5-HT on the central OT system, peripheral effects on plasma OT concentration have also been shown. For instance, intraperitoneal administration of the 5-HT releaser d-fenfluramine has been associated with an increase in plasma OT levels (). In addition, intraperitoneal administration of 6-chloro-2-[1-piperazinyl]-pyrazine (CPP) and m-chlorophenylpiperazine (mCPP) has been associated with increased plasma OT levels in rats (). While CPP is mostly known as an agonist to 5-HT2A/C receptors (; ), the mCPP has an affinity for α2-adrenoceptors and most 5-HT receptors (; ), with its strongest action reported as agonists to 5-HT2C receptors (; ; ). The effects of CPP and mCPP were attenuated by pre-treatment of the 5-HT2A/C antagonist ritanserin (). Later on, the 5-HT1A agonist alnespirone was also used to experimentally elevate plasma OT levels in conscious male rats; this effect was blocked by pre-treatment of the 5-HT1A and ß1,2-adrenoceptor antagonist pindolol (). Worth noting is that the heteroreceptor effects of 5-HT1A are possibly more important in this pharmacological action of pindolol in the human brain (), as this drug has similar efficacy at both the pre- and postsynaptic 5-HT1A receptors (). These findings indicate the influence of serotonergic elements on peripheral OT levels, with agonists of the 5-HT1A and 5-HT2 receptor subtypes increasing plasma OT concentration. Results may also point to the possible involvement of other monoamines, such as noradrenaline (see ).
The effects of altered 5-HT levels on OT functioning have also been examined via gene deletions of metabolizing enzymes. For instance, MAOA knockout mice had heightened 5-HT levels in the central nervous system and presented increased hypothalamic OT concentration, compared to intact wild-type controls (). Accordingly, another animal study showed that adult TPH2 knockout mice with experimentally reduced 5-HT levels in the central nervous system presented lowered hypothalamic OT levels (). Overall, results of the studies focusing on the action of 5-HT on OT levels in the brain and in the plasma showed that 5-HT releasers, as well as 5-HT2A/C receptors’ agonists (in contrast with antagonists), tend to stimulate OT-expressing cell activity in the hypothalamus and promote peripheral OT secretion (e.g., ; ; ).
Effect of OT intervention on central 5-HT
Akin to the animal research findings described in the previous subsection, alterations in OT levels have been associated with changes in the brain 5-HT functioning in animals and humans. For instance, OT (vs saline) administration along the brainstem was associated with a decreased 5-HT concentration in the hypothalamus and the hippocampus of adult male rats (, ). Furthermore, results of a PET study conducted in adult macaques showed that OT (vs placebo) injected along the brainstem was associated with decreased 5-HTT and increased 5-HT1A receptor availability in the amygdala and insula (). Similar results were found in a PET study conducted in human adults, where intranasal OT (vs placebo) administration was associated with increased in vivo measures of 5-HT1A receptor availability in the amygdala/(para)hippocampus complex and the raphe (). Overall, animal and human research evidence suggests that OT exerts an action on the brain 5-HT system, indicated by decreased regional 5-HTT availability and increased availability of 5-HT1A receptors in the amygdala and hippocampus. Also, prairie voles that have been injected intraperitoneally with OT (vs saline solution) during their first postnatal days displayed higher 5-HT axon length in the amygdala and hypothalamus in adolescence (). Considering that axon length is argued to contribute to the velocity of propagation of the action potentials (), further research is necessary to assess the impact of OT on 5-HT neurotransmission.
The abovementioned findings indicate that OT and 5-HT might operate within a typical neural network, including the hypothalamus, amygdala, and brainstem, contributing to maladaptive eating behaviors and related emotions. Adult rats exposed to early postnatal maternal deprivation displayed decreased 5-HT concentration in the hypothalamus, heightened anxiety, as well as decreased standard chow food intake compared to the intact control group (). Underlying such cooperation may be simultaneous alterations in the hypothalamic concentration of 5-HT and OT, especially considering that the hypothalamus is the neural root of the neuropeptides, including (but not limited to) OT (). Following this line of thought, findings of another recent study showed preliminary evidence of physical interaction between brain 5-HT2C and OXTR receptors in vitro (). However, the interaction effect between OT and 5-HT in EDs has not been studied systematically.
Discussion
Integrative summary
In the present review, we summarized the existing evidence on the involvement of both central 5-HT and OT in EDs. Animal studies have shown that pharmacological stimulation of 5-HT1B and 5-HT2C receptors in the hypothalamus and raphe nucleus of the brainstem, and 5-HT4 receptor stimulation in the NAcc was associated with lowered food intake in food-restricted and food-deprived rats (reviewed by ). In humans, PET studies have shown that the presence of AN or BN diagnoses was associated with increased 5-HT1A post-synaptic receptor density in the fronto-temporo-parietal regions (), and with decreased 5-HTT availability in such brain regions as the hypothalamus, raphe, and NAcc, as assessed in vivo (; ; ). In addition, increased OT levels in the hypothalamus and brainstem were associated with reduced food desire and intake in food-deprived rats (; ; ). To date, human studies involving individuals with EDs and healthy controls used a double-blind, placebo-controlled crossover design at intranasal OT treatment, suggesting to act through central OXTR activity (). The published findings indicate the irreproducibility of intranasal OT-related outcomes (; ). That being said, decreased food intake has been reported in humans and rats who underwent fasting following a peripheral administration of OT (; ) as well as in rats following a central administration of OT (; ), prompting the need to investigate brain OT levels in humans. However, a reliable PET radioligand to map OT in vivo remains to be established.
Central serotoninergic and oxytocinergic systems were found to exert a reciprocal effect via a common neural network. Specifically, 5-HT2A/C receptors’ activity in the brain was positively associated with OT-expressing cell activity in the hypothalamus (; ), while OT increased 5-HT1A receptor availability in the amygdala, hippocampus, and raphe () and decreased 5-HTT availability in the amygdala, insula, and hippocampus ().
Considering that both 5-HT and OT are involved in brain development (; ), they could both be susceptible to environmental stressors, especially those occurring early in life. For instance, the quality of caregiving and interaction with peers during the early postnatal period was associated with alterations in serotoninergic and oxytocinergic systems in the brain (; ; ).
Because impaired early-life social bonding has been central in interpersonal models of overeating (), we discussed the effects of a negative social environment and disrupted attachment on the brain 5-HT and OT systems. Biological embedding theory, one of the predominant theories attempting to explain the underpinning links between adverse early-life conditions and mental health, posits that stressful experiences can disrupt human developmental course by “getting under the skin” through a specific neurobiological framework, ultimately affecting individual mental health (). These effects on the brain 5-HT and OT (dys)regulation might render some people susceptible to (mal)adaptive eating behaviors and related phenotypes (e.g., anxiety, impulsivity). In addition, elements of the brain 5-HT system that have been consistently linked to brain OT functioning (e.g., ; )—particularly, 5-HT1A and 5-HT2A/C receptors and 5-HTT—have also been found to play an important role in active stress coping (; ), reflecting an organism’s ability to adapt to early-life stressors. Therefore, it could be of relevance for future treatment of EDs to concurrently target the OXTR as well as 5-HT1A and/or 5-HT2A/C receptors and 5-HTT. The preceding, however, remains to be further tested.
Relevance of 5-HT and OT interactions for a developmental biopsychosocial model of EDs
Overall, the research findings reviewed in the present review align with a developmental model of EDs. While EDs are driven by biopsychosocial pathways in which numerous hormones and neurotransmitters (and the interactions between them) are involved, concerning the role of 5-HT and OT, we conclude that vulnerability-inducing 5-HT and OT levels are shaped by early-life environmental adversity (see Figure 1). Epigenetic processes underlying environmental effects may convey alterations in the central 5-HT and OT, through their joint (emotion and reward) neural network, including the prefrontal cortex, hypothalamus, amygdala, hippocampus, NAcc, VTA, and the brainstem. Along with other risk factors, bidirectional interactions between dysregulated 5-HT and OT in the brain, especially early on during development, may contribute to risk for EDs. In addition, combined with an early-life adverse environment and various other neurobiological risk factors, alterations in the central 5-HT and OT interplay might contribute to the formation of insecure attachment. In turn, inadequate social bonding would translate to impairments in cognitive and socioemotional functioning, including lowered emotion recognition and control and heightened anxiety, which, in turn, might impede the individual’s accessibility to and use of social support. Restrictive eating, malnourishment, and food deprivation-induced neurobiological alterations are expected to further trigger the onset of the ED. The ongoing presence of ED symptoms, in turn, may further exacerbate the existing alterations in central 5-HT and OT functioning, progressively aggravating cognitive and socioemotional impairments.
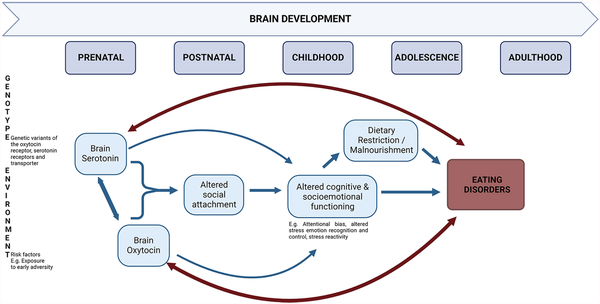
Figure 1
Schematic presentation of the role of the interaction between serotonin and oxytocin in eating disorder development from a developmental biopsychosocial perspective.
While the preceding explanation of how 5-HT and OT may interact and contribute to ED development is plausible, the proposed model requires further study, and the exact physiological mechanisms remain unclear. In addition to early-life environmental stressors, adverse life events later in life have also been associated with altered 5-HT and OT levels as well as with maladaptive eating behaviors and related cognition and affect. For instance, adverse life events at the age of 15 years have been positively associated with the drive for thinness and binge-purge behaviors at the age of 18 years (). Moreover, this effect of current life stress was found to be moderated by the short allele of the 5-HTT-linked promoter region (5-HTTLPR), coding for reduced serotonin transporter expression (). Various research findings suggest that this 5-HTTLPR interacts with stressful life events, with the short allele (as opposed to the long variant) linked to greater sensitivity to negative environmental factors (e.g., ). As for the OT system, the presence of the rs2254298 and the rs53576 minor (A) alleles of intronic polymorphisms in the OXTR gene has been associated with increased vulnerability to stress and negative mental health outcomes, including symptoms of AN and BN (e.g., ; ). By contrast, the presence of the rs53576 major (G) allele was found to enhance socioemotional functioning by promoting self-confidence, security, and a general sense of well-being (e.g., ; ; ). Such psychosocial resources would likely decrease the risk of developing an ED. Overall, research evidence supports that life stressors may heighten susceptibility to 5-HT and OT dysregulation following stress and result in disturbed eating behaviors in at-risk individuals (having “risk” gene variants, for instance). Future research is necessary to elucidate the cumulative role of early- and current-life stressors in shaping the joint vulnerability of 5-HT and OT dysfunctioning to the development of EDs. Furthermore, it would be important to further examine how a 5-HT/OT vulnerability interacts with other neurobiological risk factors, including (but not limited to) metabolic and immunological risk factors (e.g., see ).
It could be speculated that 5-HT and OT-based interventions have the joint potential to contribute to treating EDs or prevention in at-risk individuals by optimizing the functioning of brain regions involved in social rewards and emotion regulation, such as the prefrontal cortex, amygdala, and brainstem. However, refinement of methodologies and a thorough investigation of the intranasal pharmacodynamics are necessary.
Conclusion and avenues for future research
It is clear that EDs are highly multifactorial, involving numerous neurobiological substrates. As such, alterations in 5-HT, OT, and their interaction likely explain only a small fraction of the variance in ED risk for a given individual. Overall, this review highlights the importance of acknowledging the reciprocal interaction between well-studied neurobiological substrates of EDs such as 5-HT and OT. Furthermore, synthesizing relevant animal and human studies’ findings, the present review illustrated the importance of future translational research in EDs that integrates basic animal experiments with applied research in clinical populations. As such, developing more refined animal models of EDs and new methodologies to study peripheral and central 5-HT or OT in humans would be an important step forward to better understand the clinical relevance of joint administration of serotonergic and oxytocinergic drugs as a possible therapeutic or preventive intervention for EDs. Considering the clinical heterogeneity of EDs, future work also needs to consider the role of demographic and clinical factors such as sex, diagnostic status, illness duration, and psychiatric comorbidities to understand how 5-HT and OT affect (or reduce) the ultimate expression of ED-related cognitions, affect, and behaviors.
Author contributions EI designed the review, did the literature search, and drafted the manuscript. ZN revised and edited the manuscript. LB designed the review and revised and edited the manuscript. All authors approved the final manuscript.
Declaration of conflicting interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was facilitated by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) RGPIN/5025-2018 awarded to Dr. Linda Booij and by the Hungarian National Research Development and Innovation Office for bilateral science and technology cooperation project #2019-2.1.11-TÉT-2020-00242 awarded to Dr. Zsofia Nemoda.
Linda Booij
https://orcid.org/0000-0002-0863-8098
References
- Acevedo SF, Valencia C, Lutter M, et al. (2015) Severity of eating disorder symptoms related to oxytocin receptor polymorphisms in anorexia nervosa. Psychiatry Res 228: 641–648.
- Akkermann K, Kaasik K, Kiive E, et al. (2012) The impact of adverse life events and the serotonin transporter gene promoter polymorphism on the development of eating disorder symptoms. J Psychiatric Res 46: 38–43.
- Albert PR, Vahid-Ansari F (2019) The 5-HT1A receptor: Signaling to behavior. Biochimie 161: 34–45.
- Altstein M, Gainer H (1988) Differential biosynthesis and posttranslational processing of vasopressin and oxytocin in rat brain during embryonic and postnatal development. J Neurosci 8: 3967–3977.
- Amianto F, Martini M, Spalatro A, et al. (2017) Body image development within the family: Attachment dynamics and parental attitudes in cross-sectional and longitudinal studies. Acta Psychopathologica 3: 50.
- Anzengruber D, Klump KL, Thornton L, et al. (2006) Smoking in eating disorders. Eat Behav 7: 291–299.
- Arcelus J, Haslam M, Farrow C, et al. (2013) The role of interpersonal functioning in the maintenance of eating psychopathology: A systematic review and testable model. Clin Psychol Rev 33: 156–167.
- Arletti R, Benelli A, Bertolini A (1989) Influence of oxytocin on feeding behavior in the rat. Peptides 10: 89–93.
- Arletti R, Benelli A, Bertolini A (1990) Oxytocin inhibits food and fluid intake in rats. Physiol Behav 48: 825–830.
- Aydin O, Balikçı K, Taş C, et al. (2019) Assessing the relationship between attachment, parental attitude and plasma oxytocin in schizophrenia patients and their unaffected siblings. Nord J Psychiatry 73: 51–57.
- Bailer UF, Kaye WH (2011) Serotonin: Imaging findings in eating disorders. Behav Neurobiol Eat Disord 6: 59–79.
- Bardeen JR, Fergus TA (2016) The interactive effect of cognitive fusion and experiential avoidance on anxiety, depression, stress and posttraumatic stress symptoms. J Context Behav Sci 5: 1–6.
- Bartlett EA, Yttredahl AA, Boldrini M, et al. (2023) In vivo serotonin 1A receptor hippocampal binding potential in depression and reported childhood adversity. Eur Psychiatry 66: e17.
- Bartz JA (2016) Oxytocin and the pharmacological dissection of affiliation. Curr Direct Psychol Sci 25: 104–110.
- Bartz JA, Zaki J, Bolger N, et al. (2011) Social effects of oxytocin in humans: Context and person matter. Trends Cogn Sci 15: 301–309.
- Baumann MH, Ayestas MA, Dersch CM, et al. (2001) 1-(m-Chlorophenyl) piperazine (mCPP) dissociates in vivo serotonin release from long-term serotonin depletion in rat brain. Neuropsychopharmacology 24: 492–501.
- Beard R, Singh N, Grundschober C, et al. (2018) High-yielding 18 F radiosynthesis of a novel oxytocin receptor tracer, a probe for nose-to-brain oxytocin uptake in vivo. Chem Commun 54: 8120–8123.
- Blevins JE, Thompson BW, Anekonda VT, et al. (2016) Chronic CNS oxytocin signaling preferentially induces fat loss in high-fat diet-fed rats by enhancing satiety responses and increasing lipid utilization. Am J Physiol Regul Integr Comp Physiol 310: R640–R658.
- Booij L, Benkelfat C, Leyton M, et al. (2012) Perinatal effects on in vivo measures of human brain serotonin synthesis in adulthood: A 27-year longitudinal study. Eur Neuropsychopharmacol 22: 419–423.
- Booij L, Tremblay RE, Szyf M, et al. (2015) Genetic and early environmental influences on the serotonin system: Consequences for brain development and risk for psychopathology. J Psychiatry Neurosci 40: 5–18.
- Booij L, Steiger H (2020) Applying epigenetic science to the understanding of eating disorders: A promising paradigm for research and practice. Curr Opin Psychiatry 33: 515–520.
- Branchi I, Curley JP, D’Andrea I, et al. (2013) Early interactions with mother and peers independently build adult social skills and shape BDNF and oxytocin receptor brain levels. Psychoneuroendocrinology 38: 522–532.
- Brewerton TD (2022) Mechanisms by which adverse childhood experiences, other traumas and PTSD influence the health and well-being of individuals with eating disorders throughout the life span. J Eat Disord 10: 1–20.
- Bruce KR, Steiger H, Young SN, et al. (2009) Impact of acute tryptophan depletion on mood and eating-related urges in bulimic and nonbulimic women. J Psychiatry Neurosci 34: 376–382.
- Brummelte S, McGlanaghy E, Bonnin A, et al. (2017) Developmental changes in serotonin signaling: Implications for early brain function, behavior and adaptation. Neuroscience 342: 212–231.
- Bulik CM, Coleman JR, Hardaway JA, et al. (2022) Genetics and neurobiology of eating disorders. Nat Neurosci 25: 543–554.
- Caldwell HK, Aulino EA, Freeman AR, et al. (2017) Oxytocin and behavior: Lessons from knockout mice. Develop Neurobiol 77: 190–201.
- Carhart-Harris RL, Nutt DJ (2017) Serotonin and brain function: A tale of two receptors. J Pharmacol 31: 1091–1120.
- Ceccarini J, Liu H, Van Laere K, et al. (2020) Methods for quantifying neurotransmitter dynamics in the living brain with PET imaging. Front Physiol 11: 792.
- Chen CY, Chiang YA, Kuo TC, et al. (2021) Effects of intranasal oxytocin in food intake and craving: A meta-analysis of clinical trials. Clin Nutr 40: 5407–5416.
- Choy VJ, Watkins WB (1979) Maturation of the hypothalamo-neurohypophysial system. I. Localization of neurophysin, oxytocin and vasopressin in the hypothalamus and neural lobe of the developing rat brain. Cell Tissue Res 197: 325–336.
- Chruścicka B, Cowan CS, Fitzsimons SEW, et al. (2021) Molecular, biochemical and behavioral evidence for a novel oxytocin receptor and serotonin 2C receptor heterocomplex. Neuropharmacology 183: 108394.
- Cimino S, Cerniglia L, Porreca A, et al. (2016) Mothers and fathers with Binge Eating Disorder and their 18–36 months old children: A longitudinal study on parent-infant interactions and offspring’s emotional-behavioral profiles. Front Psychol 7: 580.
- Clissold KA, Choi E, Pratt WE (2013) Serotonin 1A, 1B, and 7 receptors of the rat medial nucleus accumbens differentially regulate feeding, water intake, and locomotor activity. Pharmacol Biochem Behav 112: 96–103.
- Compan V (2013) Under-to over-eating: How do serotonins receptors contribute? Future Neurol 8: 701–714.
- Compan V (2020) Serotonin in eating behavior. In: Huston JP, Steiner H (eds.) Handbook of Behavioral Neuroscience. Amsterdam, Netherlands: Elsevier, Vol. 31, pp. 489–503.
- Compan V, Walsh BT, Kaye W, et al. (2015) How does the brain implement adaptive decision making to eat? J Neurosci 35: 13868–13878.
- Connan F, Campbell IC, Katzman M, et al. (2023) A neurodevelopmental model for anorexia nervosa. Physiol Behav 79: 13–24.
- Cowdrey FA, Park RJ (2012) The role of experiential avoidance, rumination and mindfulness in eating disorders. Eat Behav 13: 100–105.
- de Lima RMS, dos Santos, Bento LV, Lugon MD, et al. (2020) Early life stress and the programming of eating behavior and anxiety: Sex-specific relationships with serotonergic activity and hypothalamic neuropeptides. Behav Brain Res 379: 112399.
- Demitrack MA, Lesem MD, Listwak SJ, et al. (1990) CSF oxytocin in anorexia nervosa and bulimia nervosa: clinical and pathophysiologic considerations. Am J Psychiatry 147: 882–886.
- Eaton JL, Roache L, Nguyen KN, et al. (2012) Organizational effects of oxytocin on serotonin innervation. Develop Psychobiol 54: 92–97.
- Ebner NC, Lin T, Muradoglu M, et al. (2019) Associations between oxytocin receptor gene (OXTR) methylation, plasma oxytocin, and attachment across adulthood. Int J Psychophysiol 136: 22–32.
- Ellegood J, Yee Y, Kerr TM, et al. (2018) Analysis of neuroanatomical differences in mice with genetically modified serotonin transporters assessed by structural magnetic resonance imaging. Mol Autism 9: 1–12.
- Erritzoe D, Frokjaer VG, Haugbol S, et al. (2009) Brain serotonin 2A receptor binding: Relations to body mass index, tobacco and alcohol use. Neuroimage 46: 23–30.
- Febo M, Ferris CF (2014) Oxytocin and vasopressin modulation of the neural correlates of motivation and emotion: Results from functional MRI studies in awake rats. Brain Res 1580: 8–21.
- Ferguson JN, Aldag JM, Insel TR, et al. (2001) Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci 21: 8278–8285.
- Francis DD, Champagne FC, Meaney MJ (2000) Variations in maternal behavior are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol 12: 1145–1148.
- Frank GK (2015) Advances from neuroimaging studies in eating disorders. CNS Spectr 20: 391–400.
- Frank GK, Shott ME, DeGuzman MC (2019) The neurobiology of eating disorders. Child Adolesc Psychiatr Clin 28: 629–640.
- Fuller RW, Snoddy HD, Robertson DW (1988) Mechanisms of effects of d-fenfluramine on brain serotonin metabolism in rats: Uptake inhibition versus release. Pharmacol Biochem Behav 30: 715–721.
- Galfalvy H, Shea E, de Vegvar J, et al. (2023) Brain serotonin 1A receptor binding: Relationship to peripheral blood DNA methylation, recent life stress and childhood adversity in unmedicated major depression. Br J Psychiatry 223: 415–421.
- Garcia-Garcia AL, Newman-Tancredi A, Leonardo ED (2014) 5-HT(1A) receptors in mood and anxiety: Recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology 231: 623–636.
- Geerling JC, Shin JW, Chimenti PC, et al. (2010) Paraventricular hypothalamic nucleus: Axonal projections to the brainstem. J Comp Neurol 518: 1460–1499.
- Gendall KA, Joyce PR (2000) Meal-induced changes in tryptophan: LNAA ratio: Effects on craving and binge eating. Eat Behav 1: 53–62.
- Ghaheri S, Niapour A, Sakhaie N, et al. (2022) Postnatal depletion of serotonin affects the morphology of neurons and the function of the hippocampus in male rats. Int J Develop Neurosci 82: 222–230.
- Gianni AD, De Donatis D, Valente S, et al. (2020) Eating disorders: Do PET and SPECT have a role? A systematic review of the literature. Psychiatry Res Neuroimaging 300: 111065.
- Giel K, Zipfel S, Hallschmid M (2018) Oxytocin and eating disorders: A narrative review on emerging findings and perspectives. Curr Neuropharmacol 16: 1111–1121.
- González-Pardo H, Arias JL, Gómez-Lázaro E, et al. (2020) Sex-specific effects of early life stress on brain mitochondrial function, monoamine levels and neuroinflammation. Brain Sci 10: 447.
- Gould BR, Zingg HH (2003) Mapping oxytocin receptor gene expression in the mouse brain and mammary gland using an oxytocin receptor–LacZ reporter mouse. Neuroscience 122: 155–167.
- Grinevich V, Knobloch-Bollmann HS, Eliava M, et al. (2016) Assembling the puzzle: Pathways of oxytocin signaling in the brain. Biol Psychiatry 79: 155–164.
- Gutnick A, Blechman J, Kaslin J, et al. (2011) The hypothalamic neuropeptide oxytocin is required for formation of the neurovascular interface of the pituitary. Develop Cell 21: 642–654.
- Hamik A, Peroutka SJ (1989) 1-(m-Chlorophenyl) piperazine (mCPP) interactions with neurotransmitter receptors in the human brain. Biol Psychiatry 25: 569–575.
- Hammock E, Levitt P (2013) Oxytocin receptor ligand binding in embryonic tissue and postnatal brain development of the C57BL/6J mouse. Front Behav Neurosci 7: 195.
- Hasselbalch KC, Lanng KR, Birkeland M, et al. (2020) Potential shortcomings in current studies on the effect of intranasal oxytocin in Anorexia Nervosa and healthy controls – A systematic review and meta-analysis. Psychopharmacology 237: 2891–2903.
- Hayashi A, Suzuki M, Sasamata M, et al. (2005) Agonist diversity in 5-HT 2C receptor-mediated weight control in rats. Psychopharmacology 178: 241–249.
- Hemrick-Luecke SK, Fuller RW (1996) Involvement of 5-HT2A receptors in the elevation of rat serum corticosterone concentrations by quipazine and MK-212. Eur J Pharmacol 311: 207–211.
- Herisson FM, Waas JR, Fredriksson R, et al. (2016) Oxytocin acting in the nucleus accumbens core decreases food intake. J Neuroendocrinol 28. https://doi.org/10.1111/jne.12381
- Hewitt KN, Lee MD, Dourish CT, et al. (2002) Serotonin 2C receptor agonists and the behavioural satiety sequence in mice. Pharmacol Biochem Behav 71: 691–700.
- Ho JM, Anekonda VT, Thompson BW, et al. (2014) Hindbrain oxytocin receptors contribute to the effects of circulating oxytocin on food intake in male rats. Endocrinology 155: 2845–2857.
- Huether G, Zhou D, Schmidt S, et al. (1997) Long-term food restriction down-regulates the density of serotonin transporters in the rat frontal cortex. Biol Psychiatry 41: 1174–1180.
- Ichise M, Vines DC, Gura T, et al. (2006) Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer-and mother-reared rhesus monkeys. J Neurosci 26: 4638–4643.
- Insel TR, Young L, Witt DM, et al. (1993) Gonadal steroids have paradoxical effects on brain oxytocin receptors. J Neuroendocrinol 5: 619–628.
- Jaenisch R, Bird A (2003) Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat Genet 33: 245–254.
- Javed A, Kamradt MC, Van de Kar LD, et al. (1999) D-Fenfluramine induces serotonin-mediated Fos expression in corticotropin-releasing factor and oxytocin neurons of the hypothalamus, and serotonin-independent Fos expression in enkephalin and neurotensin neurons of the amygdala. Neuroscience 90: 851–858.
- Jean A, Laurent L, Bockaert J, et al. (2012) The nucleus accumbens 5-HTR4-CART pathway ties anorexia to hyperactivity. Transl Psychiatry 2: e203–e203.
- Jean A, Laurent L, Delaunay S, et al. (2017) Adaptive control of dorsal raphe by 5-HT4 in the prefrontal cortex prevents persistent hypophagia following stress. Cell Rep 21: 901–909.
- Jewell T, Collyer H, Gardner T, et al. (2016) Attachment and mentalization and their association with child and adolescent eating pathology: A systematic review. Int J Eat Disord 49: 354–373.
- Jones LA, Sun EW, Martin AM, et al. (2020) The ever-changing roles of serotonin. Int J Biochem Cell Biol 125: 105776.
- Kaye WH, Gendall KA, Fernstrom MH, et al. (2000) Effects of acute tryptophan depletion on mood in bulimia nervosa. Biol Psychiatry 47: 151–157.
- Kaye WH, Barbarich NC, Putnam K, et al. (2003) Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. Int J Eat Disord 33: 257–267.
- Kaye WH, Fudge JL, Paulus M (2009) New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci 10: 573–584.
- Kendrick KM (2000) Oxytocin, motherhood and bonding. Exp Physiol 85: 111s–124s.
- Kennett GA, Curzon G (1988) Evidence that hypophagia induced by m CPP and TFMPP requires 5-HT 1C and 5-HT 1B receptors; hypophagia induced by RU 24969 only requires 5-HT 1B receptors. Psychopharmacology 96: 93–100.
- Kennett GA, Wood MD, Glen A, et al. (1994) In vivo properties of SB 200646A, a 5-HT2C/2B receptor antagonist. Br J Pharmacol 111: 797–802.
- Kim YR, Eom JS, Yang JW, et al. (2015) The impact of oxytocin on food intake and emotion recognition in patients with eating disorders: A double blind single dose within-subject cross-over design. PLoS One 10: e0137514.
- Kim YR, Kim CH, Cardi V, et al. (2014) Intranasal oxytocin attenuates attentional bias for eating and fat shape stimuli in patients with anorexia nervosa. Psychoneuroendocrinology 44: 133–142.
- Krueger F, Parasuraman R, Iyengar V, et al. (2012) Oxytocin receptor genetic variation promotes human trust behavior. Front Human Neurosci 6: 4.
- Lam DD, Garfield AS, Marston OJ, et al. (2010) Brain serotonin system in the coordination of food intake and body weight. Pharmacol Biochem Behav 97: 84–91.
- Landoni M, Dalla Muta A, Di Tella S, et al. (2022) Parenting and the serotonin transporter gene (5HTTLPR), is there an association? A systematic review of the literature. Int J Environ Res Public Health 19: 4052.
- Lawson EA, Holsen LM, Santin M, et al. (2013) Postprandial oxytocin secretion is associated with severity of anxiety and depressive symptoms in anorexia nervosa. J Clin Psychiatry 74: 14838.
- Lawson EA, Holsen LM, Santin M, et al. (2012) Oxytocin secretion is associated with severity of disordered eating psychopathology and insular cortex hypoactivation in anorexia nervosa. J Clin Endocrinol Metab 97: E1898–E1908.
- Lawson EA, Marengi DA, De Santi RL, et al. (2015) Oxytocin reduces caloric intake in men. Obesity 23: 950–956.
- Le LKD, Barendregt JJ, Hay P, et al. (2017) Prevention of eating disorders: A systematic review and meta-analysis. Clin Psychol Rev 53: 46–58.
- Lee PR, Brady DL, Shapiro RA, et al. (2007) Prenatal stress generates deficits in rat social behavior: Reversal by oxytocin. Brain Res 1156: 152–167.
- Lee TH, Jang MH, Shin MC, et al. (2002) cotine administration increases serotonin synthesis and tryptophan hydroxylase expression in dorsal raphe of food-deprived rats. Nutr Res 22: 1445–1452.
- Lefevre A, Richard N, Jazayeri M, et al. (2017) Oxytocin and serotonin brain mechanisms in the nonhuman primate. J Neurosci 37: 6741–6750.
- Leslie M, Silva P, Paloyelis Y, et al. (2018) A systematic review and quantitative meta-analysis of the effects of oxytocin on feeding. J Neuroendocrinol 30: e12584.
- Leslie M, Leppanen J, Paloyelis Y, et al. (2019) The influence of oxytocin on eating behaviors and stress in women with bulimia nervosa and binge eating disorder. Mol Cell Endocrinol 497: 110354.
- Leslie M, Leppanen J, Paloyelis Y, et al. (2020) A pilot study investigating the influence of oxytocin on attentional bias to food images in women with bulimia nervosa or binge eating disorder. J Neuroendocrinol 32: e12843.
- Lewis MW, Jones RT, Davis MT (2020) Exploring the impact of trauma type and extent of exposure on posttraumatic alterations in 5-HT1A expression. Transl Psychiatry 10: 237.
- Liu N, Hadj-Bouziane F, Jones KB, et al. (2015) Oxytocin modulates fMRI responses to facial expression in macaques. Proc Natl Acad Sci 112: E3123–E3130.
- MacDonald K, MacDonald TM (2010) The peptide that binds: A systematic review of oxytocin and its prosocial effects in humans. Harv Rev Psychiatry 18: 1–21.
- Maejima Y, Sakuma K, Santoso P, et al. (2014) Oxytocinergic circuit from paraventricular and supraoptic nuclei to arcuate POMC neurons in hypothalamus. FEBS Lett 588: 4404–4412.
- Maejima Y, Yokota S, Nishimori K, et al. (2018) The anorexigenic neural pathways of oxytocin and their clinical implication. Neuroendocrinology 107: 91–104.
- Majuri J, Joutsa J, Johansson J, et al. (2017) Serotonin transporter density in binge eating disorder and pathological gambling: A PET study with [11C]MADAM. Eur Neuropsychopharmacol 27: 1281–1288.
- Marazziti D, Dell’Osso B, Baroni S, et al. (2006) A relationship between oxytocin and anxiety of romantic attachment. Clin Pract Epidemiol Mental Health 2: 1–6.
- McMurray MS, Williams SK, Jarrett TM, et al. (2008) Gestational ethanol and nicotine exposure: Effects on maternal behavior, oxytocin, and offspring ethanol intake in the rat. Neurotoxicol Teratol 30: 475–486.
- Mele G, Alfano V, Cotugno A, et al. (2020) A broad-spectrum review on multimodal neuroimaging in bulimia nervosa and binge eating disorder. Appetite 151: 104712.
- Migliarini S, Pacini G, Pelosi B, et al. (2013) Lack of brain serotonin affects postnatal development and serotonergic neuronal circuitry formation. Mol Psychiatry 18: 1106–1118.
- Miyagawa K, Tsuji M, Fujimori K, et al. (2011) Prenatal stress induces anxiety-like behavior together with the disruption of central serotonin neurons in mice. Neurosci Res 70: 111–117.
- Mokler DJ, Galler JR, Morgane PJ (2003) Modulation of 5-HT release in the hippocampus of 30-day-old rats exposed in utero to protein malnutrition. Develop Brain Res 142: 203–208.
- Monin JK, Goktas SO, Kershaw T, et al. (2019) Associations between spouses’ oxytocin receptor gene polymorphism, attachment security, and marital satisfaction. PLoS One 14: e0213083.
- Monteleone AM, Castellini G, Volpe U, et al. (2017) The disorder of lived corporeality: A possible link between attachment style and eating disorder psychopathology. Eur Psychiatry 41: S557–S558.
- Monteleone AM, Scognamiglio P, Volpe U, et al. (2016) Investigation of oxytocin secretion in anorexia nervosa and bulimia nervosa: Relationships to temperament personality dimensions. Eur Eat Disord Rev 24: 52–56.
- Mottolese R, Redouté J, Costes N, et al. (2014) Switching brain serotonin with oxytocin. Proc Natl Acad Sci 111: 8637–8642.
- Mullis K, Kay K, Williams DL (2013) Oxytocin action in the ventral tegmental area affects sucrose intake. Brain Res 1513: 85–91.
- Muneoka K, Ogawa T, Kamei K, et al. (2001) Nicotine exposure during pregnancy is a factor which influences serotonin transporter density in the rat brain. Eur J Pharmacol 411: 279–282.
- Nagasawa M, Okabe S, Mogi K, et al. (2012) Oxytocin and mutual communication in mother-infant bonding. Front Human Neurosci 6: 31.
- Nelson CA (2013) Biological embedding of early life adversity. JAMA Pediatrics 167: 1098–1100.
- Neumann ID (2008) Brain oxytocin: A key regulator of emotional and social behaviors in both females and males. J Neuroendocrinol 20: 858–865.
- Neumann ID, Slattery DA (2016) Oxytocin in general anxiety and social fear: A translational approach. Biol Psychiatry 79: 213–221.
- Newman-Tancredi A, Chaput C, Touzard M, et al. (2001) Pindolol antagonizes G-protein activation at both pre- and postsynaptic serotonin 5-HT1A receptors. Naunyn Schmiedebergs Arch Pharmacol 363: 391–398.
- Ngai YF, Sulistyoningrum DC, O’Neill R, et al. (2015) Prenatal alcohol exposure alters methyl metabolism and programs serotonin transporter and glucocorticoid receptor expression in brain. Am J Physiol Regul Integr Comp Physiol 309: R613–R622.
- Ni YG, Miledi R (1997) Blockage of 5HT2C serotonin receptors by fluoxetine (Prozac). Proc Natl Acad Sci 94: 2036–2040.
- Noble EE, Billington CJ, Kotz CM, et al. (2014) Oxytocin in the ventromedial hypothalamic nucleus reduces feeding and acutely increases energy expenditure. Am J Physiol Regul Integr Comp Physiol 307: R737–R745.
- Olszewski PK, Klockars A, Levine AS (2016) Oxytocin: A conditional anorexigen whose effects on appetite depend on the physiological, behavioral and social contexts. J Neuroendocrinol 28. https://doi.org/10.1111/jne.12376
- Ong ZY, Alhadeff AL, Grill HJ (2015) Medial nucleus tractus solitarius oxytocin receptor signaling and food intake control: The role of gastrointestinal satiation signal processing. Am J Physiol Regul Integr Comp Physiol 308: R800–R806.
- Ott V, Finlayson G, Lehnert H, et al. (2013) Oxytocin reduces reward-driven food intake in humans. Diabetes 62: 3418–3425.
- Pichika R, Buchsbaum MS, Bailer U, et al. (2012) Serotonin transporter binding after recovery from bulimia nervosa. Int J Eat Disord 45: 345–352.
- Plasencia G, Luedicke JM, Nazarloo HP, et al. (2019) Plasma oxytocin and vasopressin levels in young and older men and women: Functional relationships with attachment and cognition. Psychoneuroendocrinology 110: 104419.
- Pratelli M, Pasqualetti M (2019) Serotonergic neurotransmission manipulation for the understanding of brain development and function: Learning from TPH2 genetic models. Biochimie 161: 3–14.
- Pratt WE, Clissold KA, Lin P, et al. (2016) A systematic investigation of the differential roles for ventral tegmentum serotonin 1-and 2-type receptors on food intake in the rat. Brain Res 1648: 54–68.
- Puglisi-Allegra S, Andolina D (2015) Serotonin and stress coping. Behav Brain Res 277: 58–67.
- Raurich A, Mengod G, Artigas F, et al. (1999) Displacement of the binding of 5-HT1A receptor ligands to pre- and postsynaptic receptors by (−)pindolol. A comparative study in rodent, primate and human brain. Synapse 34: 68–76.
- Sari Y (2004) Serotonin1B receptors: From protein to physiological function and behavior. Neurosci Biobehav Rev 28: 565–582.
- Quintana DS, Guastella AJ (2020) An allostatic theory of oxytocin. Trends Cogn Sci 24: 515–528.
- Quintana DS, Smerud KT, Andreassen OA, et al. (2018) Evidence for intranasal oxytocin delivery to the brain: Recent advances and future perspectives. Therap Deliv 9: 515–525.
- Raap D, Van de Kar LD (1999) Selective serotonin reuptake inhibitors and neuroendocrine function. Life Sci 65: 1217–1235.
- Récamier-Carballo S, Estrada-Camarena E, López-Rubalcava C (2017) Maternal separation induces long-term effects on monoamines and brain-derived neurotrophic factor levels on the frontal cortex, amygdala, and hippocampus: Differential effects after a stress challenge. Behav Pharmacol 28: 545–557.
- Riikonen RS, Nokelainen P, Valkonen K, et al. (2005) Deep serotonergic and dopaminergic structures in fetal alcoholic syndrome: A study with nor-β-CIT-single-photon emission computed tomography and magnetic resonance imaging volumetry. Biol Psychiatry 57: 1565–1572.
- Romano A, Friuli M, Cifani C, et al. (2020) Oxytocin in the neural control of eating: At the crossroad between homeostatic and non-homeostatic signals. Neuropharmacology 171: 108082.
- Russell J, Hunt GE (2023) Oxytocin and eating disorders: Knowledge gaps and future directions. Psychoneuroendocrinology 154: 106290.
- Sala M, Han K, Acevedo S, et al. (2018) Oxytocin receptor polymorphism decreases midline neural activations to social stimuli in Anorexia Nervosa. Front Psychol 9: 2183.
- Salande JD, Hawkins RC II (2017) Psychological flexibility, attachment style, and personality organization: Correlations between constructs of differing approaches. J Psychother Integr 27: 365.
- Saphier D (1991) Paraventricular nucleus magnocellular neuronal responses following electrical stimulation of the midbrain dorsal raphe. Exp Brain Res 85: 359–363.
- Saphire-Bernstein S, Way BM, Kim HS, et al. (2011) Oxytocin receptor gene (OXTR) is related to psychological resources. Proc Natl Acad Sci 108: 15118–15122.
- Saydoff JA, Rittenhouse PA, Van de Kar LD, et al. (1991) Enhanced serotonergic transmission stimulates oxytocin secretion in conscious male rats. J Pharmacol Exp Therap 257: 95–99.
- Saydoff JA, Carnes M, Brownfield MS (1993) The role of serotonergic neurons in intravenous saline-induced secretion of vasopressin, oxytocin, and ACTH. Brain Res Bull 32: 567–572.
- Schalla MA, Stengel A (2019) Activity based anorexia as an animal model for anorexia nervosa–a systematic review. Front Nutr 6: 69.
- Scharner S, Stengel A (2021) Animal models for anorexia nervosa – a systematic review. Front Human Neurosci 14: 596381.
- Schorr M, Marengi DA, Pulumo RL, et al. (2017) Oxytocin and its relationship to body composition, bone mineral density, and hip geometry across the weight spectrum. J Clin Endocrinol Metab 102: 2814–2824.
- Schwarzberg H, Kovács GL, Szabo G, et al. (1981) Intraventricular administration of vasopressin and oxytocin effects the steady-state levels of serotonin, dopamine and norepinephrine in rat brain. Endocrinol Exp 15: 75–80.
- Schwarzberg H, Kovács GL, Teledgy G (1984) The influence of oxytocin on the steady-state level and accumulation of serotonin in rat brain regions. Neuropeptides 4: 145–156.
- Seidl AH (2014) Regulation of conduction time along axons. Neuroscience 276: 126–134.
- Severinsen K, Kraft JF, Koldsø H, et al. (2012) Binding of the amphetamine-like 1-phenyl-piperazine to monoamine transporters. ACS Chem Neurosci 3: 693–705.
- Shapiro LE, Insel TR (1989) Ontogeny of oxytocin receptors in rat forebrain: A quantitative study. Synapse 4: 259–266.
- Simon JJ, Stopyra MA, Friederich HC (2019) Neural processing of disorder-related stimuli in patients with Anorexia Nervosa: A narrative review of brain imaging studies. J Clin Med 8: 1047.
- Slotkin TA, Ryde IT, Tate CA, et al. (2007) Lasting effects of nicotine treatment and withdrawal on serotonergic systems and cell signaling in rat brain regions: Separate or sequential exposure during fetal development and adulthood. Brain Res Bull 73: 259–272.
- Smith AS, Korgan AC, Young WS (2019) Oxytocin delivered nasally or intraperitoneally reaches the brain and plasma of normal and oxytocin knockout mice. Pharmacol Res 146: 104324.
- Smith KA, Fairburn CG, Cowen PJ (1999) Symptomatic relapse in bulimia nervosa following acute tryptophan depletion. Arch Gen Psychiatry 56: 171–176.
- Soumier A, Habart M, Lio G, et al. (2022) Differential fate between oxytocin and vasopressin cells in the developing mouse brain. iScience 25: 103655.
- Spetter MS, Feld GB, Thienel M, et al. (2018) Oxytocin curbs calorie intake via food-specific increases in the activity of brain areas that process reward and establish cognitive control. Scient Rep 8: 2736.
- Spetter MS, Hallschmid M (2017) Current findings on the role of oxytocin in the regulation of food intake. Physiol Behav 176: 31–39.
- Spinelli S, Chefer S, Carson RE, et al. (2010) Effects of early-life stress on serotonin1A receptors in juvenile rhesus monkeys measured by positron emission tomography. Biol Psychiatry 67: 1146–1153.
- Starr LR, Hammen C, Brennan PA, et al. (2012) Serotonin transporter gene as a predictor of stress generation in depression. J Abnorm Psychol 121: 810.
- Steiger H, Booij L, St-Hilaire A, et al. (2023) Eating disorders. In: Blaney P, Krueger R (eds.) Textbook of Psychopathology, 4th edn. Oxford: Oxford University Press, United States.
- Steiger H, Bruce KR, Groleau P (2010) Neural circuits, neurotransmitters, and behavior: Serotonin and temperament in bulimic syndromes. Behav Neurobiol Eat Disord 6: 125–138.
- Steward T, Menchon JM, Jiménez-Murcia S, et al. (2018) Neural network alterations across eating disorders: A narrative review of fMRI studies. Curr Neuropharmacol 16: 1150–1163.
- Stewart RM, Wong JW, Mahfouda S, et al. (2020) Acute tryptophan depletion Moja-De: A method to study central nervous serotonin function in children and adolescents. Front Psychiatry 10: 498160.
- Sullivan EL, Grayson B, Takahashi D, et al. (2010) Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci 30: 3826–3830.
- Suyama S, Kodaira-Hirano M, Otgon-Uul Z, et al. (2016) Fasted/fed states regulate postsynaptic hub protein DYNLL2 and glutamatergic transmission in oxytocin neurons in the hypothalamic paraventricular nucleus. Neuropeptides 56: 115–123.
- Szymanska M, Schneider M, Chateau-Smith C, Nezelof S, et al. (2017) Psychophysiological effects of oxytocin on parent–child interactions: A literature review on oxytocin and parent–child interactions. Psychiatry Clin Neurosci 71: 690–705.
- Tan Z, Wei H, Song X, et al. (2022) Positron emission tomography in the neuroimaging of autism spectrum disorder: A review. Front Neurosci 16: 806876.
- Tasca GA, Balfour L (2014) Eating disorders and attachment: A contemporary psychodynamic perspective. Psychodyn Psychiatry 42: 257–276.
- Tate K, Kirk B, Tseng A, et al. (2021) Effects of the selective serotonin reuptake inhibitor fluoxetine on developing neural circuits in a model of the human fetal cortex. Int J Mol Sci 22: 10457.
- Tauscher J, Pirker W, Willeit M, et al. (2001) [123I] β-CIT and single photon emission computed tomography reveal reduced brain serotonin transporter availability in bulimia nervosa. Biol Psychiatry 49: 326–332.
- Tops M, Koole SL, IJzerman H, et al. (2014) Why social attachment and oxytocin protect against addiction and stress: Insights from the dynamics between ventral and dorsal corticostriatal systems. Pharmacol Biochem Behav 119: 39–48.
- Treasure J, Duarte TA, Schmidt U (2020) Eating disorders. Lancet 395: 899–911.
- Vacher CM, Frétier P, Créminon C, et al. (2002) Activation by serotonin and noradrenaline of vasopressin and oxytocin expression in the mouse paraventricular and supraoptic nuclei. J Neurosci 22: 1513–1522.
- Vaidyanathan R, Hammock EA (2017) Oxytocin receptor dynamics in the brain across development and species. Develop Neurobiol 77: 143–157.
- Van de Kar LD (1991) Neuroendocrine pharmacology of serotonergic (5-HT) neurons. Ann Rev Pharmacol Toxicol 31: 289–320.
- Van de Kar LD, Javed A, Zhang Y, et al. (2001) 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. J Neurosci 21: 3572–3579.
- Van de Kar LD, Levy AD, Li Q, et al. (1998) A comparison of the oxytocin and vasopressin responses to the 5-HT1A agonist and potential anxiolytic drug alnespirone (S-20499). Pharmacol Biochem Behav 60: 677–683.
- Van den Hove DLA, Leibold NK, Strackx E, et al. (2014) Prenatal stress and subsequent exposure to chronic mild stress in rats; interdependent effects on emotional behavior and the serotonergic system. Eur Neuropsychopharmacol 24: 595–607.
- Vannucci A, Nelson EE, Bongiorno DM, et al. (2015) Behavioral and neurodevelopmental precursors to binge-type eating disorders: Support for the role of negative valence systems. Psychol Med 45: 2921–2936.
- Vickers SP, Easton N, Malcolm CS, et al. (2001) Modulation of 5-HT2A receptor-mediated head-twitch behavior in the rat by 5-HT2C receptor agonists. Pharmacol Biochem Behav 69: 643–652.
- Vidal B, Karpenko IA, Liger F, et al. (2017) [11C] PF-3274167 as a PET radiotracer of oxytocin receptors: Radiosynthesis and evaluation in rat brain. Nucl Med Biol 55: 1–6.
- Von Hausswolff-Juhlin Y, Brooks SJ, Larsson M (2015) The neurobiology of eating disorders—a clinical perspective. Acta Psychiatr Scand 131: 244–255.
- Wade TD, Bulik CM, Neale M, et al. (2000) Anorexia nervosa and major depression: Shared genetic and environmental risk factors. Am J Psychiatry 157: 469–471.
- Weinert T, Bernardoni F, King J, et al. (2023) No effects of acute tryptophan depletion on anxiety or mood in weight-recovered female patients with anorexia nervosa. Eur Arch Psychiatry Clin Neurosci 273: 209–217.
- Weltzin TE, Fernstrom MH, Fernstrom JD, et al. (1995) Acute tryptophan depletion and increased food intake and irritability in bulimia nervosa. Am J Psychiatry 152: 1668–1671.
- Wurtman RJ, Wurtman JJ, Regan MM, et al. (2003) Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. Am J Clin Nutr 77: 128–132.
- Xi TF, Li DN, Li YY, et al. (2019) Central 5-hydroxytryptamine (5-HT) mediates colonic motility by hypothalamus oxytocin-colonic oxytocin receptor pathway. Biochem Biophys Res Commun 508: 959–964.
- Xue X, Shao S, Li M, et al. (2013) Maternal separation induces alterations of serotonergic system in different aged rats. Brain Res Bull 95: 15–20.
- Yokokura M, Terada T, Bunai T, et al. (2019) Alterations in serotonin transporter and body image-related cognition in anorexia nervosa. NeuroImage Clin 23: 101928.
- Yoshimura R, Kimura T, Watanabe D, et al. (1996) Differential expression of oxytocin receptor mRNA in the developing rat brain. Neurosci Res 24: 291–304.
- Young SN (2013) Acute tryptophan depletion in humans: A review of theoretical, practical and ethical aspects. J Psychiatry Neurosci 38: 294–305.
- Zhang K, Fan Y, Yu R, et al. (2021) Intranasal oxytocin administration but not peripheral oxytocin regulates behaviors of attachment insecurity: A meta-analysis. Psychoneuroendocrinology 132: 105369.
- Zimmermann-Peruzatto JM, Lazzari VM, Agnes G, et al. (2017) The impact of oxytocin gene knockout on sexual behavior and gene expression related to neuroendocrine systems in the brain of female mice. Cell Mol Neurobiol 37: 803–815.
