Keratoconus is a corneal ectatic disorder that starts at puberty and decreases the radius of curvature of the central cornea. Kennedy et al. reported that the incidence rate is 1 per 50,000 among the population. Moreover, the pathophysiological processes underlying keratoconus have not been fully explained and proposed mechanisms including oxidative damage, proteolytic degradation in the corneal stroma, epithelial mechanical injury, immunological factors, and genetic factors have been suggested. Krachmer et al. reported an incidence of keratoconus in 3.5% to 8% of patients with Down syndrome. Furthermore, hormonal imbalances affect the corneal metabolism and may be associated with keratoconus. Likewise, thyroid gland dysfunction can frequently be associated with ocular diseases such as Graves disease.
Previous studies investigated the association between thyroid gland dysfunction and keratoconus, suggesting a positive correlation. In 1936, Appelbaum described a series of patients with keratoconus in whom there was a high prevalence of hypothyroidism symptoms, whereas King reported keratoconus after thyroidectomy. In 1990, Kahán et al. reported a possible role of tear fluid thyroxine (T4) in keratoconus development. Interestingly, T4 is important for corneal dehydration and transparency during embryonic development and regulates the synthesis of keratin sulfate proteoglycan in chickens. T4 receptors have been found in the lacrimal gland, confirming that the tear producing gland is a target organ of T4. T4 level was elevated in the tears of patients with keratoconus. More studies were published until 2018, when two new case reports by Lee et al. claimed the existence of the association.
We conducted this study to assess the prevalence of thyroid gland dysfunction among patients with keratoconus compared to normal healthy controls.
Patients and Methods
This cross-sectional case–control clinical study included 187 patients with keratoconus without previously diagnosed thyroid disease and 187 sex- and age-matched healthy controls without keratoconus. The study was conducted between May 2018 and June 2019 where patients from the different participating centers were recruited. Ethical approval was obtained from the local ethics committees of Alexandria Faculty of Medicine, Sohag Faculty of Medicine, Mansoura Faculty of Medicine, Fayoum Faculty of Medicine, Zagazig Faculty of Medicine, and Ain-Shams Faculty of Medicine. A written informed consent was obtained and the tenets of the Declaration of Helsinki were followed. The protocol of this study was registered on www.clinicaltrials.gov (NCT 03637673).
We included patients with bilateral keratoconus as the case group and healthy patients without keratoconus as the control group. The study included grades 1 to 4 keratoconus according to the Amsler–Krumeich classification. However, those who could not provide informed consent or the necessary samples for any reason were excluded. Additionally, we excluded patients with other relevant comorbidities that have been demonstrated to be associated with keratoconus.
We constructed a case report form for each included patient to register the relevant demographic, ophthalmologic, and endocrinologic data. After performing Pentacam (Oculus Optikgeräte GmbH, Wetzlar, Germany) examination, flat (K1), steep (K2), and maximum (Kmax) simulated keratometric readings were recorded for each patient.
Laboratory Investigations
For the endocrinologic data, we collected 3 mL of blood from each participant in appropriate tubes. Then, we measured the serum concentrations of thyroid-stimulating hormone (TSH), free triiodothyronine (T3), and free T4 using an enzyme-linked immunosorbent assay (DRG International, Springfeld, NJ). The normal reference ranges for TSH, free T4, and free T3 were decided to be 0.5 to 1.96 ng/mL, 3.9 to 10.9 µg/dL, and 0.25 to 5.0 ulU/mL, respectively. Suspected cases with concentrations other than the mentioned reference ranges were referred to experienced endocrinologists for further needed investigations and examination to confirm whether the patient had thyroid gland dysfunction. All investigations were handled anonymously.
Statistical Analysis
Data were analyzed using SPSS for Windows, version 25.0 (SPSS, Inc., Chicago, IL). Number and percent were used for qualitative data and mean ± standard deviation or range for quantitative data. The Student's t test was used for statistical comparisons between the study groups. For comparing categorical data, Fisher's exact test was performed because the chi-square test was not suitable due to the low numbers (2 and 10) in the cross tables. A P value of less than .05 was considered statistically significant.
Results
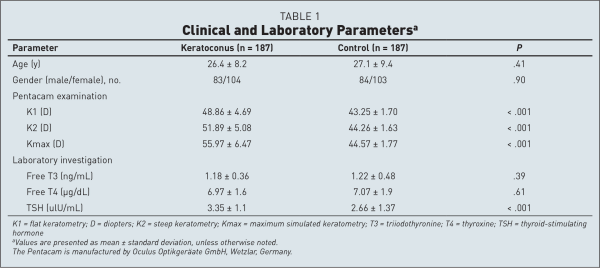
Table 1 shows comparisons between cases and controls on demographic, clinical, and laboratory parameters. One hundred eighty-seven patients with bilateral keratoconus and 187 healthy controls were analyzed. Mean age was 26.4 ± 8.2 years (range: 17 to 67 years) for patients with keratoconus and 27.1 ± 9.4 years (range: 18 to 69 years) for control patients, with no significant difference between groups. Additionally, the gender did not differ between the study groups (P = .90). We performed Pentacam examination and K1, K2, and Kmax keratometric readings were recorded for each patient. The mean K1, K2, and Kmax values differed significantly between groups (P < .001). For the case group, the mean ages of the included men and women were comparable (P = .309). The mean K1, K2, and Kmax values did not differ significantly between genders (P = .380, .827, and .492, respectively).
Our results showed that thyroid gland dysfunction prevalence was 10 of 187 patients with keratoconus (5.35%) and 2 of 187 control patients (1.1%) and this difference was statistically significant (P = .036). Eight women and two men were confirmed after endocrinologist consultation examination to have overt thyroid gland dysfunction. Six patients (2 men and 4 women) had hyperthyrosis and 4 women had hypothyrosis. For the control group, the two patients had overt hypothyrosis.
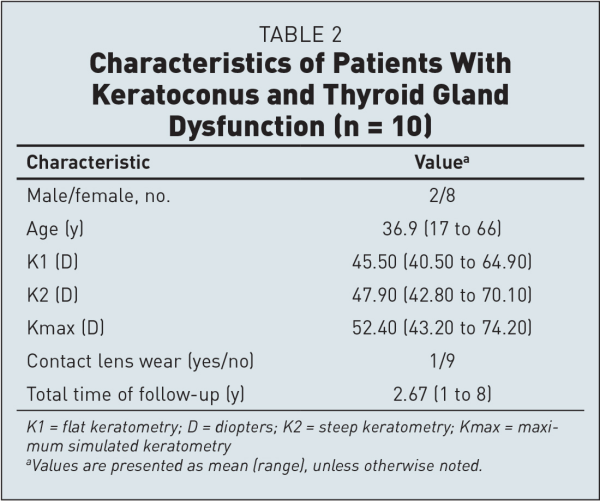
All patients started oral medications, and one of those patients was scheduled to have thyroidectomy. Table 2 provides a summary of their clinical characteristics. The mean age of the patients with both keratoconus and thyroid gland dysfunction was 36.9 years (range: 17 to 66 years). Four of those patients (4 eyes) underwent deep anterior lamellar keratoplasty and 3 of them (3 eyes) improved. Moreover, corneal cross-linking was performed for 8 patients (8 eyes), and 5 of them improved. Only 1 eye underwent both corneal cross-linking and intracorneal ring segment implantation, with no significant improvement. Furthermore, 3 patients (6 eyes) were stable during the follow-up.
Discussion
The association of thyroid and ophthalmopathy was described in the literature. The results of this study showed that thyroid gland dysfunction prevalence was 5.35% in patients with keratoconus and 1.1% in the control group. Our results for the control group were less than the previous reported prevalence of thyroid dysfunction in adults in the general population (2%). In addition, our study found a statistically significant association between thyroid gland dysfunction and keratoconus. Thus, our results are consistent with previous reports showing a positive association between keratoconus and thyroid gland dysfunction. For example, Kahán et al. reported that, in 50 patients with keratoconus, 27 had euthyrosis, 6 had hyperthyrosis, and 17 had hypothyrosis in terms of their blood serum thyroxine level. Moreover, the tear thyroxine levels of patients with keratoconus were found to be 2 to 50 times higher than those of patients without ocular diseases and levels were higher during the progression of keratoconus and declined once cornea curvature fulfilled a new steady value. Furthermore, a study by Thanos et al. showed that thyroid gland dysfunction prevalence among patients with keratoconus was 13.6%. Additionally, other studies by Tabibian et al. and Gatzioufas and Thanos support our study results. However, Alhawari et al. found no association between autoimmune thyroid disease and keratoconus. Our study was limited by the small sample, which may affect the power and subsequently the results of the study.
Although the difference was statistically significant in our study, thyroid disease may be responsible for only 4% of causative subfactors for keratoconus development and/or progression. Hence, this study highlights the multifactorial nature of keratoconus. In addition to being idiopathic in some cases, there are many subfactors for keratoconus, such as eye rubbing, genetic predisposition, environmental conditions, systemic diseases, and hormones. Such subfactors stimulate the main factor, which leads to changes of the cornea. The main factor may be matrix metalloproteinases. Higher matrix metalloproteinase levels in the tear fluid of developing or progressive keratoconus may change the corneal structure. It is stimulated by inflammation and some hormones influence inflammation. There is a causative link between inflammation and increased matrix metalloproteinase production. Higher levels of proinflammatory or inflammatory cytokines were shown.
Dias et al. reported that T4 receptors have been found in the lacrimal gland, confirming that the tear producing gland is a target organ of T4 and the T4 level was elevated in the tears of patients with keratoconus. Likewise, Thanos et al.'s study, in which tear samples were collected from all patients with keratoconus and thyroid gland dysfunction, from a group of patients with keratoconus but without thyroid gland dysfunction, and from a control group with both keratoconus and thyroid gland dysfunction, compared tear T4 in thyroid gland dysfunction and immunohistochemical staining of its receptors between patients with keratoconus and controls. They found a higher tear T4 level in keratoconus, and keratocyte and T4 receptors were elevated in keratoconus compared with controls. In the same study, organotypic tissue cultures from monkey corneas were studied to investigate the significance of T4 for corneal metabolism. Core proteins such as collagen and cytokeratins were altered equally in both patients with keratoconus and the cultured corneas substituted with T4, which showed a clear role of T4 in keratoconus pathophysiology via T4 receptors.
To our knowledge, this is the first study with this unique design that compared cases and controls. We controlled for sex, age, and relevant comorbidities. However, we encountered some limitations, mainly our inability to include tear T4 analysis due to cost-related issues. We also acknowledge that our study did not observe the patients after treatment of thyroid eye disease to detect the influence of treatment on keratoconus stability or progression. Furthermore, we could not perform more investigations for our study population to define thyroid gland dysfunction more accurately. However, we managed to fully examine those with positive laboratory results. Therefore, more clinical studies avoiding these limitations are highly encouraged.
Our study showed that there is an association between keratoconus and thyroid gland dysfunction, but more studies are needed to build upon these results.
AUTHOR CONTRIBUTIONS
Study concept and design (AE, MFD, MI, OF, OMS, MOY, AEB, AT, AS); data collection (AE, MFD, MI, OF, OMS, MOY, AEB, AT, AS); analysis and interpretation of data (AE, MFD, MI, OF, OMS, MOY, AEB, AT, AS); writing the manuscript (AE, MFD, MI, OF, OMS, MOY, AEB, AT, AS); critical revision of the manuscript (AE, MFD, MI, OF, OMS, MOY, AEB, AT, AS); statistical expertise (MFD); administrative, technical, or material support (AE, OF, AS); supervision (AE, OF, AS)
References
- 1. . Keratoconus. Surv Ophthalmol. 1998;42(4):297–319.
- 2. . A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101(3):267–273.
- 3. . Keratoconus. Contact lens or keratoplasty? Ophthalmology. 1988;95(4):487–492.
- 4. . Gene expression in keratoconus: initial results using DNA microarrays [article in German]. Ophthalmologe. 2003;100(7):545–549.
- 5. . Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28(4):293–322.
- 6. . Keratoconus. Arch Ophthalmol. 1936;15(5):900–921.
- 7. . Keratoconus following thyroidectomy. Trans Opthal Soc UK. 1953;73:31–39.
- 8. . The possible role of tear fluid thyroxine in keratoconus development. Exp Eye Res. 1990;50(4):339–343.
- 9. . Bilateral keratoconus associated with Hashimoto's disease, alopecia areata and atopic keratoconjunctivitis. Eur J Ophthalmol. 1999;9(2):130–133.
- 10. Thyroxine affects expression of KSPG-related genes, the carbonic anhydrase II gene, and KS sulfation in the embryonic chicken cornea. Invest Ophthalmol Vis Sci. 2006;47(1):120–132.
- 11. . Corneal development. 3. The role of the thyroid in dehydration and the development of transparency. Exp Eye Res. 1964;3(2):105–114.
- 12. Influence of thyroid hormone on thyroid hormone receptor beta-1 expression and lacrimal gland and ocular surface morphology. Invest Ophthalmol Vis Sci. 2007;48(7):3038–3042.
- 13. . Bilateral, asymmetric keratoconus induced by thyrotoxicosis with long-term stability after corneal cross-linking. J Refract Surg. 2018;34(5):354–356.
- 14. . Bilateral keratoconus induced by secondary hypothyroidism after radioactive iodine therapy. J Refract Surg. 2018;34(5):351–353.
- 15. Antibody-dependent cell-mediated cytotoxicity against orbital target cells in thyroid-associated ophthalmopathy and related disorders; close relationship between serum cytotoxic antibodies and parameters of eye muscle dysfunction. J Endocrinol Invest. 1996;19(6):334–341.
- 16. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf). 1977;7(6):481–493.
- 17. Role of thyroxine in the development of keratoconus. Cornea. 2016;35(10):1338–1346.
- 18. Pregnancy-induced changes in corneal biomechanics and topography are thyroid hormone related. Am J Ophthalmol. 2017;184:129–136.
- 19. . Acute keratoconus induced by hypothyroxinemia during pregnancy. J Endocrinol Invest. 2008;31(3):262–266.
- 20. Autoimmune thyroid disease and keratoconus: is there an association? Int J Endocrinol. 2018;2018:7907512.
- 21. Increased hair cortisol concentrations in patients with progressive keratoconus. J Refract Surg. 2017;33(6):383–388.
- 22. . What causes keratoconus? Cornea. 2012;31(6):716–719.
- 23. . Matrix metalloproteinases in keratoconus—too much of a good thing? Exp Eye Res. 2019;182:137–143.
- 24. . Interaction of the endocrine system with inflammation: a function of energy and volume regulation. Arthritis Res Ther. 2014;16(1):203.
- 25. Overexpression of tear inflammatory cytokines as additional finding in keratoconus. Mediators Inflamm. 2018;2018:4285268.
- 26. . Keratoconus: an inflammatory disorder? Eye (Lond). 2015;29(7):843–859.