The basic principle of trifocal intraocular lenses (IOLs) is a dioptric addition for intermediate vision that is half of the addition for near vision. In this way, the first harmonic focus of the intermediate power coincides with the near focus, reinforcing the near image and reducing light dispersion. The efficacy of this design has been widely demonstrated in the literature and has promoted further studies and developments of different multifocal IOL optics. More recently, a new diffractive pentafocal IOL was described, the Intensity SeeLens IOL (Hanita Lenses), which uses negative diffraction orders for distance vision, the optical principles of which have been recently reported.
Early clinical studies with this IOL provided positive results in terms of visual acuity, defocus curves, and patient satisfaction. However, there are no studies comparing this new pentafocal IOL with trifocal IOLs. We report the results of a consecutive series comparing the Intensity IOL (with C loop) to a reference model for diffractive trifocal technology, the FineVision POD F IOL (with double C loop) (PhysIOL).
Patients and Methods
Patients
This comparative study is part of a larger prospective study designed to evaluate advanced optics IOLs. It included patients who had asked for spectacle independence at the time of cataract surgery and who received bilateral implantation of either the Intensity IOL or the FineVision IOL, according to the IOL supplier at the time of their cataract surgery. No clinical criteria were adopted to drive IOL selection; that was the result of a change in the IOL supplier. The study was approved by the local institutional review board and was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients. Surgeries took place between January 1, 2021 and December 31, 2022.
The inclusion criteria adopted for spherical multifocal IOL implantation were: age 21 years or older, regular corneal astigmatism less than 1.00 diopter (D) with-the-rule or less than 0.50 D against-the-rule, no ocular anomalies or pathologies that could affect either vision or IOL stability (ie, severe dry eye, pseudo-exfoliation, iridodonesis, glaucoma, maculopathies, or previous ocular surgery), no intraoperative or postoperative complications, no general diseases potentially affecting vision such as diabetes or affecting patient-reported outcomes such as dementia. In addition, to harmonize the two study groups we only included patients with axial length between 21.0 and 26.0 mm. Patients had been informed about the surgery and about the diffractive nature of the IOL to be implanted.
IOLs
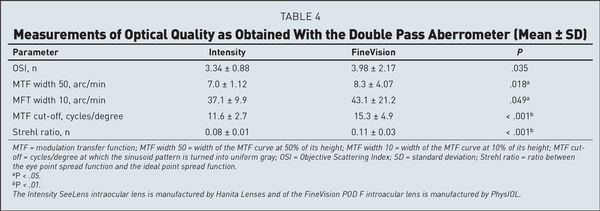
The Intensity SeeLens IOL is a 25% hydrophilic acrylic, foldable single-piece IOL with two “C” haptics, with 1.45 refractive index and −0.13 D of spherical aberration. It has an aspheric diffractive posterior surface and a spherical anterior surface. The IOL uses refraction to produce the intermediate focus, and has 12 concentric diffractive steps in the zone between 1 and 5.2 mm in diameter. The diffractive steps have different heights varying across the lens radius, with a maximum step height of 3.6 microns, and produce four additional foci: −0.75 and −1.50 D for intermediate-distance and for distance vision, and +0.75 and +1.50 D for intermediate-near and for near vision. Despite receiving the largest part of the light transmitted to the retina, the distance focus is obtained by diffraction. The light distribution at 3.5-mm aperture diameter is 46% for distance, 22% for intermediate, and 24% for near vision. The suggested “A” constant is 118.4.
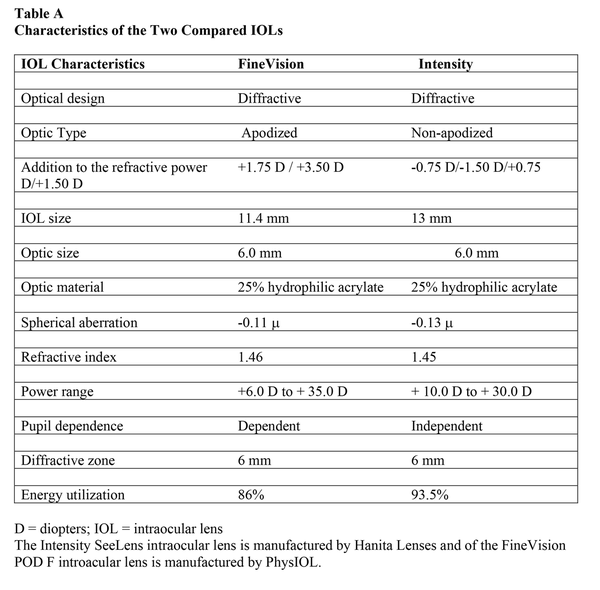
The FineVision POD F IOL is a 26% hydrophilic acrylic, foldable single-piece IOL with four “C” haptics, with 1.46 refractive index and −0.11 D of spherical aberration. The IOL has the refractive power directed to the distance focus and 26 concentric diffractive steps in the zone between 1 and 5.5 mm in diameter. The diffractive steps produce two additional foci: +1.75 D for intermediate and +3.50 D for near vision. The distribution of the transmitted light is 42% for distance, 15% for intermediate, and 29% for near vision. The suggested “A” constant is 118.95. Further characteristics of the two IOLs are collected in Table A and Figure A.
Figure
A:. The two intraocular lenses (IOLs) included in the study: (A) FineVision POD F IOL (PhyIOL) and (B) Intensity SeeLens IOL (Hanita Lenses).
Preoperative Examination
All patients were examined preoperatively with the slit lamp to verify the anatomical status of the anterior segment. Refraction was obtained with the Topcon RM800 autorefractometer (Topcon). Uncorrected (UDVA) and corrected (CDVA) distance visual acuity (logMAR) were measured with an electronic optotype (Vision Chart; CSO) in photopic conditions: room lighting 200 lux, optotype luminance 85 cd/m2. Corneal tomography was obtained by anterior optical coherence tomography (MS-39; CSO) to measure the amount and regularity of the corneal astigmatism. The “A” constant of the two IOLs provided by the manufacturer was adopted during this study. Posterior segment analysis was conducted by ophthalmoscopy through dilated pupil and macular optical coherence tomography (Cirrus HDOCT 5000; Carl Zeiss Meditec AG). Ocular biometry was obtained with the IOLMaster 500 (Carl Zeiss Meditec AG), and the IOL power was calculated with the Barrett Universal II formula (available at https://iolcalculator.escrs.org). The IOL power expecting to produce the smallest hyperopic error was selected.
Surgical Procedure
The surgical procedures were performed by the same experienced surgeon (RB) under topical anesthesia. The 2.2-mm main corneal incision was located at the steepest meridian to minimize postoperative astigmatism. A viscoadaptive viscosurgical device (Fidial Plus; Fidia) was used to maintain space during surgery. The Stellaris phaco machine (Bausch & Lomb) with a micro-incision phaco tip was employed to fragment the lens nucleus, remove the lens material, and irrigate/aspirate the remaining cortex. The IOLs were implanted with the Accujet 2.2 injector (Medicel), using the wound-assisted technique. All patients received 0.2 mL of 1% cefuroxime injection into the anterior chamber at the end of surgery. Post-operatively, patients were given a combination of 1% dexamethasone 3% netilmicin eye drops four times a day for 1 week, and 0.9% bromfenac twice a day for 2 weeks. First and second eye surgeries were performed 4 weeks apart.
Postoperative Examination
The study examination was performed 30 ± 7 days after the second eye surgery. All of the eyes were examined with the slit lamp by one of the authors (CB or RB).The objective refraction was obtained with the RM800 automated refractor. The subjective refraction was obtained using the duochromatic test and the Jackson cross-cylinder test. The pupil size was measured with the automated refractor. Monocular and binocular UDVA and CDVA (at 4 m), intermediate (UIVA and DCIVA, at 60 cm), and near (UNVA, DCNVA, at 40 cm) under the same lighting conditions as preoperatively. The defocus curve was obtained in distance vision by recording the visual acuity after adding plus and minus lenses to the distance correction in 0.50-D steps from +1.00 to −4.00 D. Binocular contrast sensitivity in photopic conditions (room lighting 200 lux, background test luminance 85 cd/m2) were obtained in distance vision with the sine wave test of the electronic optotype. Wavefront refraction at 3.5 and 5 mm and optical aberration up to the 4th Zernike order were measured with the Zywave machine (Bausch & Lomb) with the pupil dilated. The objective optical quality of the two IOLs was measured with the OQAS machine (Visiometrics) at 4-mm aperture diameter, considering the Objective Scattering Index (OSI), the width of the modulation transfer function (MTF) at 10% and 50% of its height, the MTF cut-off (the value in cycles/degree at which the modulation of the image is lost), and the Strehl ratio (the ratio between the eye point spread function and the ideal point spread function). Patients' opinions were collected with the Quality of Vision (QoV) questionnaire developed by McAlinden et al to investigate the frequency, severity, and bothersomeness level of 10 visual symptoms in a scale from 0 (never, not at all) to 3 (often, severe). All of the numerical parameters were obtained as the means of three consistent measurements.
Statistical Analysis
The minimum sample size was calculated considering binocular vision, error of 0.05, and error (power) of 0.80. For visual acuity, a mean error in the measurement of 0.1 logMAR and a minimum significant difference of 0.1 logMAR produced a minimum sample size of 26 eyes. For contrast sensitivity, a mean error in the measurement of 0.2 log units and a minimum significant difference of 0.2 log units produced a minimum sample size of 26 eyes. For optical aberration, a mean error in the measurement of 0.06 and a minimum significant difference of 0.1 produced a minimum sample size of 9.36 eyes. For the MTF cut-off, a mean error in the measurement of 5.0 cycles/degree and a minimum significant difference of 5.0 cycles/degree produced a minimum sample size of 26 eyes. For visual symptoms, a mean error in the evaluation of 0.52 points and a minimum significant difference of 0.50 points produced a minimum sample size of 28 participants. After all of these calculations, we decided to include 30 patients (60 eyes) per group in this analysis.
Statistical analysis was performed using SPSS software (version 28; SPSS, Inc). All data were presented as mean ± standard deviation. Normality distribution was evaluated by performing the Kolmogorov–Smirnov test. One-way analysis of variance and Kruskal–Wallis tests were used to compare data that were normally and non-normally distributed, respectively. Chi-squared tests were used to compare demographic and clinical data between patients. Values are expressed as the number of patients or as the mean ± standard deviation. Dunn–Bonferroni tests were performed for post-hoc comparisons. A P value less than .01 was considered highly statistically significant, and a P value less than .05 was considered statistically significant.
Results
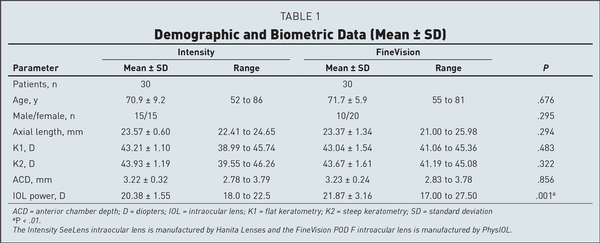
A total of 60 patients (120 eyes) were included in this study, 30 per group. Preoperative demographic and biometric characteristics of the two groups are reported in Table 1. None of the considered items showed statistically significant differences except for the implanted IOL power, which was lower in the Intensity IOL group because of small differences in the eye parameters and the different “A” constant of the two IOLs. Moreover, preoperative refractive spherical equivalent was −1.72 ± 2.31 D in the Intensity group and −1.48 ± 3.52 in the FineVision group; the CDVA was 0.26 ± 0.24 logMAR in the Intensity group and 0.25 ± 0.23 logMAR in the FineVision group. These two latter parameters also were statistically comparable.
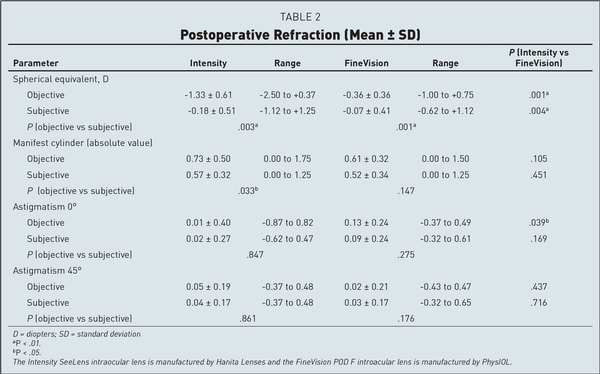
At the study visit, the pupil diameter measured by the autorefractometer was 2.95 ± 0.23 mm in the Intensity eyes, and 3.01 ± 0.24 mm in the FineVision eyes (P = n.s.). The objective refraction (Table 2) with the Intensity IOL was averagely more myopic by −0.97 D than with the FineVision IOL; the subjective refraction was −0.11 D more myopic with the Intensity IOL. Both differences were statistically significant (P < .01). The difference between the objective and the subjective refraction was −1.15 D with the Intensity IOL and −0.29 D with the FineVision IOL.
The subjective refraction was within ±0.50 D in 43 (72%) eyes with the Intensity IOL and in 50 (83.3%) eyes with the FineVision IOL (P = n.s., Figure 1). The manifest cylinder, both objective and subjective, was similar with either IOL. The difference between the objective cylinder and the (lower) subjective cylinder was 0.16 D with the Intensity IOL (P = .033) and was 0.09 D with the FineVision IOL (P = .147). A low objective against-the-rule astigmatism was noted in the FineVision group (0.13 D, P = .039), but the subjective astigmatism was similar with either IOL.
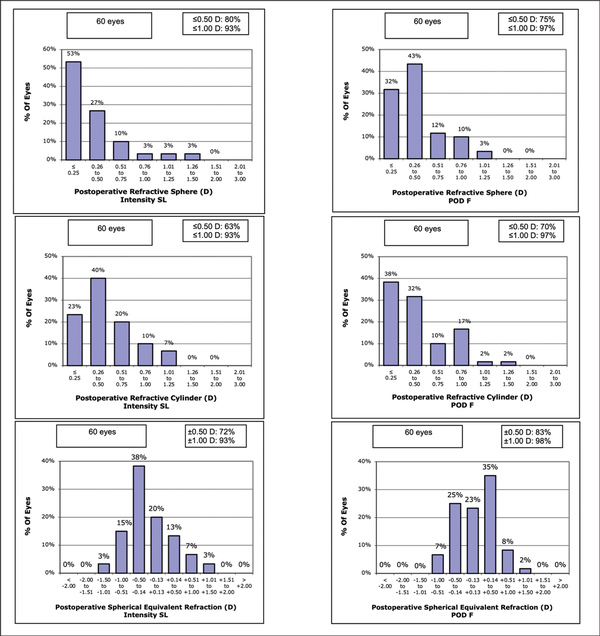
Figure 1
The 1-month postoperative refractive data of the Intensity SeeLens (Hanita Lenses) and the FineVision POD 5 (PhysIOL) groups. D = diopters
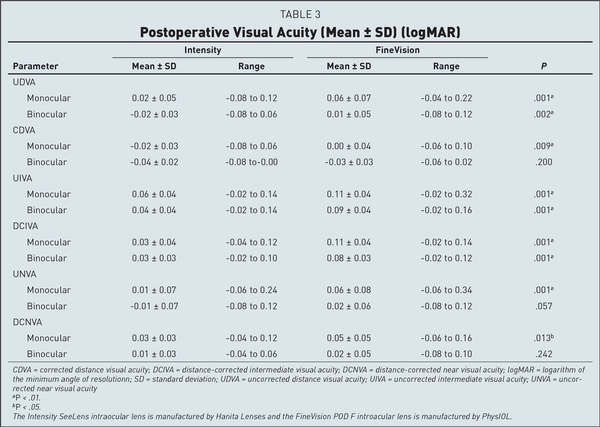
Visual acuity data (Table 3) indicated slightly better UDVA and monocular CDVA with the Intensity IOL, but no difference was found for binocular CDVA. However, both UIVA and DCIVA were clinically and statistically better for the pentafocal Intensity IOL. Nine patients with the Intensity IOL and 1 patient with the FineVision IOL had binocular UIVA and binocular DCIVA of 0.00 logMAR or better (P = .015), but no difference was detected at the 0.1 log-MAR level (Figure 2). UNVA and DCNVA were better for the Intensity IOL, but only the difference in monocular visual acuity reached statistical significance (Table 3).
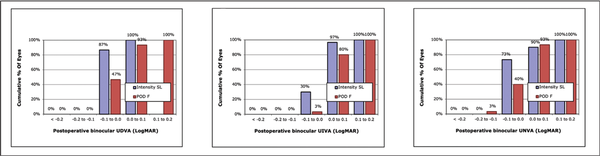
Figure 2
Postoperative binocular uncorrected distance visual acuity (UDVA), uncorrected intermediate visual acuity (UIVA), and uncorrected near visual acuity (UNVA) at the follow-up visits of the two groups. The Intensity SeeLens intraocular lens is manufactured by Hanita Lenses and the FineVision POD F introacular lens is manufactured by PhysIOL. **P < .01; *P < .05.
The binocular distance-corrected defocus curve is reported in Figure 3. The Intensity IOL provided better visual acuity at +1.00 and at +0.50 D defocus, and within the interval −0.50 to −2.50 D defocus, with a maximum difference of 0.107 logMAR at −1.00 D defocus. The FineVision IOL was superior by 0.39 logMAR at −4.00 D defocus (P = .001). No difference was detected at 0.00 D and at −2.50, −3.00, and −3.50 D defocus.
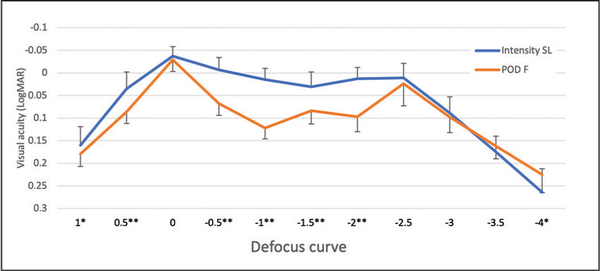
Figure 3
Defocus curves of the Intensity SeeLens intraocular lens (IOL) (Hanita Lenses) and of the FineVision POD F IOL (PhysIOL) at the follow-up visit. **P < .01; *P < .05.
The contrast sensitivity curves measured under photopic conditions (200 lux room lighting, 85 cd/m2 test luminance) are reported in Figure 4, and indicate no difference between the two IOLs.
Concerning aberrometry assessments, the Hartmann-Shack aberration analysis in the two groups found little or no difference between the two IOLs (Figure 5). Both lenses induced a slightly negative spherical aberration; horizontal coma was higher with the Intensity IOL, although small in magnitude. The wavefront-based refraction was −0.90 ± 0.66 D with the Intensity IOL and 0.29 ± 0.73 D with the FineVision IOL at 3.5-mm aperture diameter (P < .001). At a 5-mm aperture diameter the wavefront-based refraction was −1.18 ± 0.29 and 0.05 ± 0.77 D, respectively. The myopic increase was −0.29 ± 0.28 and −0.22 ± 0.17 D, respectively, with no difference between IOLs (P = n.s.).
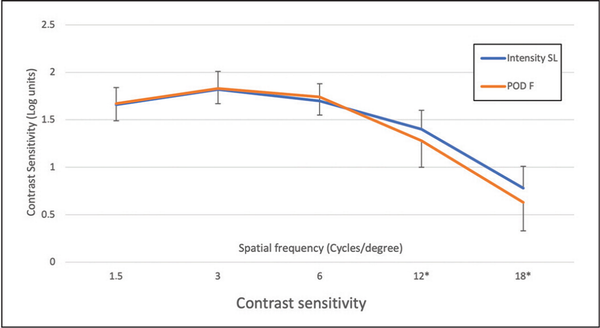
Figure 4
Contrast sensitivity curves of Intensity SeeLens intraocular lens (IOL) (Hanita Lenses) and of the FineVision POD F IOL (PhysIOL) groups measured under photopic conditions. **P < .01; *P < .05.
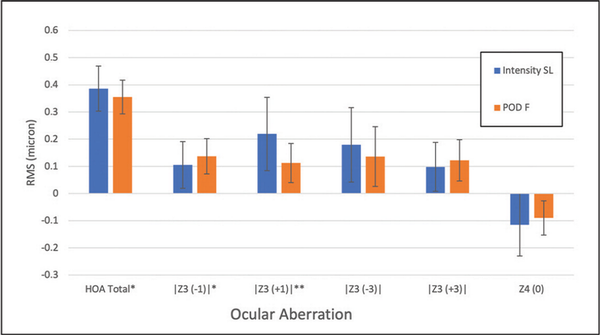
Figure 5
Ocular aberration at 3.5-mm aperture diameter. The Intensity SeeLens intraocular lens is manufactured by Hanita Lenses and the FineVision POD F introacular lens is manufactured by PhysIOL. HOA Total = total higher order aberrations; RMS = root mean square; Z3 (−1) = vertical coma; Z3 (+1) = horizontal coma; Z3 (−3) = vertical trefoil; Z3 (+3) = horizontal trefoil; Z4 (0) = spherical aberration
The results obtained with the Double Pass aberrometer (OQAS) are collected in Table 4. The OSI and the width of the MTF curve were slightly better with the Intensity IOL, but the MTF cut-off value and the Strehl ratio were definitely better with the FineVision IOL.
Figure 6 reports the result of the questionnaire concerning the visual disturbances. All the reported values and all the differences between the groups were generally low, however a few symptoms were less emphasized with the Intensity IOL: the symptoms frequency for halos, starburst, and fluctuation; the symptoms severity for glare, halos, starburst, and fluctuation; the symptoms bother for glare, halos, hazy vision, blurred vision, distortion, and fluctuation.
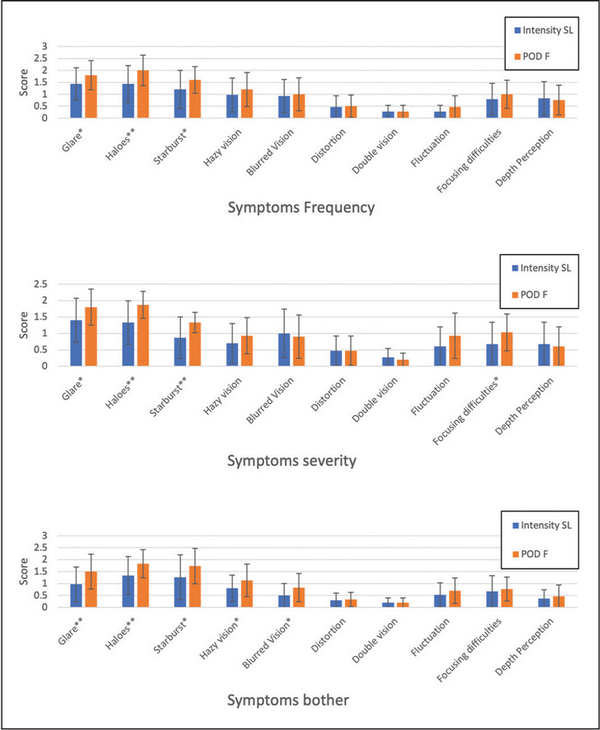
Figure 6
The results of the questionnaire concerning the quality of vision obtained for the symptoms frequency (top), severity (middle), and discomfort (bottom). The Intensity SeeLens intraocular lens is manufactured by Hanita Lenses and the FineVision POD F introacular lens is manufactured by PhysIOL.
Discussion
In this study we compared the refractive, clinical, and aberrometric outcomes obtained with two diffractive IOLs of different optic design implanted during cataract extraction: the pentafocal Intensity IOL with diffractive distant focus and the trifocal FineVision IOL with refractive distant focus. The two lenses are similar for the hydrophilic acrylic material and for the negative asphericity of the base optical power but are different in their optical design.
As for refraction, the Intensity IOL produced a myopic shift at automated refraction typical of IOLs with important diffractive light transmission. The measured subjective refraction was also slightly more myopic with the Intensity IOL (Table 2 and Figure 1), suggesting some optimization of the “A” constant is needed for this IOL in our area. We found no relevant differences in astigmatism between the two IOLs (Table 2). All of these results are in agreement with those obtained by most authors with the same IOLs, whereas other authors had slightly hyperopic refractive results with the FineVision IOL.
The visual acuity results in Table 3 point out the optical differences between the two IOLs. UDVA, UIVA, UNVA, CDVA, and DCNVA were similar or slightly better with the Intensity IOL (Table 3 and Figure 2). In contrast, monocular and binocular DCIVA were definitely better with the Intensity IOL, with a statistically significant difference (Table 3). Because of the slight myopia we found in the Intensity group, we cannot say that intermediate uncorrected vision is better in this group. However, DCIVA was definitively better in the Intensity group. This finding is also evident from the shape of the defocus curve.
Published data about visual acuity with the Intensity IOL is scarce and indicates better figures than ours for distance and for near, and the same for intermediate visual acuity, a difference largely due to the better uncorrected visual acuity obtained in the study of Nov et al. As for the FineVision IOL, the published figures are similar to ours, with small differences among authors.
In this study, the Intensity IOL provided a flatter defocus curve than the FineVision IOL (Figure 3), with statistically significant differences in the interval from −0.50 to −2.00 D defocus. This better intermediate vision appears to be strongly related with the pentafocal IOL optics and with the light distribution typical of this IOL that incorporates an intermediate refractive focus. It may help intermediate vision (eg, for computer reading).
The photopic contrast sensitivity curves were also similar, with small differences in favor of the Intensity IOL at 12 and 18 cycles/degree (Figure 4). All of the obtained values are in agreement with published data.
The Hartmann-Shack aberration study showed low differences between IOLs (Figure 5), with no prevalence of either IOL. We could not find other studies about the same IOLs to compare our results with, but our data are in line with other types of multifocal diffractive IOLs.
The OQAS double pass aberrometer measured the same OSI and produced nearly the same MTF curve in the two groups of eyes, but better MTF cut-off and better Strehl ratio were obtained with the FineVision IOL (Table 4). This result is important because the MTF cutoff value is the level of MTF in cycles/degree at which the black and white modulation appears uniformly gray. It is in contrast with the visual acuity result and may be related both to the more diffractive nature of the Intensity IOL and to the particular optical principle of the OQAS machine, which is based on Badal lenses and not on Hartmann-Shack sensors.
The most reported everyday life complaints in this study were glare, halos, and starburst (Figure 6), the frequency of which was similar with either IOL, whereas the severity and the subjective bothersomeness were lower for the Intensity IOL for halos and starburst. As for these parameters, we can say that the Intensity IOL has provided some improvement in the subjective quality of vision as compared with the FineVision IOL. This result may be due to the different optical design of the two IOLs and/or to the lower number of diffractive rings of the Intensity IOL. In a study comparing three trifocal IOLs and using the same questionnaire, Ribeiro and Ferreira found complaints in line with what we found. Worse results were obtained by Paul et al, who reported night driving difficulties in 40% of patients implanted with the trifocal Acri-LISA IOL.
In the context of evaluating the performance of diffractive IOLs, it is essential to consider aberrometry, contrast sensitivity, and defocus factors, because measurements obtained using infrared light may not accurately reflect the optical behavior of these lenses under normal visual conditions. Although aberrometry with infrared light remains a valuable tool in many areas of ophthalmic evaluation, its application in assessing the visual quality provided by diffractive IOLs requires careful interpretation. For studies or clinical evaluations focusing on the outcomes of diffractive IOL implantation, relying on assessments that simulate or directly measure the optical performance in the visible light spectrum would provide more relevant insights into the patient's visual experience.
The peculiarities introduced by negative diffraction orders have a specific implication: they can effectively reduce the amount of light contributing to the primary (positive) focal points, particularly the one for distance vision. To compensate for this, the “carrier” IOL, or the base design of the diffractive IOL without considering the diffractive elements, must possess a higher refractive power than an equivalent monofocal IOL intended solely for distance vision. This excess power in the carrier IOL is necessary to offset the light distribution affected by negative diffraction, ensuring that enough light is focused at the distance vision point, even after accounting for the diffraction-induced dispersion of light to other focal points. This may account for the discrepancies between objective (infrared based) and subjective (visible based) refraction.
This is one of the first studies comparing the pentafocal Intensity IOL with the trifocal FineVision IOL, and it has limitations. The constant of the study IOLs was not optimized, and this resulted in slight postoperative myopia with the Intensity IOL. This myopia affected UDVA, and therefore CDVA is a better indicator of the differences. The contrast sensitivity was studied with an electronic test that did not allow for mesopic conditions. The aberration test, despite using common clinical equipment and looking for differences rather than for absolute values, was performed with infrared light of 785 nm (Zywave) and 780 nm (OQAS) and may not be ideal to study diffractive IOLs in a human eye.
Despite these limitations, we can conclude that in patients with low astigmatism the pentafocal Intensity IOL gave better DCIVA than the trifocal FineVision IOL, with no decrease in photopic contrast sensitivity. The flatter defocus curve we obtained with the new IOL gave seamless vision from distance to near to our patients and could clinically compensate for some residual hyperopia. The patient-reported outcome was better with the Intensity IOL, possibly for the lower number of diffractive rings. The optical aberration in the implanted eyes was similar with either IOL, but the optical quality study gave contradictory results. Further investigation is required to better understand the optical properties of the new IOL in the implanted eyes.
AUTHOR CONTRIBUTIONS
Study concept and design (CB, RB); data collection (CB); analysis and interpretation of data (CB, PM, SAT, SG, RB); writing the manuscript (CB, RB); critical revision of the manuscript (PM, SAT, SG); administrative, technical, or material support (CB); supervision (SG)
References
- 1. Gatinel D, Houbrechts Y. Comparison of bifocal and trifocal diffractive and refractive intraocular lenses using an optical bench. J Cataract Refract Surg. 2013;39(7):1093–1099–. PMID:
- 2. Lee S, Choi M, Xu Z, Zhao Z, Alexander E, Liu Y. Optical bench performance of a novel trifocal intraocular lens compared with a multifocal intraocular lens. Clin Ophthalmol. 2016;10:1031–1038–.
- 3. Amigo-Frances A, Castillo-Gomez A, Carmona-Gonzalez D, Martinez-Sorribes P, Amigó A. Comparative study of visual results obtained with two trifocal lens models in cataract surgery. Journal of Clinical Research and Ophthalmology. 2020;7(2):054–060–.
- 4. Ferreira TB, Ribeiro FJ. Prospective comparison of clinical performance and subjective outcomes between two diffractive trifocal intraocular lenses in bilateral cataract surgery. J Refract Surg. 2019;35(7):418–425–. PMID:
- 5. Serdiuk V, Ustymenko S, Fokina S, Ivantsov I. Comparison of three different presbyopia-correcting intraocular lenses. Rom J Ophthalmol. 2020;64(4):364–379–. PMID:
- 6. Gabric N, Gabric I, Gabric K, Bišcevic A, Piñero DP, Bohac M. Clinical outcomes with a new continuous range of vision presbyopia-correcting intraocular lens. J Refract Surg. 2021;37(4):256–262–. PMID:
- 7. Nov E, Rubowitz A, Dar N, Sharon T, Assia EI. Visual performance of a novel optical design of a new multifocal intraocular lens. J Refract Surg. 2022;38(3):150–157–. PMID:
- 8. Bianchi GR. A prospective study of a new presbyopia pseudo-phakic intraocular lens: Safety, efficacy and satisfaction. Indian J Ophthalmol. 2022;70(9):3305–3310–. PMID:
- 9. Ribeiro F, Ferreira TB. Comparison of clinical outcomes of 3 trifocal IOLs. J Cataract Refract Surg. 2020;46(9):1247–1252–. PMID:
- 10. Savini G, Calossi A, Schiano-Lomoriello D, Barboni P. Precision and normative values of a new computerized chart for contrast sensitivity testing. Sci Rep. 2019;9(1):16537.
- 11. Saad A, Saab M, Gatinel D. Repeatability of measurements with a double-pass system. J Cataract Refract Surg. 2010;36(1):28–33–. PMID:
- 12. McAlinden C, Pesudovs K, Moore JE. The development of an instrument to measure quality of vision: the Quality of Vision (QoV) questionnaire. Invest Ophthalmol Vis Sci. 2010;51(11):5537–5545–. PMID:
- 13. Bellucci R, Cargnoni M, Bellucci C. Clinical and aberrometric evaluation of a new extended depth-of-focus intraocular lens based on spherical aberration. J Cataract Refract Surg. 2019;45(7):919–926–. PMID:
- 14. Bellucci C, Mora P, Tedesco SA, Gandolfi S, Bellucci R. Automated and subjective refraction with monofocal, multi-focal, and EDOF intraocular lenses: review. J Cataract Refract Surg. 2023;49(6):642–648–. PMID:
- 15. Cochener B. Prospective clinical comparison of patient outcomes following implantation of trifocal or bifocal intraocular lenses. J Refract Surg. 2016;32(3):146–151–. PMID:
- 16. Poyales F, Pérez R, López-Brea I, Zhou Y, Rico L, Garzón N. Comparison of visual performance and patient satisfaction outcomes with two trifocal IOLs with similar optical design but different materials. Clin Ophthalmol. 2020;14:3237–3247–.
- 17. Nagy ZZ, Popper-Sachetti A, Kiss HJ. Comparison of visual and refractive outcomes between hydrophilic and hydrophobic trifocal intraocular lenses sharing the same optical design. J Cataract Refract Surg. 2019;45(5):553–561–. PMID:
- 18. Ang RET. Long term clinical outcomes of hydrophilic and hydrophobic versions of a trifocal IOL with the same optical design. Clin Ophthalmol. 2023;17:623–632–.
- 19. Chen T, Yu F, Lin H , , et al. Objective and subjective visual quality after implantation of all optic zone diffractive multifocal intraocular lenses: a prospective, case-control observational study. Br J Ophthalmol. 2016;100(11):1530–1535–. PMID:
- 20. Liao X, Lin J, Tian J, Wen B, Tan Q, Lan C. Evaluation of optical quality: ocular scattering and aberrations in eyes implanted with diffractive multifocal or monofocal intraocular lenses. Curr Eye Res. 2018;43(6):696–701–. PMID:
- 21. Paul C, Gläser S, Kiraly L, Bechmann M, Sel S, Sekundo W. Patient-reported quality of life and satisfaction after refractive lens extraction using a diffractive trifocal IOL: a multicenter retrospective cohort study. J Refract Surg. 2021;37(11):768–774–. PMID:
- 22. Schwiegerling J, DeHoog E. Problems testing diffractive intraocular lenses with Shack-Hartmann sensors. Appl Opt. 2010;49(16):D62–D68–. PMID: