INTRODUCTION
Sepsis is defined as life-threatening organ dysfunction caused by dysregulated host responses to infection.[] Despite the reported positive correlation between genetic polymorphism and sepsis outcome,[] a recent genome-wide association study that included 2534 patients identified only one gene variant (i.e., single nucleotide polymorphism [SNP]) that differed between survivors and non-survivors.[] Histones, positively charged nucleoproteins, are released into the extracellular space following cell injury from processes such as necrosis, apoptosis, pyroptosis, and NETosis. In sepsis, extracellular histones have been shown to have direct toxicity, acting as damage-associated molecular patterns (DAMPs) and interacting with receptors, such as Toll-like receptor 2 (TLR2), TLR4, and TLR9, to trigger various proinflammatory pathways and the complementary and coagulation systems and impair gut and blood-brain barrier function, leading to multi-organ failure and death.[] Mounting evidence has shown that epigenetic changes, particularly histone modifications, play a crucial role in the regulation of gene expression during sepsis and septic shock (SS).[] Epigenetic activation and inhibition may occur in the early phase of the inflammatory response; indeed, the expression of more than 3700 genes was induced within 2 h in healthy volunteers receiving intravenous lipopolysaccharide (LPS).[] In addition, so-called “endotoxin tolerance” (i.e., altered responsiveness to bacterial endotoxin)[] and the late development of an immunosuppressive phase during sepsis can also be regulated by epigenetic mechanisms.[,]
Histone modification is a posttranslational event that includes processes such as methylation, acetylation, phosphorylation, ubiquitination, malonylation, propionylation, butyrylation, crotonylation, and lactylation.[] Histone modifications can positively or negatively influence gene transcription. Histone acetylation is typically associated with active promoter regions and destabilizes DNA-protein interaction, transforming condensed chromatin into a looser structure and facilitating transcriptional activation.[] Histone methylation can lead to chromatin condensation with varying effects, depending on its location on the N-terminal tail.[] Citrullinated histone, particularly histone H3 (H3Cit), is mainly catalyzed by peptidyl arginine deiminases 4 and promotes the rapid formation of neutrophil extracellular traps (NETs) during chromatin decondensation.[] Consequently, H3Cit may serve as a biomarker for NET production in sepsis and for the severity of sepsis; it may also represent a potential therapeutic target.[] Whether the neutralization of histones can influence posttranslational histone modification is unknown.
Histone modifications are also involved in various cardiovascular conditions, including cardiac hypertrophy, arrhythmias, atherosclerosis, myocardial infarction, and myocardial ischemia/reperfusion (I/R) injury.[] Cardiogenic shock (CS), a severe condition characterized by inadequate tissue perfusion due to cardiac failure, involves complex pathophysiological mechanisms, including calcium and proton overload, endoplasmic reticulum stress, mitochondrial dysfunction, activation of apoptotic pathways, oxidative stress, inflammation, and metabolic dysregulation.[] Epigenetic modifications, particularly histone modifications, also play a significant role in the regulation of gene expression and the pathogenesis of CS.[] The expression of genes related to inflammatory response is upregulated after myocardial infarction, as a result of the activation of the nuclear factor-κB (NF-κB) and tumor necrosis factor (TNF) signaling pathways.[,] Despite these shared inflammatory pathways in CS and SS, little is known about whether epigenetic modification patterns, specifically histone H3 modifications, are the same in SS and CS.
The administration of sodium-β-O-methyl cellobioside sulfate (mCBS) significantly decreased circulating nucleosome concentrations and vasopressor requirements, improved microcirculation and tissue oxygenation, and decreased inflammation and organ dysfunction in a clinically relevant SS model.[] We explored the temporal dynamics of nucleosomes and posttranslational H3 modifications (including H3K27ac, H3K27me3, and H3R8Cit) in experimental models of SS and CS. We also studied the effects of mCBS administration on posttranslational H3 modifications during SS. SS experiments can theoretically be conducted on smaller mammals, such as rodents or rabbits; however, sheep and pigs offer a more translational animal model due to their closer resemblance to human anatomy and physiology. In sheep, sepsis induces clinical manifestations—fever, tachycardia, hypotension, and lactic acidosis—paralleling those observed in human sepsis patients. Moreover, the use of clinical-grade measurement tools, such as Swan-Ganz pulmonary artery catheters, necessitates an animal model with appropriately scaled organs.
Pigs, in particular, provide a superior model for cardiovascular research due to their anatomical and physiological similarity to humans. Key features include comparable cardiac chamber and vessel sizes, a lack of significant coronary artery collateral networks, a similar heart rate range, and autonomic regulation of blood pressure. These factors, along with analogous inflammatory responses to myocardial ischemia and hypoxemia, make pigs ideal for validating findings and ensuring clinical relevance. Additionally, clinical measurement devices—such as echocardiography, pressure-volume loop catheters, transesophageal Doppler, micro-dialysis catheters, and PbtO2 electrodes—are optimized for use in larger species with human-like organ dimensions, further supporting the choice of pigs for this research.
METHODS
Study setting
The study followed the European Union (EU) Directive (2010/63/EU) for animal experiments and was approved by the local animal ethics committee, Comité Ethique du Bien-Être Animal of the Université Libre de Bruxelles (ULB) in Brussels, Belgium (Protocol number 740N for sheep model and protocol number 712N for pig model). Experiments were performed in the Experimental Laboratory of Intensive Care (LA1230406).
SS model
Detailed methods are provided in the supplementary material http://links.lww.com/JTCCM/A26. Briefly, SS was induced in 24 female sheep (Suffolk, female, 35 kg, age 6-9 months, Local farm, Belgium) by fecal peritonitis. Pain was carefully monitored with pain tests during the whole experiment. We chose only female animals because of the difficulty of male bladder catheterization and to maintain homogeneity. In the current protocol, 4 pilot animals did not have any adverse effects (expected or unexpected, such as APTT prolongation or allergic reaction). After the formal protocol started, animals were allocated to three groups according to the previous day’s randomization. The randomization procedure in the current protocol was conducted using blinded envelope draws, with each envelope containing a predefined animal number. No additional confounders were considered or controlled in this process. Investigators were blinded to the randomization: control, early mCBS administration, and late mCBS administration (n = 8 each). mCBS was given as a bolus (1 mg/kg) followed by a continuous infusion (1 mg/kg/h) immediately after sepsis induction (early group) or 4 h later (late group). The experimental period spanned 24 h, during which plasma samples were collected at baseline and every 4 h.[]
Cardiogenic shock model
Detailed methods are provided in the supplementary material http://links.lww.com/JTCCM/A26. Briefly, CS was induced in 8 pigs (Sus scrofa domestics, male/female, 65 kg, age 4-6 months, RA-SE Genetics, Belgium) of either sex by partial occlusion of the anterior interventricular and circumflex arteries using two angioplasty balloons for 120 min. The CS experiment lasted for 12 h, with serum samples collected at baseline, post-ischemia, and thereafter every 4 h. All animals were mechanically ventilated, hemodynamically monitored, fluid resuscitated and given norepinephrine throughout the experiment. Pain tests were performed regularly during the experiment.
Biological measurements
The levels of circulating nucleosomes (Nu H3.1), H3R8Cit, H3K27ac, and H3K27me3 were measured using an enzyme-linked immunosorbent assay (ELISA, Volition, Isnes, Belgium) in plasma for the SS animals and in serum for the CS animals. The lower limits of quantification for H3.1, H3R8Cit, H3K27ac, and H3K27me3 are 3 µg/L, 4.8 µg/L, 1 µg/L, and 2.8 µg/L, respectively. Measurements have been validated in mice, pigs, horses, dogs, and rabbits, while as a corn histone, H3 is highly conserved across different species. Measurements were made 30 months after sample collection for SS and 22 months after sample collection for CS.
Statistical analysis
Given the absence of prior studies on large animals, an a priori convenience sample size of 8 animals per group was considered adequate for the study aims. Statistical analysis was performed using Prism 9 (Version 9.1.2, San Diego, CA, USA). Continuous variables are presented as mean ± standard deviation (SD) or median. To estimate the effects of SS and CS on circulating nucleosomes and posttranslational H3 modifications, a one-way analysis of variance (ANOVA) was used. To estimate the effect of mCBS administration, a mixed-effects model with Greenhouse-Geisser correction was used. The effects of time and group, as well as the interactions between groups and time, were tested as fixed effects, and animals were introduced as random effects. If there were significant differences, the two-stage linear procedure of Benjamini, Krieger, and Yekutieli, with individual variances, was used to compare the means of these variables for the groups at each time point. A P value of < 0.05 was considered statistically significant.
RESULTS
General parameters for the SS and CS models
Four hours after the induction of sepsis, the animals exhibited SS, as suggested by the presence of fever, tachycardia, hypotension, and decreased cardiac output. Throughout the subsequent 24-h observation period, despite full fluid resuscitation, vasopressors, antibiotics, and peritoneal lavage, the animals had a decreased PaO2/FiO2 ratio, indicative of impaired oxygenation, and metabolic acidosis, as evidenced by elevated lactate levels [Figure 1]. Two animals died from refractory shock 14 and 16 h after sepsis inducement. In the pigs, 2 h of coronary ischemia led to the development of CS characterized by hypotension, decreased cardiac output, and pulmonary hypertension [Figure 1]. All the 8 animals survived until the end of the experiment. Baseline measurements of body weight, core temperature, cardiac output, mean arterial pressure (MAP), mean pulmonary artery pressure (MPAP), PaO2/FiO2, and arterial lactate levels indicated that the animals were hemodynamically stable and within species-relevant physiological ranges. The baseline characteristics of the SS and CS animals are shown in Table 1.
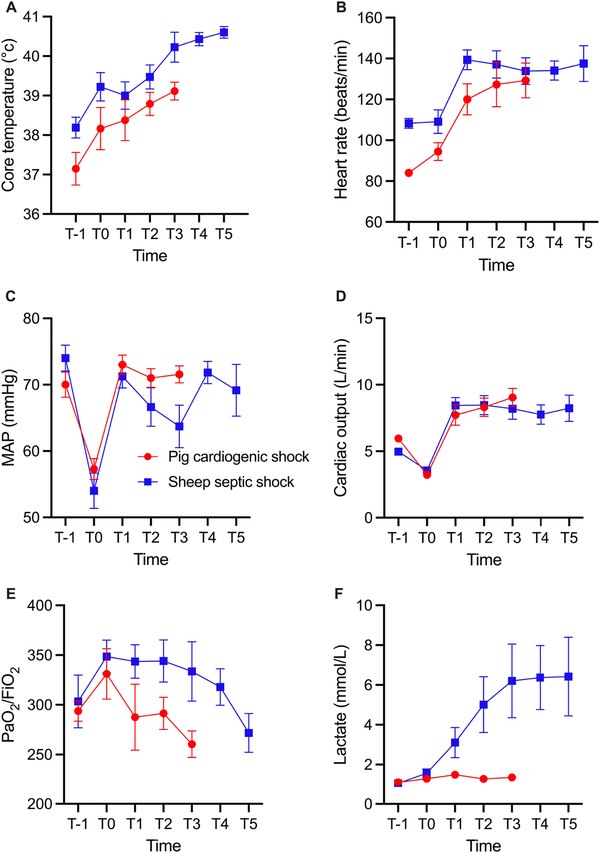
Figure 1.
Core body temperature (A), heart rate (B), MAP (C), cardiac output (D), PaO2/FiO2 (E), and arterial lactate levels (F) in the SS and CS groups. For the SS group, T-1, T0, T1, T2, T3, T4, T5 represent baseline, 4, 8, 12, 16, 20, and 24 h, respectively, after sepsis inducement. For the CS group, T-1, T0, T1, T2, represent baseline, after 120 min of ischemia, and 4 and after ischemia, respectively. Values given as mean ± standard error. MAP, mean arterial pressure; CS, cardiogenic shock; SS, septic shock.
Table 1.
Baseline characteristics in the SS and CS animals
| Variables | Sheep sepsis model (n = 8) | Pig cardiogenic shock model (n = 8) |
|---|---|---|
| Male/female | 0/8 | 5/3 |
| Body weight (kg) | 35.9 ± 0.7 | 58.5 ± 7.1 |
| Temperature (℃)* | 38.2 ± 0.3 | 37.2 ± 1.2 |
| Heart rate (beats/min) | 108 ± 2 | 84 ± 5 |
| MAP (mmHg) | 74 ± 2 | 70 ± 5 |
| MPAP (mmHg) | 19 ± 3 | 25 ± 2 |
| PaO2/FiO2 (mmHg/%) | 303 ± 27 | 294 ± 29 |
| Arterial lactate (mmol/L) | 1.1 ± 0.1 | 1.1 ± 0.2 |
Circulating nucleosomes and post-translational histone modifications in the two models
In the control SS sheep, the circulating nucleosome concentrations were 11.7 ± 3.7 µg/L at baseline. After sepsis induction, they increased to 158.7 ± 148.8 µg/L and remained elevated until the end of the experiment, although the differences were not statistically significant [Table 2]. The two animals that died early had markedly higher levels than the other animals [Figure 2]. H3K27ac concentrations were very low at baseline (0.4 ± 0.1 µg/L), peaked around 8-12 h after sepsis, and then decreased until the end of the experiment (P = 0.34, Table 2). However, values for 24 of 51 samples (53%) were below the lower limit of quantification. H3R8Cit concentrations were also low at baseline (2.6 ± 0.8 µg/L), peaked at 8 h post-sepsis induction, and then gradually decreased (P = 0.46, Table 2). H3K27me3 concentrations were low at baseline (6.4 ± 1.8 µg/L), doubled by 12 h after sepsis, and remained elevated until the end of the experiment [Table 2].
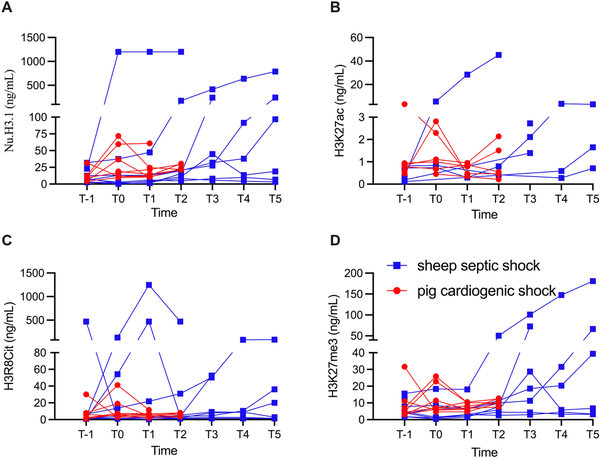
Figure 2.
Individual concentrations of circulating nucleosomes (NU.H3.1, A), H3K27ac (B), H3R8Cit (C), and H3K27me3 (D) at different time points in the SS and CS groups. For the SS group, T-1, T0, T1, T2, T3, T4, T5 represent baseline, 4, 8, 12, 16, 20, and 24 h, respectively, after sepsis inducement. For the CS group, T-1, T0, T1, T2, represent baseline, after 120 min of ischemia, and 4 and 8 h after ischemia, respectively. Values are given for each individual. CS, cardiogenic shock; SS, septic shock.
Table 2.
Circulating concentrations of nucleosomes (Nu. H3.1), H3K27ac, H3R8Cit, and H3K27me3 in the CS and SS groups
| Group | T-1 | T0 | T1 | T2 | T3 | T4 | T5 | P value | |
|---|---|---|---|---|---|---|---|---|---|
| H3.1 (μg/L) | CS | 12.3 ± 4.1 | 29.5 ± 8.5 | 20.8 ± 6.0 | 24.7 ± 1.7 | - | - | - | 0.31 |
| SS | 11.7 ± 3.7 | 158.7 ± 148.8 | 160.8 ± 148.6 | 182.4 ± 146.8 | 110.9 ± 59.7 | 132.3 ± 101.8 | 193.2 ± 124.9 | 0.95 | |
| H3K27ac (μg/L) | CS | 1.1 ± 0.4 | 1.4 ± 0.4 | 0.6 ± 0.1 | 1.0 ± 0.4 | - | - | - | 0.49 |
| SS | 0.4 ± 0.1 | 2.4 ± 1.6 | 14.4 ± 14.1 | 15.5 ± 14.9 | 2.1 ± 0.4 | 1.5 ± 1.1 | 1.8 ± 0.7 | 0.34 | |
| H3R8cit (μg/L) | CS | 7.6 ± 3.9 | 11.6 ± 4.6 | 5.7 ± 1.0 | 6.3 ± 0.8 | - | - | - | 0.54 |
| SS | 2.6 ± 0.8 | 25.8 ± 16.2 | 218.5 ± 157.9 | 64.9 ± 58.0 | 18.4 ± 8.6 | 19.2 ± 12.8 | 24.5 ± 13.4 | 0.46 | |
| H3K27me3 (μg/L) | CS | 9.3 ± 3.9 | 11.3 ± 3.0 | 7.9 ± 0.8 | 10.5 ± 0.8 | - | - | - | 0.77 |
| SS | 6.4 ± 1.8 | 5.4 ± 2.5 | 5.9 ± 2.2 | 13.2 ± 6.3 | 34.2 ± 14.3 | 35.5 ± 22.9 | 50.0 ± 28.1 | 0.13 | |
In pigs, circulating nucleosome concentrations were 12.3 ± 4.1 µg/L at baseline, increased after ischemia, and remained elevated until the end of the experiment, although these differences were not statistically significant [Table 2]. There were no significant changes in circulating H3R8Cit (P = 0.49), H3K27ac (P = 0.54), or H3K27me3 levels (P = 0.77) during the 12-h experiment [Table 2 and Figure 2].
There was a trend toward lower absolute circulating nucleosome concentrations (P = 0.14), H3R8Cit (P = 0.12), and H3K27me3 levels (P = 0.08) in the SS animals treated with mCBS than in the control animals [Figure 3]. H3K27ac values were below the lower quantification limit in 115 of 163 samples (71%), with no significant differences among the groups.
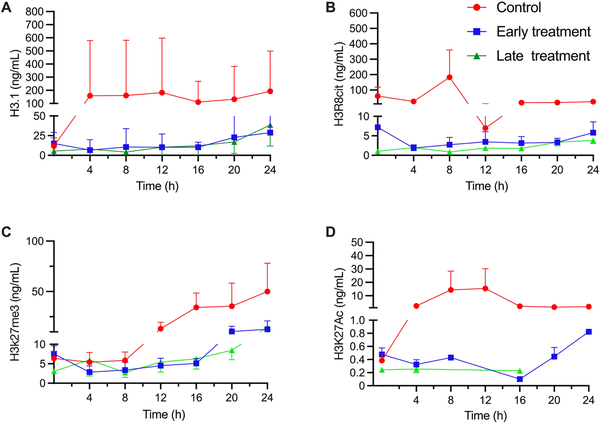
Figure 3.
Circulating concentrations of nucleosomes (NU. H3.1) (A), H3R8Cit (B), H3K27ac (C), and H3K27me3 (D) in the control group, and the two mCBS groups in the SS animals. Values given as mean ± standard error. mCBS, sodium-β-O-methyl cellobioside sulfate; SS, septic shock.
DISCUSSION
The main findings of the current study in these two large animal, clinically relevant SS and CS models, are as follows: (1) Circulating nucleosome levels increased in SS in sheep but not in CS in pigs. (2) Circulating H3R8Cit levels peaked at 8 h after sepsis induction and decreased thereafter in SS; there were no differences in values over time in CS. (3) Circulating H3K27ac levels showed a trend toward an increase in SS (71% of samples had undetectable levels), and there were no differences over time in CS. After mCBS administration, H3K27ac levels were below the quantification limit in 79% of the SS samples. (4) Circulating H3K27me3 levels increased slowly until the end of the experiment in SS but not in CS. (5) Neutralization of histones with mCBS was associated with a trend toward decreased levels of circulating nucleosomes, H3R8Cit, and H3K27me3 in SS.
Circulating nucleosome levels have emerged as a promising biomarker for cell injury and death, with the potential for detecting and monitoring NETosis and sepsis severity, and as a prognostic marker that may help guide personalized therapy in SS.[] We corroborated this possibility by demonstrating increased nucleosome levels after the development of SS, with a larger increase in the two animals that died. Histone neutralization with mCBS in sepsis has been shown to lower vasopressor requirements, improve microcirculation, and reduce multi-organ dysfunction in a sheep sepsis model.[] Unlike in the sepsis model, nucleosome levels did not increase during CS. In a rat heart ischemia/reperfusion (I/R) model, mCBS administration was associated with decreased circulating histone levels and infarct size.[] Several mechanisms may explain these apparent differences between SS and CS: (1) a marked increase in autophagy during the reperfusion phase in CS;[] (2) cell death processes, including apoptosis, necrosis, and pyroptosis, might occur less frequently in the acute phase of CS than SS; (3) different sources (serum versus plasma) for measurements of circulating nucleosome and histone levels in the two models; and (4) the shorter observation period in the CS studies may not capture these changes: extensive transcriptional changes have been documented 24 and 48 h after I/R (45 min ischemia) in a murine myocardial model.[]
H3R8Cit is implicated in the modulation of gene expression and inflammatory processes. Bacterial LPS activates neutrophils and subsequently catalyzes the intracellular citrullination of histone arginine by peptidyl arginine deiminase 4 (PAD4).[] In our SS model, circulating H3R8Cit levels peaked about 8 h after sepsis induction. Similarly, in a mouse model of LPS-stimulated sepsis, the number of H3Cit-positive cells in the liver peaked at 12 h, as observed by immunohistochemistry, while flow cytometry showed a significant increase in these cells in the peripheral blood 4 h after LPS injection.[] Intra-individual elevations of plasma H3Cit levels have also been observed in a human model of LPS-induced inflammation.[] Interestingly, the two animals that died had very high H3Cit values. These results are consistent with those seen in sepsis patients in whom high H3Cit levels were correlated with poor prognoses.[,] In contrast, there were no significant differences in H3R8Cit levels over time in the CS model, suggesting that H3R8 citrullination may be less frequent in the acute phase of CS.
Interestingly, histone acetylation has also been reported to be associated with increased NETosis,[] whereas the loss of histone 3 acetylation and tri-methylation has been shown to be correlated with endotoxin tolerance.[] Histone acetylation is generally recognized for its positive regulation of gene transcription by enhancing DNA accessibility.[] Specifically, the acetylation of histone H3 at lysine 27 (H3K27Ac) is a well-established marker of active promoters and enhancers, playing a crucial role in the transcriptional activation of genes involved in inflammatory processes.[,] In an in vitro model of inflammation, neutrophils stimulated by LPS exposure exhibited high H3K27Ac activity, which enhanced their ability to respond more robustly and precisely to proinflammatory cytokines at inflammation sites.[] Conversely, active H3K27Ac was significantly reduced in cardiomyocytes exposed to ischemic conditions.[] Ischemia significantly reduces total acetylation,[] whereas reperfusion may enhance it. In a mouse model of cecal ligation and puncture-induced polymicrobial sepsis, pretreatment with histone deacetylase inhibitors (HDACi), such as trichostatin A or sodium butyrate, improved lung injury, improved survival rates, and decreased circulating interleukin 6 (IL-6) levels during sepsis.[] Prophylactic inhibition of histone deacetylation alone or combined with the inhibition of histone methylation reduced capillary leak and pulmonary edema in vivo and substantially reduced lung histopathology in experimental acute lung injury.[] Similarly, in cardiac ischemia/reperfusion, HDACi induce a range of protective effects, reducing infarct size.[] Levels of acetylation in our study were under the quantification limit in 53% of samples in the control group and 79% of samples in the mCBS treatment groups. Furthermore, the loss of acetylation has been related to the overexpression of adhesion molecules and decreased endothelial permeability in a murine model of acute lung injury-induced sepsis.[] An additional explanation might be the short observation period because in an observational study of 59 cardiac surgery patients, serum H3K27ac levels were elevated at 24 h and 7 d compared with baseline values.[] Additionally, H3K27ac was significantly reduced in cardiomyocytes exposed to ischemic conditions.[]
Histone methylation impacts gene expression differently depending on the site and degree of methylation. H3 is the major site of histone methylation[] and plays an important role in NF-κB-dependent inflammatory responses.[] Methylation of histone H3K27me is associated with gene expression silencing and often acts as a repressive marker.[,] Histone lysine methylation has emerged as a critical factor in the regulation of gene expression, cell cycle progression, genome stability, and nuclear architecture.[] Hypermethylation (increased methylation) of gene promoters and enhancers is frequently associated with gene repression.[] H3K27me3 is a key epigenetic modifier in the I/R process. Both H3K27me3 and its methyltransferase, polycomb repressor complex 2 (PRC2), were upregulated in myocardial tissues following I/R.[] Inhibition of H3K27me3, and its methyltransferase could be a potential strategy for myocardial I/R injury intervention.[] Oxygen deprivation typically leads to a significant increase in H3K27me3 levels. In a fully resuscitated SS model, we observed a marked increase in H3K27me3 concentrations, indicating reperfusion injury, as evidenced by the occurrence of pulmonary edema and impaired gas exchange.[] In an in vitro model of inflammation, in which neutrophils were stimulated by LPS exposure, a lower activity of H3K27me3 was observed, suggesting that H3K27me3 might limit neutrophil activation within the vessel, allowing additional time for chemotaxis, diapedesis, and infiltration.[] In our SS model, H3K27me3 concentrations increased during the 12- and 24-h observation periods in the SS control group, while no increase was observed in the CS model during the first 12 h. This discrepancy may be attributed to the duration of the observation period, as active histone modifications (H3K27ac, H3K4me1, H3K4me3, and H3K9ac) were positively correlated with gene expression changes at both 24 and 48 h post-I/R in a murine heart I/R model.[]
The current studies involving sheep SS and pig CS models have several limitations. First, acute models were used with short observation periods (24 h for SS and 12 h for CS), which limits the ability to extrapolate these early observations to the late phases of circulatory failure. Second, the animals used in both models were healthy and young, which may not accurately reflect the clinical reality in which patients often have diverse comorbidities. Third, no sham animals were included in the study to control for the effect of instrumentation and/or prolongated anesthesia; however, this aligns with the principle of the three Rs (replacement, reduction, and refinement) to minimize the number of animals used. Fourth, only circulating concentrations of nucleosome and post-translational H3 modifications were measured; organ- and tissue-specific levels were not assessed, limiting interpretation. Fifth, serum, rather than plasma, was used for measurements in the CS animals, providing some bias, but serum nucleosome levels have been reported to be higher than plasma levels, probably because the clotting process generally destroys leukocytes, which results in more released fragments.[] Sixth, the small number of animals used in the current study (8 per group) might explain why a trend rather than significance was found. Seventh, the small sample population (8 animals in each group) might add statistical bias. Finally, these studies were performed in large animals, but it is still difficult to generalize the findings directly to patients, which raises the request for clinical trials to confirm current results.
CONCLUSION
The two clinically relevant models had different patterns of circulating nucleosomes and H3 PTMs, which might suggest that a neutralization of histone strategy should be different for SS and CS. Neutralization of histones with mCBS showed a trend to reduce H3 PTMs; however, its mechanism and clinical relevance warrant further investigation.
Author contributions
Su F: Conceptualization, Formal analysis, Writing—Original draft, Investigation, Supervision, Project administration, Funding acquisition, Writing—Review and Editing. Garcia B: Investigation (septic shock), Writing—Review and Editing. Herpain A, Moiroux-Sahraoui A, Manicone F: Investigation (cardiogenic shock), Writing—Review and Editing. Vincent J-L, Creteur J, Taccone FS: Supervision, Writing—Review and Editing. Moreau A, Annoni F, Giuseppe C, Picod A: Writing—Review and Editing. All authors have read and approved the final version.
Source of funding
This protocol was funded by Volition, Isnes, Belgium and Grandpharma, Wuhan, China
Ethics approval
The study followed the European Union Directive (2010/63/EU) for animal experiments and was approved by the local animal ethics committee, Comité Ethique du Bien-Être Animal of the Université Libre de Bruxelles (ULB) in Brussels, Belgium (Protocol number 740N for sheep model and protocol number 712N for pig model). Experiments were performed in the Experimental Laboratory of Intensive Care (LA1230406).
Conflict of interest
Fuhong Su is a medical adviser for Grandpharma, China. The other authors declare they have no competing interests.
Data availability statement
Data used to support the findings of this study are available from the corresponding author upon request.
Protocol registration
The current study design was not predefined, after the registered protocol 712N & 740N finished, the positive findings of those two studies guided us to make further investigation, resulting in the current study.
How to cite this article: Su FH, Herpain A, Garcia B, et al. Post-translational histone 3 modification patterns in experimental septic and cardiogenic shock. J Transl Crit Care Med. 2024;6:e24-00031. doi: 10.1097/JTCCM-D-24-00031
REFERENCES
1.
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801-810.2.
Shimada T, Oda S, Sadahiro T, et al. Outcome prediction in sepsis combined use of genetic polymorphisms-A study in Japanese population. Cytokine. 2011;54(1):79-84.3.
Rautanen A, Mills TC, Gordon AC, et al. Genome-wide association study of survival from sepsis due to pneumonia: An observational cohort study. Lancet Respir Med. 2015;3(1):53-60.4.
Li Y, Wan D, Luo X, et al. Circulating histones in sepsis: potential outcome predictors and therapeutic targets. Front Immunol. 2021;12:650184.5.
Cross D, Drury R, Hill J, Pollard AJ. Epigenetics in sepsis: Understanding its role in endothelial dysfunction, immunosuppression, and potential therapeutics. Front Immunol. 2019;10:1363.6.
Calvano SE, Xiao W, Richards DR, et al. A network-based analysis of systemic inflammation in humans. Nat. 2005;437(7061):1032-1037.7.
Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nat. 2007;447(7147):972-978.8.
El Gazzar M, Liu T, Yoza BK, McCall CE. Dynamic and selective nucleosome repositioning during endotoxin tolerance. J Biol Chem. 2010;285(2):1259-1271.9.
Liu R, Wu J, Guo H, et al. Post-translational modifications of histones: Mechanisms, biological functions, and therapeutic targets. Med Comm. 2023;4(3):e292.10.
McKinsey TA. Therapeutic potential for HDAC inhibitors in the heart. Annu Rev Pharmacol Toxicol. 2012;52:303-319.11.
Costello KR, Schones DE. Chromatin modifications in metabolic disease: Potential mediators of long-term disease risk. Wiley Interdiscip Rev Syst Biol Med. 2018;10(4):e1416.12.
Tang J, Zhuang S. Histone acetylation and DNA methylation in ischemia/reperfusion injury. Clin Sci (Lond). 2019;133(4):597-609.13.
Verdone L, Caserta M, Di Mauro E. Role of histone acetylation in the control of gene expression. Biochem Biol Cell. 2005;83(3):344-353.14.
Leshner M, Wang S, Lewis C, et al. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front Immunol. 2012;3:307.15.
Li Y, Liu Z, Liu B, et al. Citrullinated histone H3: A novel target for the treatment of sepsis. Surg. 2014;156(2):229-234.16.
Li Y, Wang C, Li T, et al. The whole transcriptome and proteome changes in the early stage of myocardial infarction. Cell Death Discov. 2019;5:73.17.
Brener MI, Rosenblum HR, Burkhoff D. Pathophysiology and advanced hemodynamic assessment of cardiogenic shock. Methodist Debakey Cardiovasc J. 2020;16(1):7-15.18.
Escate R, Padró T, Suades R, et al. MiR-619-5p and cardiogenic shock in patients with ST-segment elevation myocardial infarction. Eur J Clin Invest. 2024;54(8):e14186.19.
Wang X, Su J, Lin Z, Liu K, Zhuang Y. PINCH1 knockout aggravates myocardial infarction in mice via mediating the NF-κB signaling pathway. Exp Ther Med. 2022;23(1):62.20.
Garcia B, Su F, Dewachter L, et al. Neutralization of extracellular histones by sodium-Β-O-methyl cellobioside sulfate in septic shock. Crit Care. 2023;27(1):458.21.
Su F, Moreau A, Savi M, et al. Circulating nucleosomes as a novel biomarker for sepsis: A scoping review. Biomed. 2024;12(7):1385.22.
Shah M, He Z, Rauf A, et al. Extracellular histones are a target in myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2022;118(4):1115-1125.23.
Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Diwan A. Autophagy is impaired in cardiac ischemia-reperfusion injury. Autophagy. 2012;8(9):1394-1396.24.
Ni L, Lin B, Zhang Y, et al. Histone modification landscape and the key significance of H3K27me3 in myocardial ischaemia/reperfusion injury. Sci China Life Sci. 2023;66(6):1264-1279.25.
Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207(9):1853-1862.26.
Nomura K, Miyashita T, Yamamoto Y, et al. Citrullinated histone H3: early biomarker of neutrophil extracellular traps in septic liver dam age. J Surg Res. 2019;234:132-138.27.
Thalin C, Daleskog M, Göransson SP, et al. Validation of an enzyme-linked immunosorbent assay for the quantification of citrullinated histone H3 as a marker for neutrophil extracellular traps in human plasma. Immunol Res. 2017;65(3):706-712.28.
Tian Y, Russo RM, Li Y, et al. Serum citrullinated histone H3 concentrations differentiate patients with septic verses non-septic shock and correlate with disease severity. Infection. 2021;49(1):83-93.29.
Paues Göranson S, Thalin C, Lundström A, et al. Circulating H3Cit is elevated in a human model of endotoxemia and can be detected bound to microvesicles. Sci Rep. 2018;8(1):12641.30.
Hamam HJ, Khan MA, Palaniyar N. Histone acetylation promotes neutrophil extracellular trap formation. Biomol. 2019;9(1):32.31.
Zhao S, Allis CD, Wang GG. The language of chromatin modification in human cancers. Nat Rev Cancer. 2021;21(7):413-430.32.
Roadmap Epigenomics Consortium, Kundaje A, Meuleman W, et al. Integrative analysis of 111 reference human epigenomes. Nat. 2015;518(7539):317-330.33.
Neville JJ, Orlando J, Mann K, McCloskey B, Antoniou MN. Ubiquitous Chromatin-opening Elements (UCOEs): Applications in biomanufacturing and gene therapy. Biotechnol Adv. 2017;35(5):557-564.34.
Piatek P, Namiecinska M, Lewkowicz N, et al. Histone H3 posttranslational modified enzymes defined neutrophil plasticity and their vulnerability to IL-10 in the course of the inflammation. J Inflamm (Lond). 2024;21(1):16.35.
Randhawa PK, Rajakumar A, Futuro de Lima IB, Gupta MK. Eugenol attenuates ischemia-mediated oxidative stress in cardiomyocytes via acetylation of histone at H3K27. Free Radic Biol Med. 2023;194:326-336.36.
Zhang L, Jin S, Wang C, Jiang R, Wan J. Histone deacetylase inhibitors attenuate acute lung injury during cecal ligation and puncture-induced polymicrobial sepsis. World J Surg. 2010;34(7):1676-1683.37.
Thangavel J, Malik AB, Elias HK, et al. Combinatorial therapy with acetylation and methylation modifiers attenuates lung vascular hyperpermeability in endotoxemia-induced mouse inflammatory lung injury. Am J Pathol. 2014;184(8):2237-2249.38.
Zhang L, Wang H, Zhao Y, et al. Myocyte-specific overexpressing HDAC4 promotes myocardial ischemia/reperfusion injury. Mol Med. 2018;24(1):37.39.
Aune SE, Herr DJ, Mani SK, Menick DR. Selective inhibition of class I but not class IIb histone deacetylases exerts cardiac protection from ischemia reperfusion. J Mol Cell Cardiol. 2014;72:138-145.40.
Herr DJ, Baarine M, Aune SE, et al. HDAC1 localizes to the mitochondria of cardiac myocytes and contributes to early cardiac reperfusion injury. J Mol Cell Cardiol. 2018;114:309-319.41.
Bomsztyk K, Mar D, An D, et al. Experimental acute lung injury induces multi-organ epigenetic modifications in key angiogenic genes implicated in sepsis-associated endothelial dysfunction. Crit Care. 2015;19(1):225.42.
Laudanski K, Liu D, Hajj J, Ghani D, Szeto WY. Serum level of total histone 3, H3K4me3, and H3K27ac after non-emergent cardiac surgery suggests the persistence of smoldering inflammation at 3 months in an adult population. Clin Epigenetics. 2022;14(1):112.43.
Jambhekar A, Dhall A, Shi Y. Roles and regulation of histone methylation in animal development. Nat Rev Mol Cell Biol. 2019;20(10):625-641.44.
Yu L, Fang F, Dai X, et al. MKL1 defines the H3K4Me3 landscape for NF-κB dependent inflammatory response. Sci Rep. 2017;7(1):191.45.
Howard C, Yehudit B. Linking DNA methylation and histone modification: Patterns and paradigms. Nat rev Genet. 2009;10:295-304.46.
Black JC, Van Rechem C, Whetstine JR. Histone lysine methylation dynamics: Establishment, regulation, and biological impact. Mol Cell. 2012;48(4):491-507.47.
Jaenisch R, Bird A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat genet. 2003;33 Suppl:245-254.48.
Mittra I, Nair NK, Mishra PK. Nucleic acids in circulation: Are they harmful to the host? J Biosci. 2012;37(2):301-312.
