Introduction
Atherosclerotic diseases such as myocardial infarction and stroke are still the major cause of mortality globally []. Diabetes, hyperlipidemia, and hypertension are the major risk factors which exacerbate the development of atherosclerosis [-]. Meanwhile, the prevalence of hypertension continues to increase worldwide, and hypertensive patients are expected to reach 1.5 billion by 2025 []. In general, hypertension alone without elevated plasma cholesterol levels was not able to induce atherosclerosis in elastic and muscular arteries such as the aorta and carotid and coronary arteries [], suggesting that hypertension is an enhancer for the development of atherosclerosis in hypertensive patients.
To elucidate the pathophysiology of hypertension, Goldblatt et al. [] were the first to introduce unilateral constriction of the renal artery in dogs, and this method was also used in mice [], rats [], and rabbits []. In addition, angiotensin II (AngII) infusion into mice via osmotic minipumps was developed to elucidate the mechanisms underlying hypertension [, ]. However, using this method, it is difficult to investigate the effect of mechanical stress on the arterial wall because other biological functions of elevated AngII also affect the development of atherosclerosis.
Cholesterol-fed rabbits are a unique model for the study of human atherosclerosis because rabbits are sensitive to a cholesterol diet and rapidly develop atherosclerosis in aortas and coronary arteries [, ]. Furthermore, in contrast to rodents (mice and rats), many features of the lipid metabolism in rabbits are similar to those in humans. Moreover, atherosclerotic lesions in cholesterol-fed rabbits are characterized by macrophage-derived foam cells, which mimic human fatty streaks []. Chobanian et al. [, ] showed that a short period of hypertension enhanced aortic atherosclerosis in Watanabe heritable hyperlipidemic (WHHL) rabbits whereas captopril, an angiotensin-converting enzyme inhibitor, inhibited hypertension-induced atherosclerosis. However, it is still not clear whether elevated blood pressure (BP) affects lipid metabolism and the development of coronary atherosclerosis. To examine the effect of hypertension on coronary atherosclerosis, we performed the current study using cholesterol-fed rabbits in which coronary atherosclerosis can be observed []. We compared the atherosclerotic features of both aorta and coronary arteries of hypertensive (HTN) cholesterol-fed rabbits with normotensive (NTN) cholesterol-fed rabbits. Our results demonstrated that hypertension not only enhanced aortic atherosclerosis but also induced coronary atherosclerosis and arteriolosclerosis.
Materials and Methods
Experimental Design
To investigate the effects of hypertension on the development of atherosclerotic lesions, we used cholesterol-fed Japanese white rabbits (4 months old, male) (Japan SLC Inc., Shizuoka, Japan) and divided them into two groups: the HTN group and the NTN group. Hypertension was induced by surgical removal of the left kidney and partial ligation of the right renal artery (1K1C) while sham surgery was conducted in the normotensive group as a control []. Rabbits were anesthetized by ketamine/medetomidine by ear vein injection and placed in dorsal recumbent position; subsequently, the abdominal hair was shaved and sterilized with Betadine. After opening the abdominal cavity, the left kidney was removed with the adrenal gland remaining, and the right renal artery was partially ligated using a silver restriction clip with a gap of 0.508 mm []. After a 2-week recovery period, the HTN group (n = 7) and the NTN group (n = 10) rabbits were fed a chow diet supplemented with 0.8% cholesterol and 3% soybean (w/w) (CLEA Japan Inc., Tokyo, Japan) for 16 weeks.
Determination of Blood Pressure, Plasma Lipids, and Lipoproteins
BP and heart rate were examined every for 4 weeks during the experiment as described previously [, ]. To avoid environmental stress, BP measurements were conducted in a separate room. Rabbits were placed in a quiet room at least 30 min before BP measurement. BP and heart rate were recorded using a transducer, and data were collected from each rabbit around 20 min using a BP amplifier (AD Instruments, Tokyo, Japan). The data were analyzed using Chart5 Pro v5.5 software (AD Instruments).
Blood was collected from an auricular artery after 16 h of fasting. Plasma lipids including total cholesterol, triglycerides, and high-density lipoprotein (HDL)-cholesterol (HDL-C) were measured every 4 weeks using Wako assay kits (Wako Pure Chemical Industries, Osaka, Japan). Apolipoprotein (apo) AI, apoB, and apoE contents in the EDTA-plasma were analyzed by Western blotting. Plasma (1 μL) was loaded on 4–20% SDS-PAGE under denaturing and reducing conditions, followed by immunoblotting with polyclonal antibodies (Abs) against apoAI (Bio-Rad Co., Kidlington, UK), apoB (Rockland Co., Limerick, PA, USA), apoE (Rockland) (working dilution 1: 1,000), and immune-complexed apolipoproteins were identified by reaction with a horseradish peroxidase-conjugated donkey Ab against goat IgG, followed by enhanced chemiluminescence detection [].
Pathological Analysis
After 16 weeks of cholesterol diet feeding, all rabbits were sacrificed by an overdose injection of sodium pentobarbital (100 mg/kg). The aortic tree was isolated along with the heart. Aortas were longitudinally opened and fixed in 10% neutral formalin solution. The fresh hearts were weighed, photographed first, and then fixed in 10% neutral formalin. After fixing, whole aortic trees with lumen opened were stained with Sudan IV for the evaluation of gross atherosclerotic lesions. The en face sudanophilic area relative to the whole surface area of each aorta was quantified using the WinRoof image system (Mitani Co., Tokyo, Japan).
For microscopic analysis of the aortic lesion size and quality, the whole aorta was separated into 3 segments including aortic arch and thoracic and abdominal aorta []. Each segment was cut serially into sections (8–10 for the arch and 24–28 for the thoracic and abdominal aorta) and then embedded in paraffin. Specimens were cut in 3-µm-thick sections. For intimal lesion size evaluation, serial sections were stained with elastic van Gieson (EVG) staining. For lesion quality analysis, sections were stained with hematoxylin and eosin (HE) or immunohistochemically with monoclonal Abs against rabbit macrophages RAM11 (Dako Co., Carpinteria, CA, USA) (1: 400 dilution), α- smooth muscle actin (Dako Co.) (1: 300 dilution), von Willebrand factor (Abbexa Co., Cambridge, UK) (1: 100 dilution), or copper oxidized-LDL (oxLDL; kindly supplied by Prof. Hiroyuki Itabe, Showa University) (1: 400 dilution). In addition, we classified the lesions into early and advanced stages based on the classification of the American Heart Association []. Early-stage lesions include fatty streaks (type II: mainly composed of foam cells) and fibrotic lesions (type III: mainly composed of smooth muscle cells, SMCs, and extracellular matrix). Advanced lesions (types IV or V) are characterized by fibrous plaques with lipid cores and calcification. The length of different-typed lesions on each specimen was measured.
Analysis of Coronary Atherosclerotic Lesions
For coronary lesion analysis, hearts were dissected into 5 blocks as described previously []. Blocks I and II, which contained the main trunks of the left and right coronary arteries, were used to quantify coronary stenosis. Block III, VI, and V were used to analyze small coronary arteries. Stenosis (%) was defined as atherosclerotic lesion area/lumen area. Block V was used to analyze heart hypertrophy and myocardial fibrosis. The thickness of the interventricular septum and the left ventricular wall was measured and averaged. Myocardial fibrosis was evaluated by Masson’s trichrome staining.
Statistical Analysis
All values are expressed as means ± SEM. Data were examined by Shapiro-Wilk test to verify their distribution. Unpaired-sample t test was used to analyze parametric distributed data, and Mann-Whitney U test was used to analyze nonparametric distributed data. In all cases, a p value of less than 0.05 was considered statistically significant.
Results
Effects of Hypertension on Plasma Lipids
For the first 4 weeks, HTN rabbits gained less body weight than NTN rabbits possibly due to the reduced food consumption after surgery. Thereafter, they showed similar food consumption, and body weight gain was comparable to that in NTN rabbits (online suppl. Fig. S-1, see http://www.karger.com/doi/10.1159/000/498897 for all online suppl. material). HTN rabbits showed constantly high BP during the experiment: both systolic BP and diastolic BP were significantly increased compared with control rabbits (Fig. 1). Heart rate was faster in HTN than NTN rabbits.
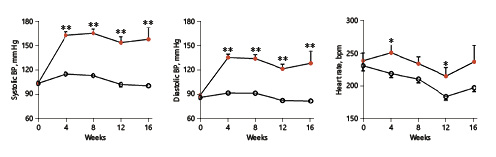
Fig. 1
Blood pressure and heart rate measurement. Systolic/diastolic blood pressure (BP) and heart rate were measured 4, 8, 12 and 16 weeks after nephrectomy. Values are means ± SEM, n = 10 and 7 for the normotensive (NTN; empty circles) and the hypertensive (HTN) group (full circles) of rabbits, respectively. *°p < 0.05, **°p < 0.01 vs. NTN rabbits.
During cholesterol diet feeding, hypertension did not affect plasma lipids: plasma lipid levels, including total cholesterol, triglycerides, and HDL-C levels, were essentially similar in HTN and NTN rabbits (Fig. 2). In addition, cholesterol diet feeding led to no change in apoAI but increased plasma levels of apoB-100 and apoE (Fig. 2) (nonsignificant), suggesting that hypertension did not affect lipid metabolism.
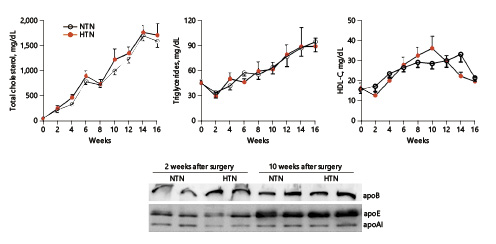
Fig. 2
Plasma lipid levels and apolipoproteins. Total cholesterol, triglycerides, and HDL-C levels in plasma were measured biweekly. Apolipoproteins in plasma, including apoB, apoE, and apoAI, were analyzed by Western blotting. Values are means ± SEM, n = 10 and 7 for the normotensive (NTN; empty circles) and the hypertensive (HTN) group (full circles) in plasma lipid analysis and n = 4 for each group in apolipoprotein analysis.
Influence of Hypertension on Aortic Atherosclerosis
After 16 weeks of cholesterol diet feeding, all rabbits were sacrificed for the analysis of aortic atherosclerosis. Gross lesion area defined by Sudan-IV staining was significantly increased in HTN rabbits: total aortic lesions were increased by 200% (35% increase in the aortic arch, 427% in the thoracic aorta, and 271% in the abdominal aorta) compared to NTN rabbits (Fig. 3a). Microscopically, the lesions of aortic atherosclerosis were composed of macrophage-derived foam cells, SMCs, and extracellular matrix in both groups. Using EVG-stained specimens, we quantified the lesion size and found that intimal lesion size was strikingly increased in HTN rabbits: 2.6-fold↑ in the aortic arch, 10.8-fold↑ in the thoracic aorta, and 10.1-fold↑ in the abdominal aorta compared with NTN rabbits. Increased intimal lesions in HTN rabbits were accompanied by concomitant increases in macrophage and SMC levels: 2.8-fold↑ in macrophages and 2.5-fold↑ in SMCs in the aortic arch, 21.9-fold↑ in macrophages and 9.1-fold↑ in SMCs in the thoracic aorta, and 10.1-fold↑ in macrophages and 8.7-fold↑ in SMCs in the abdominal aorta of HTN compared with NTN rabbits (Fig. 3b). In addition, we quantified the lesion quality (fatty streaks vs. advanced lesions) and found that both early- and advanced-stage lesions were significantly increased in HTN versus NTN rabbits (Fig. 3c). In contrast to NTN rabbits in which 86.7% of the lesions were those of fatty streaks, HTN rabbits had more advanced lesions with calcification (45.7% in the HTN group vs. 13.3% in the NTN group) (Fig. 3c). Calcification was often observed in fatty streaks in HTN rabbits (Fig. 3c). Furthermore, oxLDL contents in the lesions were also significantly increased in HTN rabbits; oxLDL was mainly colocalized with macrophages and occasionally with endothelial cells (Fig. 4).
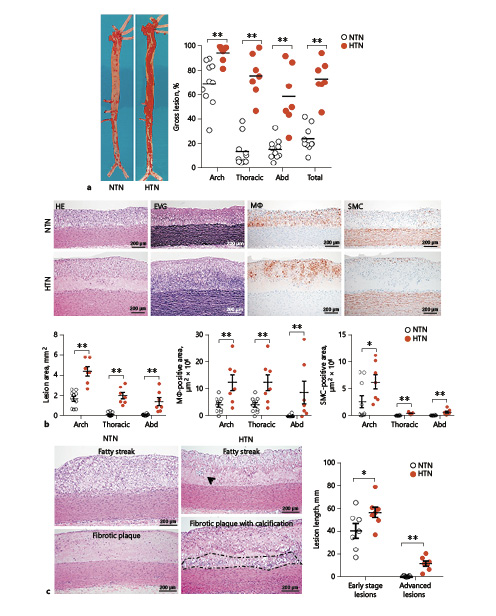
Fig. 3
Pathological analysis of aortic atherosclerotic lesions. a Representative pictures of aortas stained with Sudan IV are shown on the left. Gross lesion areas were quantified using an image analysis system. Each dot represents the data of an individual rabbit. Abd, abdominal. b Representative micrographs of the aortic arch are shown in the top panels. Serial paraffin sections were stained with hematoxylin and eosin (HE), elastica van Gieson (EVG), and immunohistochemically with monoclonal antibodies against either macrophages (Mϕ) or smooth muscles cells (SMC). Bottom panels: intimal lesion areas in each segment of the aorta along with positively stained areas for Mϕ or SMC were quantified using an image analysis system. c Representative lesions of fatty streaks (type II lesion), fibrotic plaques, and advanced lesions (fibrous plaques with calcification) are shown on the left. The results of lesion length measurements are depicted on the right. Arrows indicate calcification foci, and areas surrounded by dotted lines show severe calcification. Values are means ± SEM, n = 10 and 7 for the normotensive (NTN) and the hypertensive (HTN) group of rabbits, respectively. *°p < 0.05, **°p < 0.01 vs. NTN rabbits.
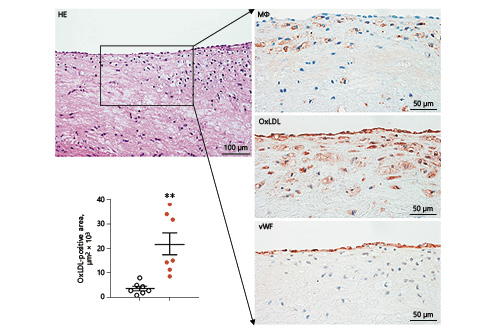
Fig. 4
Demonstration of oxidized (Ox)LDL in lesions of hypertensive rabbits. Representative images of serial paraffin sections of the aortic arch from hypertensive rabbits were stained with hematoxylin and eosin (HE), and immunohistochemically with monoclonal antibodies against either macrophages (Mϕ), OxLDL, or von Willebrand factor (vWF). Values are means ± SEM, n = 7 for both the normotensive (empty circles) and the hypertensive group (full circles) of rabbits, respectively. ** p < 0.01 vs. normotensive rabbits.
Influence of Hypertension on the Heart and Coronary Artery
Pathological analysis revealed that hypertension showed a pronounced effect on rabbit hearts. Grossly, hearts of HTN rabbits were larger in size, and average heart weight was increased 1.7-fold compared with NTN rabbits (Fig. 5). On the cutting surface, interventricular septum and left ventricle thickness were 2.0-fold and 1.6-fold greater in HTN than NTN rabbits. Microscopic observations using Masson trichrome-stained specimens showed that focal myocardial fibrosis of the left ventricle was frequently seen in HTN rabbits but not in NTN rabbits. Capillary muscles also showed focal myocardial fibrosis (6/7 in HTN rabbits vs. 3/7 in NTN rabbits) (online suppl. Fig. S-3). Taken together, hypertension induces myocardial hypertrophy.
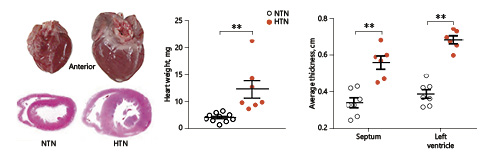
Fig. 5
Pathological analysis of the left ventricle in hypertensive (HTN) and normotensive (NTN) rabbits. Rabbit hearts were harvested, weighed, and photographed. Left: anterior view of the whole heart (left ventricle on the right side). Middle: fresh hearts were weighed, and each dot represents the weight of an individual heart. Block V of the heart sections was stained with hematoxylin and eosin and used to measure the thickness of the interventricular septum and the left ventricle. Values are means ± SEM; n = 10 and 7 for the NTN and the HTN group, respectively. **°p < 0.01 vs. NTN rabbits.
Next, we examined whether hypertension affected coronary atherosclerosis. The stenosis rate of the left coronary artery was 1.5-fold higher in HTN rabbits, whereas macrophage contents in lesions were 2.9-fold higher than in NTN rabbits, although the SMC content was not changed (Fig. 6). A similar trend was also observed in the right coronary artery (online suppl. Fig. S-2). Except for the main trunk of coronary arteries, hypertension enhanced the lesion development of small arteries and arterioles. In blocks III–V, arterial diameters were < 200 µm, and their stenosis rate was significantly increased (Fig. 7a). Histological analysis showed that many small arteries in HTN rabbits exhibited hyaline arteriolosclerosis with foam cell formation (Fig. 7b).
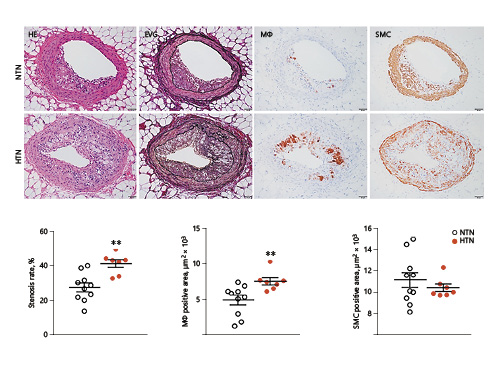
Fig. 6
Microscopic analysis of left coronary atherosclerotic lesions in hypertensive (HTN) and normotensive (NTN) rabbits after a cholesterol diet for 16 weeks. Top: serial paraffin sections of the left coronary artery were stained with hematoxylin and eosin (HE), elastica van Gieson (EVG), and immunohistochemically with monoclonal antibodies against macrophages (Mϕ) or smooth muscle cells (SMC). Stenosis rate (lesion area/total lumen area) and areas with positive staining for Mϕ and SMC were quantified with an image analysis system. Values are means ± SEM; n = 10 and 7 for the NTN and the HTN group, respectively. **°p < 0.01 vs. NTN rabbits.
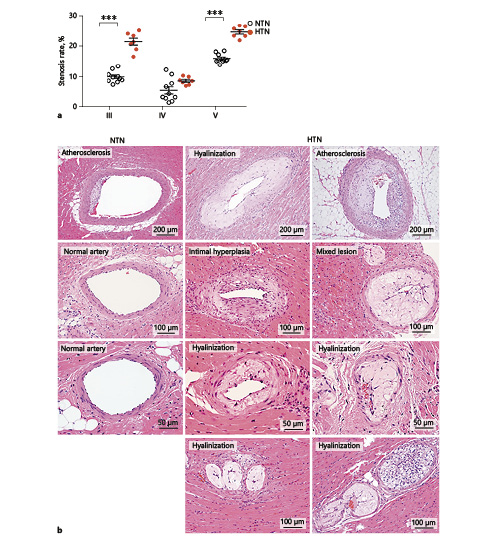
Fig. 7
Stenosis and histological features of hyaline arteriosclerosis. Blocks III, IV, and V were stained with hematoxylin and eosin and elastica van Gieson, and stenosis rate of coronary arteries was calculated (a). Representative micrographs of coronary arteriolosclerosis in normotensive (NTN) and hypertensive (HTN) rabbits (b). Small arteries and arterioles in HTN rabbits showed various changes, including intimal hyperplasia, hyalinization, fatty streaks, and extensive stenosis, and mixed lesions which contained both fatty streaks and hyalinization. Values are means ± SEM; n = 10 and 7 for the NTN and the HTN group, respectively. *** p < 0.001 vs. NTN rabbits.
Discussion
Although it has been known for a decade that elevated BP promotes the development of atherosclerosis in experimental animals [-], it has not been clarified whether hypertension would affect lipid metabolism and the development of coronary atherosclerosis. Taking advantage of the rabbit model, we examined these issues using HTN rabbits. Compared with NTN rabbits, we found that hypertension did not affect lipid metabolism, suggesting that this renovascular hypertension model differs from the unilateral renal vascular constriction model, in which the renin-angiotensin-aldosterone system was activated, and this activation in turn changed cholesterol metabolism and eventually reduced total cholesterol levels in plasma [, ]. AngII infusion via osmotic minipumps resulted in high BP but reduced plasma total cholesterol levels in mice []. In the current HTN rabbit model, hypertension was mainly induced by sympathetic nervous system activity [, ], which did not mediate lipid metabolism thus making it possible to study the exclusive effect of mechanical strength on atherosclerotic development.
In the current study, we found that hypertension remarkably enhanced atherosclerotic development in the aorta of HTN rabbits, suggesting that mechanical stress caused by hypertension is a strong enhancer for aortic lesion development. It has been shown that hypertension can induce aortic aneurysm formation []. Meanwhile, our data showed that hypertension increases the oxLDL deposition in the lesions, and oxLDL was not only colocated with macrophages but also with endothelial cells. oxLDL is an inducer of reactive oxygen species, which exhibited a number of biological functions in the arterial wall such as endothelial dysfunction and monocyte infiltration []. It is currently unknown how hypertension enhances oxLDL deposition in the lesions, but it is possible that increased mechanical stress enhances endothelial permeability of blood LDLs into the intima, which in turns results in more monocyte infiltration and eventually lesion enhancement [].
Our previous work using aged WHHL rabbits, a mutant rabbit with genetically deficient LDL receptor functions, showed that hypertension led to significant enhancement in the progression of atherosclerosis []. Several mechanisms may be operative in the development of atherosclerosis increased by hypertension. First, elevated BP may directly induce endothelial dysfunction or activation []. These activated endothelial cells expressed high levels of intercellular adhesion molecule-1 (ICAM-1) and endothelial-leukocyte adhesion molecule-1 (E-selection) which facilitates monocyte migration into the intima []. Our current study also showed that macrophage contents in the lesions were significantly increased in HTN rabbits. Second, hypertension increases atherogenic lipoproteins such as LDL influx into the intima either dependent on or independent of endothelial injury []. As shown in the current study, there was increased oxLDL in the lesions of HTN rabbits. Third, mechanical stress can stimulate SMC proliferation which also aggravates atherosclerosis []. It has been reported that hypertension would change the phenotypes of SMCs from the contractile type in the media into the synthetic type in the intima, which secrete extracellular matrix []. Therefore, hypertension enhanced lesion expansion through concerted mechanisms. It is worth noting that in the HTN group, the advanced lesions contained calcified foci but lacked typical necrotic/lipid cores or plaque rupture. These features are different from AngII-infused WHHL rabbits, in which coronary erosion/rupture can be induced []. On the other hand, HTN WHHL rabbits exhibited a higher frequency of intraplaque hemorrhages in the aorta []. Taking these observations together, hypertension enhances lesion development in cholesterol-fed rabbits and destabilizes the atherosclerotic plaques in WHHL rabbits which have well-developed lesions.
It is well known that chronic hypertension causes compensatory hypertrophy of the heart which constitutes a major risk factor of heart failure [] and often results in myocardial damage and fibrosis []. Consistent with these observations, our study also revealed that HTN rabbits showed myocardial hypertrophy accompanied by myocardial damage or fibrosis in the left ventricle and papillary muscle, which was rarely observed in NTN rabbits. In addition to these changes, we demonstrated that hypertension significantly increases the development of atherosclerosis in coronary arteries. Therefore, our study has provided experimental evidence that elevated BP is a risk factor for coronary heart disease []. It should be pointed out that hypertension not only enhances stenosis of the left/right epicardial coronary arteries but also affects hyaline arteriosclerosis in small arteries. In these small arteries, the lesions are often complicated by foam cell formation.
In conclusion, our current study demonstrated that hypertension induced by nephrectomy combined with renal artery constriction showed no effects on plasma lipid metabolism. Hypertension led to the enhancement of atherosclerosis in both aorta and coronary arteries. These results suggest that effective control of high BP would be beneficial for hypertensive patients to prevent atherosclerotic complications.
Acknowledgments
We thank Zhengchao Liu, Bo Ning, and Dedong Kang for their help with rabbit surgery and experiments.
Statement of Ethics
Animal experiments were performed following approval from the Animal Care Committee of the University of Yamanashi and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Disclosure Statement
The authors have no conflicts of interest to declare.
Author Contributions
J.F. designed the study and participated in the analysis and interpretation of the results; Y.C., A.B.W., H.Y., Y.W., and J.L. performed experiments and analyzed the data. J.F., Y.C., and J.L. drafted the manuscript.
Funding Sources
This work was supported in part by a research grant from the National Natural Science Foundation of China (Nos. 81770457 and 81570392), JSPS-CAS under the Japan-China Research Cooperative Program, a JSPS KAKENHI grant (15H04718), the National Key Research and Development Program of China (No. 2016YFE0126000), and the Natural Science Foundation of Shaanxi Province (2017JZ028).
References
- 1. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–25.
- 2. Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37(4):1053–9.
- 3. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et alINTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52.
- 4. Aha A. National Heart L, Blood I, Smith SC, Jr., Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, Hiratzka L, Jones D, Krumholz HM, Mosca L, Pearson T, Pfeffer MA, Taubert KA: AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2006;47:2130–9.
- 5. Franco OH, Peeters A, Bonneux L, de Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women: life course analysis. Hypertension. 2005;46(2):280–6.
- 6. Chobanian AV. The influence of hypertension and other hemodynamic factors in atherogenesis. Prog Cardiovasc Dis. 1983;26(3):177–96.
- 7. Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on Experimental Hypertension : I. The Production of Persistent Elevation of Systolic Blood Pressure by Means of Renal Ischemia. J Exp Med. 1934;59(3):347–79.
- 8. Wiesel P, Mazzolai L, Nussberger J, Pedrazzini T. Two-kidney, one clip and one-kidney, one clip hypertension in mice. Hypertension. 1997;29(4):1025–30.
- 9. Leenen FH, de Jong W. A solid silver clip for induction of predictable levels of renal hypertension in the rat. J Appl Physiol. 1971;31(1):142–4.
- 10. Chobanian AV, Lichtenstein AH, Nilakhe V, Haudenschild CC, Drago R, Nickerson C. Influence of hypertension on aortic atherosclerosis in the Watanabe rabbit. Hypertension. 1989;14(2):203–9.
- 11. Lerman LO, Chade AR, Sica V, Napoli C. Animal models of hypertension: an overview. J Lab Clin Med. 2005;146(3):160–73.
- 12. Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105(11):1605–12.
- 13. Fan J, Chen Y, Yan H, Niimi M, Wang Y, Liang J. Principles and Applications of Rabbit Models for Atherosclerosis Research. J Atheroscler Thromb. 2018;25(3):213–20.
- 14. Fan J, Kitajima S, Watanabe T, Xu J, Zhang J, Liu E, et al Rabbit models for the study of human atherosclerosis: from pathophysiological mechanisms to translational medicine. Pharmacol Ther. 2015;146:104–19.
- 15. Chobanian AV, Haudenschild CC, Nickerson C, Drago R. Antiatherogenic effect of captopril in the Watanabe heritable hyperlipidemic rabbit. Hypertension. 1990;15(3):327–31.
- 16. Waqar AB, Koike T, Yu Y, Inoue T, Aoki T, Liu E, et al High-fat diet without excess calories induces metabolic disorders and enhances atherosclerosis in rabbits. Atherosclerosis. 2010;213(1):148–55.
- 17. Li S, Wang YN, Niimi M, Ning B, Chen Y, Kang D, et al Angiotensin II Destabilizes Coronary Plaques in Watanabe Heritable Hyperlipidemic Rabbits. Arterioscler Thromb Vasc Biol. 2016;36(5):810–6.
- 18. Wang C, Nishijima K, Kitajima S, Niimi M, Yan H, Chen Y, et al Increased Hepatic Expression of Endothelial Lipase Inhibits Cholesterol Diet-Induced Hypercholesterolemia and Atherosclerosis in Transgenic Rabbits. Arterioscler Thromb Vasc Biol. 2017;37(7):1282–9.
- 19. Koike T, Liang J, Wang X, Ichikawa T, Shiomi M, Sun H, et al Enhanced aortic atherosclerosis in transgenic Watanabe heritable hyperlipidemic rabbits expressing lipoprotein lipase. Cardiovasc Res. 2005;65(2):524–34.
- 20. Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, et al A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92(5):1355–74.
- 21. Pick R, Johnson PJ, Glick G. Deleterious effects of hypertension on the development of aortic and coronary atherosclerosis in stumptail macaques (Macaca speciosa) on an atherogenic diet. Circ Res. 1974;35(3):472–82.
- 22. Koletsky S, Roland C, Rivera-Velez JM. Rapid acceleration of atherosclerosis in hypertensive rats on high fat diet. Exp Mol Pathol. 1968;9(3):322–38.
- 23. Heptinstall RH, Barkley H, Porter KA. The relative roles of blood cholesterol level and blood pressure level in the production of experimental aortic atheroma in rabbits. Angiology. 1958;9(2):84–7.
- 24. Arruda RM, Peotta VA, Meyrelles SS, Vasquez EC. Evaluation of vascular function in apolipoprotein E knockout mice with angiotensin-dependent renovascular hypertension. Hypertension. 2005;46(4):932–6.
- 25. Nogueira BV, Peotta VA, Meyrelles SS, Vasquez EC. Evaluation of aortic remodeling in apolipoprotein E-deficient mice and renovascular hypertensive mice. Arch Med Res. 2007;38(8):816–21.
- 26. Weiss D, Kools JJ, Taylor WR. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation. 2001;103(3):448–54.
- 27. Taylor CL, Yuan Z, Selman WR, Ratcheson RA, Rimm AA. Cerebral arterial aneurysm formation and rupture in 20,767 elderly patients: hypertension and other risk factors. J Neurosurg. 1995;83(5):812–9.
- 28. Mitra S, Goyal T, Mehta JL. Oxidized LDL, LOX-1 and atherosclerosis. Cardiovasc Drugs Ther. 2011;25(5):419–29.
- 29. Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119(1):136–45.
- 30. Ning B, Chen Y, Waqar AB, Yan H, Shiomi M, Zhang J, et al Hypertension enhances advanced atherosclerosis and induces cardiac death in Watanabe heritable hyperlipidemic rabbits. Am J Pathol. 2018;188(12):2936–47.
- 31. Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, et al Effects of severe hypertension on endothelial and platelet microparticles. Hypertension. 2003;41(2):211–7.
- 32. Franck G, Mawson T, Sausen G, Salinas M, Masson GS, Cole A, et al Flow Perturbation Mediates Neutrophil Recruitment and Potentiates Endothelial Injury via TLR2 in Mice: Implications for Superficial Erosion. Circ Res. 2017;121(1):31–42.
- 33. Chapman MJ, Sposito AC. Hypertension and dyslipidaemia in obesity and insulin resistance: pathophysiology, impact on atherosclerotic disease and pharmacotherapy. Pharmacol Ther. 2008;117(3):354–73.
- 34. Sarzani R, Brecher P, Chobanian AV. Growth factor expression in aorta of normotensive and hypertensive rats. J Clin Invest. 1989;83(4):1404–8.
- 35. Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28(5):812–9.
- 36. Hein S, Arnon E, Kostin S, Schönburg M, Elsässer A, Polyakova V, et al Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107(7):984–91.
- 37. Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target?Circulation. 2004;109(13):1580–9.
- 38. Dawber TR, Moore FE, Mann GV. II. Coronary Heart Disease in the Framingham Study. Int J Epidemiol. 2015;44(6):1767–80.
Yajie Chen and Ahmed Bilal Waqar contributed equally to this study.
