1 INTRODUCTION
Platelets, the smallest anucleate blood cells (2‐4μm), are produced by megakaryocytes and have a short lifespan of 7‐10 days, during which they perform a variety of essential biological functions. It has been reported that approximately 50% of total platelet production occurs in the lungs, where a significant number of megakaryocytes are present. As integral components of the immune system, platelets interact with various immune cells, including neutrophils, natural killer (NK) cells, monocytes, and dendritic cells (DCs),, , , through diverse mechanisms such as membrane molecule binding and factor secretion. These interactions not only influence the body's innate immune response but also play a crucial role in shaping lymphocyte‐mediated adaptive immunity by affecting antigen presentation, cell differentiation, and antibody secretion.
The idea that platelets can promote tumor cells during their metastasis came out has been proposed for a long time. French doctor Armand Trousseau observed excessive blood clotting in patients with occult cancer, and first described Trousseau's syndrome (cancer‐associated thrombosis) in 1865, which is considered as a high‐risk event of venous thromboembolism (VTE) in patients with malignant tumor. One century later, Richard and colleagues compared the blood coagulation and platelet function between patients with cancer or nonmalignant diseases, and then found an abnormal platelet aggregation and higher platelet counts in patients with cancer. And in 1973, Gasic showed that platelets are the pre‐condition of tumor metastasis in mice, and decreasing the platelet response to aggregating agents could release the reaction in metastasis.
In recent decades, more theories of how platelets being activated, aggregating, and adhering have been established. Platelet activation is typically induced by various agonists with differing capacities, such as thrombin, collagen, Thromboxane A2 (TXA2), Adenosine diphosphate (ADP), and even high shear forces in blood., A multitude of diverse molecules on platelets mediate adhesion between cells and between cells and the extracellular matrix (ECM). It has been suggested that the adhesion between CTCs and platelets may aid tumor cells in escaping the immune system., This escape mechanism is not only due to the platelet coating but also involves the transfer of the major histocompatibility complex (MHC) from platelets to CTCs., Additionally, platelets facilitate the adhesion of CTCs to the blood vessel endothelium and may increase the likelihood of epithelial‐to‐mesenchymal transition (EMT)., Furthermore, a number of preclinical research have found that both indirect and direct interactions between platelets and tumor cells can help tumor cells pro‐liferation and metastasis., , Thrombocytosis is more prevalent in many cancer patients, so increased platelet counts may serve as a predictor of cancer in patients with an occult malignancy, and were proved to be connected with tumor progression across various cancer types., ,
The link between platelets and tumor cells is bidirectional. Platelets possess a comprehensive endocytosis mechanism, enabling them to uptake and store tumor cell‐associated proteins, RNA, and other small molecules, thereby altering their transcriptome and proteome. And in turn, the stimulation activates platelets secreting activating soluble factors, promoting tumor metastasis and progression. In this review, we summarize the interaction mechanisms between platelets and immune cells, as well as the critical role of platelets in tumor cell metastasis. We also propose some antitumor therapeutic strategies targeting platelets and novel platelet‐derived drug delivery systems. Additionally, since tumor‐educated platelets (TEPs) have been shown to be associated with tumor initiation and progression, the significant potential of platelets as an auxiliary tool for tumor detection on the “liquid biopsy” platform will also be discussed here.
2 PLATELET PHYSIOLOGY
2.1 Platelet production, maturation and structure
Platelets are the smallest cells (2‐4 µm) in the blood stream, which lack a nucleus but are rich in granules and organelles such as mitochondria and endosomal, falling off from mature megakaryocytes (MKs) and entering the circulation as pro‐platelets. The normal platelet counts in human blood ranges from 150,000 to 450,000 per microliter, and the lifespan of platelets is only 7–10 days. Megakaryocytes are considered as multifunctional hematopoietic cells that not only produce a large number of platelets but also exist in different subtypes, capable of regulating the body's immune functions. MKs are the largest cells in the bone marrow (50–100 µm). To assemble and release platelets, MKs undergo endomitosis, where the number of chromosomes increases, and the volume of the cytoplasm enlarges without cell division, resulting in polyploidy. During this process, most of the cytoplasm of MKs is remodeled and packaged into units called pro‐platelets, and then undergo fission to produce platelets (Figure 1, left side). Numerous pieces of evidence suggest that cytoskeletal reorganization provides the driving force for platelet extension, and cytoplasmic dynein is utilized to drive the extension of pro‐platelets. Although platelets are anucleate cell fragments, resting platelets contain a variety of organelles and granules, including mitochondria, lysosomes, α‐granules, and dense granules. Resting platelets typically contain about 5‐8 mitochondria, and the number of α‐granules is linearly correlated with platelet size. Platelets generate energy through glycolysis and mitochondrial respiration. Interestingly, recent studies have shown that platelet mitochondria undergo fission events during activation, and this fission is dependent on dynamin‐related protein 1 (Drp1). Additionally, MutT Homolog 1 (MTH1) has been recently identified as providing protection against mitochondrial oxidative stress, and platelets lacking MTH1 exhibit reduced aggregation and thrombin‐induced calcium mobilization.
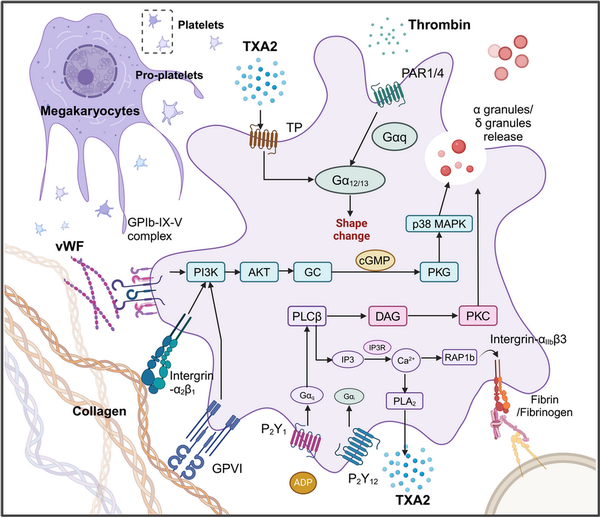
Figure 1
The signaling pathways involved in platelet activation and adhesion. Platelet activation is a complex, well‐regulated process involving multiple signaling pathways. Key pathways include those mediated by glycoprotein receptors, integrins, and soluble agonists like ADP, thrombin, and TXA2. Platelets have glycoprotein receptors on their surface, notably GPIb‐IX‐V and GP IIb/IIIa. GPIb‐IX‐V is crucial for the initial adhesion of platelets to the exposed subendothelial matrix at injury sites, primarily interacting with von Willebrand factor (vWF) anchored to collagen., This interaction triggers signaling pathways that promote platelet activation and the release of granules. Collagen, exposed when blood vessels are injured, interacts with platelet receptors like GPVI and integrin α2β1,, , initiating signaling cascades that lead to platelet activation and intracellular calcium release, enhancing platelet aggregation. Integrin αIIbβ3, when activated, binds fibrinogen, aiding in aggregation and thrombus formation. ADP, released from platelet dense granules and damaged red blood cells, binds to P2Y1 and P2Y12 receptors on platelets, promoting shape change and stabilizing aggregation by reducing cAMP levels and increasing intracellular calcium. Thrombin, generated through the coagulation cascade, activates platelets via PARs, primarily PAR1 and PAR4, leading to increased intracellular calcium and further platelet activation., , , , , TXA2, synthesized from arachidonic acid via the COX pathway, binds to thromboxane receptors (TP), promoting platelet activation, shape changes, and vasoconstriction at the injury site.
2.2 Platelet activation and adhesion
Platelets activation is amplified in different cancers and inflammatory response which describes the processes that platelets change from a smooth and nonviscous state to a viscous, irregular shape. Although there are many types of platelet receptors, including integrins, leucine‐rich repeats receptors, selectins, tyrosine kinase receptors, transmembrane receptors, prostaglandin receptors, lipid receptors, immunoglobulin superfamily receptors, tetraspanins and other platelet receptors (Figure 1). The most important glycoproteins are considered to be GPIb‐IX‐V complex, integrin αIIbβ3 and GPVI, which play important roles in platelet activation, adhesion and aggregation (Figure 1). Early platelet adhesion is coordinated and mediated by the binding of GPIbα to von Willebrand factor (VWF) and integrins to collagen. These glycoprotein receptors bind thrombin, and the signals are transmitted through PARs, a specific family of G protein‐coupled receptors (GPCRs), to activate platelets, , , , , (Figure 1). The extracellular matrix protein collagen which acts as another strong platelet aggregation inducer exposed by injured vessel endothelium is able to interact with the collagen receptor on the platelet surface. Additionally, GPVI plays a major role in platelet‐collagen adhesion while also activating different adhesion‐related receptors, including integrin α2β1, which further enhances this adhesion process., , It has been proved that galectin‐9 (Gal‐9) stimulates aggregation in human and mouse platelets in a dose‐dependent way, which is mediated by GPVI and CLEC‐2. GPVI acts as the primary receptor for collagen, and upon binding to collagen, it triggers downstream signaling pathways, such as the phospholipase Cγ2 (PLCγ2) pathway. This leads to the release of calcium ions and the activation of protein kinase C (PKC), resulting in platelet shape change and the secretion of pro‐coagulant substances, including ADP and TXA2. Subsequently, TXA2 binds to TXA2 receptors, while ADP interacts with P2Y1 and P2Y12 receptors on the platelet surface, thereby further enhancing platelet activation (Figure 1). However, high concentrations of collagen can induce weak platelet aggregation without the participation of TXA2 and ADP. ADP, one of the components in the dense granules released from activated platelets,, acts as a weak agonist connecting to GPCR‐P2Y1 and P2Y12, and there is an intricate signal interaction between P2Y1 and P2Y12 to maintain the delicate balance between the activation and inhibition of platelets, and the adequate platelet aggregation cannot be achieved without their co‐activation, , , (Figure 1). High shear stress in the blood vessels could also lead to the activation of platelets. vWF is a kind of glycoprotein present in platelet α‐granules and subendothelial connective tissue, which plays an essential role in hemostasis., When vascular injury occurs, vWF is exposed and binds to the exposed collagen. This binding provides an anchoring point for platelets, and the bridging function of vWF and its interaction with the GPIb‐IX‐V complex ensure that platelets can effectively adhere and aggregate, particularly under high shear stress conditions, thereby forming a stable thrombus., Binding of fibrinogen to GPIIb/IIIa on the surface of activated platelets enables platelets adhesion to endothelial cells, resulting in the interaction between and intercellular adhesion molecule (ICAM)‐1 receptors of activated endothelial cells.
3 COMMUNICATIONS OF PLATELET WITH IMMUNE CELLS
In addition to their well‐known hemostatic functions, the role of platelets in immune defense cannot be overlooked. Platelets engage in cellular interactions with immune cells through membrane surface ligands, platelet‐derived microparticles (PMPs), lipids, growth factors, and chemokines, thereby serving as mediators of immune responses in the blood. Platelets interact with various immune cells and participate in both innate and adaptive immune responses, to facilitate immune reactions.
3.1 Platelet in innate immune response
Platelets play an indispensable role in the innate immune system, serving as signaling messengers for leukocytes and amplifiers of inflammation (Figure 2). Previous studies have reported that platelet activation promotes various inflammatory conditions, including sepsis, peritonitis, and pancreatitis., ,
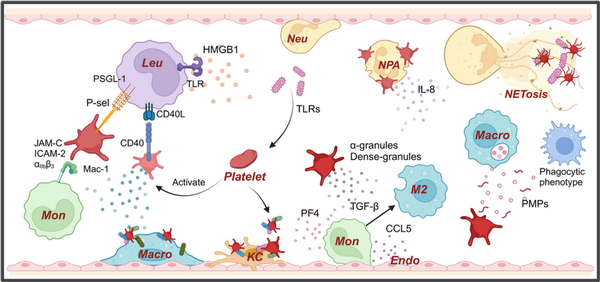
Figure 2
Platelets play a crucial role in innate immune response. Platelets, as the smallest immune cells, also play a significant role in the body's autoimmune responses. The adhesive properties of platelets help them adhere to pathogens and interact with neutrophils through the P‐selectin–PSGL‐1 axis, releasing IL‐8 to recruit neutrophils and promoting the formation of NETs., Simultaneously, platelets can encapsulate bacteria and direct them to Kupffer cells and NETs for pathogen clearance. Additionally, platelets interact with leukocytes through CD40 and P‐selectin, forming platelet‐leukocyte aggregates. Activated platelets can release alpha‐granules and dense granules, in which PF4 promotes endothelial cells to release CCL5, recruiting monocytes. TGF‐β further promotes the polarization of monocytes into M2 macrophages. Moreover, HMGB1 released by activated platelets can bind to TLRs on the surface of leukocytes, promoting inflammation.
Platelets can act as cellular scavengers by using adhesion receptors on their surface to scan for pathogens on the vascular surface, enhancing the activity of phagocytic cells, and exacerbating tissue damage due to inflammation. Additionally, platelets can form transient interactions through their surface GPIb with vWF expressed by Kupffer cells (KCs). This interaction allows KCs to rapidly capture bacteria adhered to platelets and activate the platelets, enabling GPIIb to adhere to KCs, thereby encapsulating the bacteria (Figure 2).
Under certain inflammatory stimuli, platelets can directly interact with leukocytes through the surface expression of P‐selectin or CD40, binding to PSGL‐1 and CD40L on the leukocyte surface, forming platelet‐leukocyte aggregates. Platelets primarily form these aggregates with circulating monocytes or neutrophils, promoting the innate immune response (Figure 2, left side). In humans, neutrophils are the most abundant leukocyte in blood, which take an important part in the innate immune system. And the interaction between platelets and neutrophils are crucial for inflammation and immunity. The interaction between platelets and neutrophils happens through P‐selectin and PSGL‐1. Platelets in the bloodstream can be activated via TLR4, TLR2, and TLR1,, which can induce the formation of neutrophil‐platelet aggregates (NPA), leading to the robust activation of neutrophils and the formation of Neutrophil extracellular traps (NETs) (Figure 2, right side).
Moreover, the stimulation of TLR2 can increase the upregulation of P‐selectin expression thereby further promoting inflammation and the innate immune response. High‐mobility group box 1 (HMGB1), released by activated platelets, enhances inflammation by interacting with TLR on immune cells, contributing to reactive oxygen species (ROS) burst and inflammation. In local inflammatory responses, platelets activate neutrophils by promoting adhesion and secretion of IL‐8, leading to neutrophil recruitment to infection sites and promoting the formation of NETs, aiding in pathogen capture and neutralization.
NETs are web‐like structures released by activated neutrophils consist of DNA fibers, histones and various antimicrobial proteins, playing a crucial role in immobilizing and killing microbes and facilitating their destruction by neutrophils. Platelet adhesion, activation, and aggregation within NETs promote thrombus formation,, which further elucidates the close association between inflammation and thrombosis.
Monocytes, serving as a bridge between innate and adaptive immunity, are demonstrated to form platelet‐monocyte aggregates and regulated by platelets through vari influencing inflammation and pathogen clearance., , , Upon platelet activation, the formation of platelet‐monocyte aggregates is initiated by the interaction between P‐selectin and PSGL‐1. Additionally, GPIbα, αIIbβ3, ICAM‐2, and JAM‐3 on the platelet surface can further bridge with Mac‐1 on monocytes, thereby facilitating the formation of platelet‐leukocyte aggregates, , , , (Figure 2, left side). Furthermore, activated platelets locally release transforming growth factor‐β (TGF‐β), inducing CD16 upregulation in monocytes, promoting their differentiation into M2‐like macrophages and participating in antibody‐dependent cellular phagocytosis (ADCP).,
Macrophages, derived from monocytes and mainly distributed in tissues, play a crucial role in phagocytosis and digestion of cellular debris and pathogens. Platelet factor 4 (PF4) is an abundant chemokine released from activated platelet α‐granules, demonstrated to have numerous effects on myeloid leukocytes, proved to be a ligand for the integrin Mac‐1, can turn monocytes to macrophages with high capacity for unspecific phagocytosis, and mediate oxidative burst. Chatterjee et al. found enhanced aggregation of platelets and platelet‐macrophage aggregates in peritoneal fluid after inducing peritonitis in mice, with platelet‐derived CXCL12 modulating monocyte‐macrophage function through interactions with PF4 and CXCR7.
Additionally, PMPs are the most abundant microparticles in blood, which contain multiple kinds of bioactive molecules such as cytokines, lipids and RNAs, induce transcriptional changes in macrophages, reprogramming their functions towards a phagocytic phenotype (Figure 2, right side).
Atherosclerosis, representing the interaction between arterial lipid deposition and unresolved inflammation, is a chronic vascular inflammatory disease, which is closely linked to the formation of monocyte‐platelet aggregates (MPA). Barrett et al. found that platelets induce monocyte migration and recruitment to atherosclerotic plaques, leading to the formation of MPA within the plaque, promoting SOCS3 protein expression in macrophages, enhancing inflammatory cytokine production, and impairing macrophage function, contributing to plaque growth. Moreover, PF4 plays an important role in accelerating atherosclerosis by recruiting monocytes on endothelial cells in conjunction with CCL5, suppressing CD163 expression in macrophages and upregulating endothelial E‐selectin expression, further promoting atherosclerosis.
Platelets also induce monocyte differentiation into SMCs/smooth muscle‐like cells, further promoting plaque stability. During arterial thrombus formation, eosinophils rapidly recruit and interact with platelets, forming extracellular eosinophil traps (EETs), which further activate platelets, promoting atherosclerotic plaque formation and thrombus generation.
3.2 Platelets in adaptive immune response
Our current understanding of platelet interactions with immune cells has primarily focused on the connection between platelets and innate immune cells. However, platelets also play diverse direct or indirect roles in adaptive immunity. A key aspect of adaptive immunity is the timely presentation of antigens and the rapid recruitment of lymphocytes to sites of infection or tissue injury. In a murine model of allergic inflammation, the interaction between P‐selectin on the platelet surface and PSGL‐1 promotes the formation of platelet‐leukocyte aggregates, which, along with eosinophils, participate in the recruitment of lymphocytes.
Circulating lymphocytes can enter lymph nodes and be rapidly transported to pathogen‐exposed sites due to their ability to roll along High Endothelial Venules (HEVs). Activated platelets can bind to circulating lymphocytes via P‐selectin on their surface, mediating their rolling within HEVs.
T‐cells are broadly classified into CD8+ cytotoxic T cells and CD4+ T helper cells. CD8+ cytotoxic T cells produce effector cytokines and release cytotoxic granules upon antigen stimulation, directly killing target cells. CD4+ T helper cells are further classified based on their secreted cytokines into Th1, Th2, Th17, and T regulatory (Treg) cells. T helper (Th) cells are the primary effector cells in transplant rejection, with the chemokine PF4 capable of limiting Th17 differentiation, thereby reducing the immune response to transplantation. PF4‐deficient and platelet‐deficient mice demonstrate a heightened immune response to cardiac transplantation, characterized by an increased number of Th17 cells and elevated plasma IL‐17 levels. However, when human CD4+ T cells were cocultured with autologous platelets, platelets enhanced the secretion of IL‐10 and other characteristic cytokines by Th1 (secreting IFN‐γ) and Th17 (IL‐17) cells, as well as the differentiation of Th1, Th17, and Treg cells (Figure 3i).
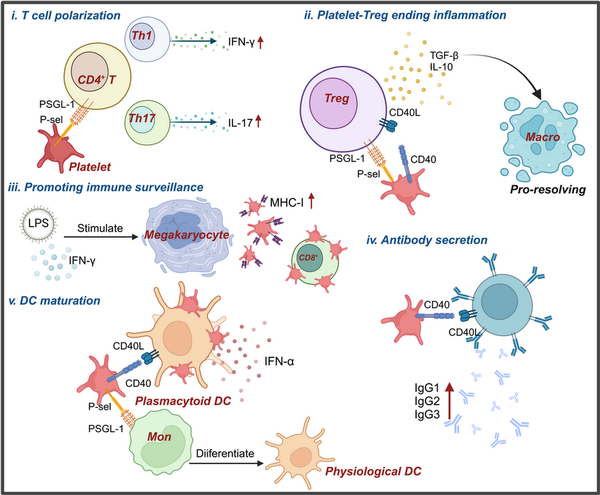
Figure 3
Platelets modulate adaptive immune response via multifaceted regulatory ways. Platelets can influence adaptive immunity through various mechanisms. (i) Platelets interact with CD4+ T cells through the ligand–receptor pair PSGL‐1 and P‐selectin, influencing their differentiation into Th1 and Th17 T cells. (ii) After interacting with Treg cells, platelets promote the secretion of TGF‐β and IL‐10, leading to the polarization of phagocytes into a pro‐resolving phenotype, thereby terminating the inflammatory response. (iii) The expression of MHC‐I on platelets generated from megakaryocytes under LPS and IFN‐γ stimulation is significantly increased, enhancing immune surveillance. (iv) Platelets can also affect antibody secretion by interacting with B cells through CD40‐CD40L. (v) Platelets interact with monocytes, leading to their transformation into physiological DCs, and further promote DC maturation through interactions with DCs.,
These experimental findings suggest that platelets and PF4 play crucial roles in the development and differentiation of CD4+ T cells. CD4+ Tregs can suppress inflammation following severe trauma, and the disruption of PAR4 on platelets led to increased activation of CD4+ Tregs post‐trauma in a mouse model with third‐degree burn injury of 25% of the total body area. In a mouse pneumonia model, platelet‐Treg aggregates were found to be recruited to the lungs during the resolution of inflammation, with the formation of these aggregates mediated by sCD40L and PSGL‐1 expressed on Tregs. Platelets induced Tregs to produce IL‐10 and TGF‐β, further polarizing alveolar macrophages towards a pro‐resolving phenotype, thus contributing to the termination of inflammation (Figure 3ii). During sepsis, both human and mouse platelets, as well as platelets generated in vitro from human megakaryocytes stimulated with LPS and IFN‐γ, showed significantly increased MHC‐I expression, which enhanced antigen cross‐presentation and binding to antigen‐specific CD8+ T cells, regulating CD8+ T cell function and numbers (Figure 3iii).
Moreover, platelets are essential for immune cell development, as demonstrated by severe proliferation defects in lymphatic endothelial cells of CLEC‐2‐deficient mice, resulting in the absence of lymph nodes at birth. In systemic lupus erythematosus (SLE) patients, activated platelets contribute to autoimmunity by interacting with plasmacytoid dendritic cells (pDCs) via the CD40‐CD40L pathway, forming immune aggregates and enhancing IFN‐α secretion. Platelets also activate the cross‐presentation program in peripheral blood monocytes through P‐selectin and PSGL‐1, rapidly driving monocytes to differentiate into phDCs, indicating that platelets can mediate cytokine‐independent DC maturation (Figure 3v).
In vitro coculture experiments show that platelets and B lymphocytes engage in mutual activation via CD40‐CD40L, with increased expression of CD62p on platelets and CD86 on B cells, alongside elevated secretion of IgG1, IgG2, and IgG3 by B cells (Figure 3iv), suggesting that platelets may have additional, yet undiscovered, roles in adaptive immunity.
Moreover, recent findings have shown that in patients with metastatic colorectal cancer, Erbin expression is significantly elevated in platelets. Knockdown of Erbin in platelets or megakaryocytes can enhance B cell‐mediated antitumor immune responses via the Erbin–mitochondria axis, thereby inhibiting pulmonary metastasis in mice. This suggests that there may be additional, yet undiscovered roles for platelets in adaptive immunity.
4 ROLE OF PLATELETS IN TUMOR PROGRESSION
4.1 Tumor‐induced platelet activation
Tumor cells can secrete soluble mediators such as ADP and TXA2 to promote tumor cell‐induced platelet aggregation (TCIPA). TXA2 is generally considered as a potent agonist for platelet activation and a regulator of platelet aggregation, exerting its effects by binding to the thromboxane prostanoid receptor (TP). ADP from ‐granules released by activated platelets can further stabilize platelet aggregation, while ADP produced by tumor cells can also promote platelets activation and the formation of TCIPA. Haemmerle et al. demonstrated that the hypoxia‐induced ADP production and platelets infiltration of tumor would promote tumor growth after discontinuation of antiangiogenic drugs in a mouse model of ovarian cancer, while focal adhesion kinase (FAK) in platelets could promote the migration of platelets into the tumor microenvironment (TME). More studies also demonstrated that the manner in which platelet activation triggered by colon cancer cells depends on tissue factor (TF) expression, thrombin generation, activation of the protease‐activated receptor 4 (PAR4) on platelets and consequent release of ADP and TXA2. The expressions of TF, thrombin and PAR1 is upregulated in breast fibroblasts before the infiltration of breast cancer cells and thrombin accelerated tumor progression by enhancing the adhesion of tumor cells and increasing their metastatic potential. As a crucial promoter of coagulation response, TF is commonly overexpressed in tumor cells. TF, considered as a transmembrane protein, which is primarily expressed in subendothelial cells, triggers the coagulation cascade by forming a complex with its endogenous ligand coagulation factor VII (FVII), mediating the conversion from prothrombin to thrombin. Multiple tumor cells express TF and spontaneously releasing TF+ microparticles (MPs) into bloodstream, which may explain the increased probability of VTE in patients with cancer. There are other mechanisms by which tumor cells mediate platelet activation and aggregation. For instance, P‐selectin known to promote TCIPA, which sends signals through its receptor, P‐selectin glycoprotein ligand 1 (PSGL‐1), on leukocytes, inducing the production of high procoagulant TF+MPs, and it is also associated with fibrin formation., Soluble P‐selectin has been clinically proven to be strongly associated with VTE and can serve as an evaluation factor for cancer‐associated thrombosis (Figure 4). In vivo studies have found that P‐selectin can promote platelet deposition within solid tumors and enhance tumor growth by activating platelet integrins.
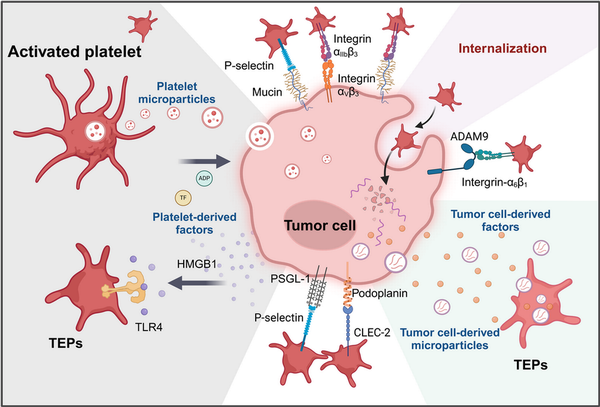
Figure 4
The interaction between platelets and tumor cells can activate platelets and promote tumor progression. The interactions between platelets and tumor cells can occur both directly and indirectly. First, tumor cells can activate platelets by interacting with various platelet surface receptors, such as CLEC‐2, P‐selectin (below), and integrin α6β1 (right),, , thereby enhancing platelet adhesion. Additionally, tumor cells can release a range of platelet agonists, including ADP, various factors, and tumor‐derived microparticles, which promote the formation of tumor‐educated platelets (TEPs) (right). Furthermore, activated platelets release a variety of platelet‐associated granule contents and platelet‐derived microparticles, which can be absorbed by tumor cells through multiple pathways,, , , endowing tumor cells with certain platelet‐like biological characteristics and influencing tumor proliferation (left).
C‐type lectin‐like receptor 2 (CLEC‐2) plays a significant role in platelet activation and can be activated by the transmembrane protein podoplanin (PDPN) on the surface of tumor cells, which is upregulated in various tumor types, (Figure 4). This interaction signals through ITAM can activate downstream signaling molecules protein kinase C and integrin‐αIIbβ 3 as well as the subsequent granule release. In vivo experiments in mice have shown that inhibiting this interaction can effectively suppress platelet aggregation and tumor metastasis., , ,
Furthermore, matrix metalloproteinases (MMPs) on tumor cells are proved to be involved in the formation of TCIPA, and tumor cell ‐released HMGB1 was found to mediate platelet‐tumor interaction by interacting with TLR4 on platelets, promoting tumor progression and metastasis (Figure 4).
Platelets are capable of efficiently absorbing tumor‐derived extracellular vesicles (Figure 4). In vitro studies have demonstrated that, following the uptake of tumor cell‐derived exosomes, platelets undergo robust activation via a CD63‐RPTPα‐dependent pathway. Moreover, through this mechanism, platelets can acquire tumor‐associated RNA, functional proteins, and even experience alterations in their transcriptional profile.
4.2 Platelet‐tumor cell adhesion
For patients with malignancy, cancer‐associated thrombosis (CAT) is the leading cause of death besides cancer progression itself. The connection between cancer and thrombosis was proposed more than 100 years ago by Dr. Armand Trousseau, who highlighted the link between cancer and the formation of platelet‐rich microthrombi in the vascular system, hence the disease is also called Trousseau syndrome. Since then, a lot of studies have confirmed the increased probability of VTE in patients with cancer, and it is considered that the development of CAT is associated with bad prognosis. According to current studies, it was demonstrated that the amounts of leukocytes, platelets, and TF and microvesicles (MVs) are factors which increase CAT in patients with cancer, and soluble P‐selectin can also be recognized as a biomarker of CAT., Moreover, platelets activated by surgical stress may enhance the formation of platelet‐tumor cell aggregates. The primary reason for the elevated risk of CAT is that tumor cells can activate platelets through various mechanisms, forming TCIPA, a phenomenon observed in multiple cancers, including lung, colorectal, breast, and pancreatic cancers., , , Platelets protect CTCs by forming clots, which create a physical barrier that shields CTCs from the high shear stress of blood flow and immune cell attack, thereby promoting tumor metastasis to some extent (Figure 2). Numerous studies have demonstrated that some substances released by tumor cells can activate platelets and thus trigger the formation of TCIPA.
Platelets adhere to tumor cells through multiple pathways, a process mediated by several platelet activation‐related receptors, including GPVI, integrins, CLEC‐2, and P‐selectin. GPVI is a platelet‐specific receptor that interacts with collagen and fibrin, triggering platelet activation via the ITAM signaling pathway, which promotes platelet adhesion and aggregation. In vivo studies have demonstrated that platelet GPVI interacts with Galectin‐3 expressed on tumor cells, facilitating the extravasation of colorectal and breast cancer cells. Knockout of GPVI expression significantly reduces platelet accumulation within metastatic tumors. Additionally, CLEC‐2 on the platelet surface can bind to transmembrane protein PDPN expressed on various tumor cells, promoting tumor metastasis. Furthermore, in vivo research has shown that integrin α6β1 significantly enhances lung metastasis by binding to A disintegrin and A metalloprotease (ADAM) 9 on tumor cells, while targeting α6β1 effectively inhibits tumor metastasis, and ADAM9 has been found in various solid tumors (Figure 4). P‐selectin on the platelet surface plays a critical role in platelet‐tumor adhesion and aggregation. Platelet P‐selectin can interact with tumor cells in a manner independent of PSGL‐1 and CD24, and integrin αIIbβ3 on the platelet surface can stabilize this interaction into a firm adhesion, thereby promoting the formation of TCIPA (Figure 4). Moreover, integrin αVβ3 on tumor cells cooperates with platelets promoting tumor cells metastasis and extravasation, which can be mediated by GPIIb/IIIa on platelets (Figure 4). Mucins, which are heavily glycosylated proteins, are associated with poor prognosis in tumor cells,, , and their binding to platelet P‐selectin may mediate the production of TCIPA (Figure 4). Injection of cancer‐associated mucins into P‐selectin‐deficient mice results in a significant reduction in microthrombus formation.
4.3 Platelet‐mediated CTC immune evasion
Distal metastasis first requires tumor cells to shed from primary tumor site and enter the vascular system as CTCs, where the tumor cells can arrest on the vessel wall and then invade into the distant tissues, thus CTCs are also described as the seeds of tumor metastasis. Only a very small number of CTCs could survive under the high shear forces of blood stream and the killing of immune system, indicating that the interplay between platelets and lymphocytes can significantly influence tumor progression. Platelet‐derived selectins facilitate interactions between tumor cells and platelets/leukocytes, activating endothelial cells and inducing the production of chemokine CCL5, contributing to the formation of a pro‐metastatic microenvironment. Furthermore, platelet‐induced formation of TEPs promotes M2 polarization of tumor‐associated macrophages (TAMs), reshaping the tumor microenvironment and facilitating pulmonary metastasis.
Upon entering the bloodstream and turning into CTCs, tumor cells, now stripped of the protective TME, are exposed to the immune system as well as the threat of high shear forces in the blood stream, making them susceptible to cytolytic lymphocytes. However, it was found in vivo that the coculture of platelets with lymphocytes decreased the production of IFN‐γ, TNF‐α, T lymphocytes proliferation and the expression of CD25, PD‐L1, which reduced inflammatory responses.
NK cells are vital cytotoxic immune cells of the innate immune system, play a crucial role in eliminating tumor cells and mitigating tumor metastasis. Nieswandt et al. first demonstrated that platelets protect CTCs from NK cell lysis in mice. Initially, it was posited that platelets provide physical protection to CTCs from NK cells by forming a dense coat around the tumor cells. However, more mechanisms of platelets have been found to immediate the immune evasion of tumor cells. Placke et al. showed that platelets coating on the surface of tumor cells at the ultrastructural level may cause transfer of platelet‐derived MHC‐I onto the tumor cells surface, thereby disrupting NK cell recognition of tumors and impeding IFN‐γ production (Figure 5). The interaction between G proteins (Gi and G13) on the surface of platelets and GPCRs on tumor cells can promote tumor growth and metastasis. In a mouse lung metastasis model, it was observed that mice deficient in fibrin(ogen) and Gq exhibited significantly reduced lung metastasis. This reduction, however, could be reversed by the depletion of NK cells, which demonstrated that the platelet‐fibrin (ogen) axis enhances metastatic potential by impeding the elimination of tumor cells by NK cells. Additionally, they also demonstrated that TF‐mediated metastasis might be facilitated through fibrinogen and platelet‐dependent restriction of micrometastasis clearance by NK cells.
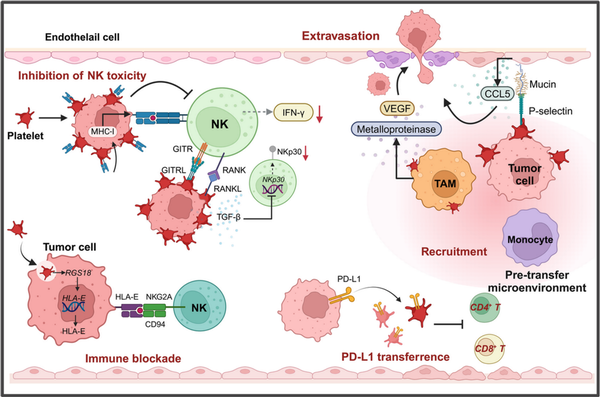
Figure 5
Platelets facilitate tumor immune evasion. Platelets can help circulating tumor cells evade immune surveillance by forming a protective coating around them and transferring MHC‐I to the surface of tumor cells (left). This interferes with the immune recognition of tumor cells by NK cells and reduces the secretion of IFN‐γ (left). Additionally, TEPs secrete TGF‐β, which decreases the expression levels of NKp30 on the surface of NK cells, inhibiting their cytotoxicity, (left). Furthermore, after tumor cells endocytose platelets, the expression of HLA‐E on the surface of tumor cells is upregulated (left). The immune checkpoint molecule interaction between HLA‐E and CD94‐NKG2A blocks NK cell‐mediated killing of tumor cells. In the pro‐metastatic microenvironment formed within blood vessels, platelets interact with TAMs and other cells, promoting TAMs to secrete matrix metalloproteinases and VEGF, facilitating tumor cell extravasation (right). Moreover, PD‐L1 can be transferred to platelets, suppressing the activity of CD4+ and CD8+ T lymphocytes (right).
Besides, Platelets upregulate RANKL expression upon contact with tumor cells, and platelet‐derived RANKL has been found to inhibit the tumor‐killing activity of normal NK cells (Figure 5). Similarly, ligands of immune checkpoint GITR, GITRL has been demonstrated to be upregulated on platelet surface and the formation of tumor cell‐platelet coating confers tumor cells GITRL pseudo‐expression, which inhibits the toxicity of NK cells and IFN‐ secretion, and upregulation of platelet GITRL expression in patients with breast cancer is found to be specifically regulated, having connection with tumor progression, (Figure 5). Interestingly, recent studies also demonstrated that platelet coating will reduce the expression of NKG2D ligands on the surface of tumor cells, resulting in impaired recognition of NK cells and, thus, reduced lysis of tumor cells, which could suggest that platelet‐mediated shedding of NKG2DL may be involved in the immune escape of metastatic tumor cells from NK cells, and, further studies found that shed NKG2D ligands are induced to be secreted into the TME and promote immune evasion., However, our recent research found that platelet‐derived RGS18 could drive upregulation of HLA‐E expression on CTCs, which acquire RGS18 by endocytosing platelets, evading immune surveillance from NK cells through a special immune checkpoint molecule pair, HLA‐E:CD94‐NKG2A, which overturns the previous perception and suggests a novel mechanism by which platelets promote tumor metastasis (Figure 5).
It was suggested that there might be a connection between tumor PD‐L1 expression and platelet count, which help regulate tumor progression, and that PD‐L1 is expressed on platelets of both patients with cancer and healthy individuals. Platelet‐derived PD‐L1 can protect PD‐L1‐negative solid tumors from cytotoxicity of T lymphocytes, thereby modulating the immune system to support tumor growth. Furthermore, when tumor cells interact with platelets, PD‐L1 is transferred from tumor cells to platelets via fibronectin 1, integrin αVβ1 and GPIbα‐dependent ways, which resulting in high PD‐L1 expression in the platelets of non‐small cell lung cancer (NSCLC) patients, which subsequently suppresses the activity of CD4+ and CD8+ T lymphocytes (Figure 5). In addition, platelets can also inactivate antitumor immune responses by upregulated PD‐L1 expression on ovarian cancer cells through contact‐dependent NF‐κB signaling pathway and the contact‐independent TFG‐βR1/Smad signaling pathway. Additionally, platelet‐derived factors such as PF4 can influence T cell differentiation, thereby modulating immune responses., ,
Platelet is a major source of TGF‐β and activation of TGF‐β1 occurs when subjected to agitation or shear stress. The platelet‐specific deletion of Lrrc32, the gene coding glycoprotein A repetitions predominant (GARP), reduces TGF‐β activity at tumor sites, enhancing protective immunity against melanoma and colorectal cancer, revealing that platelets can suppress the immunity of T lymphocytes and promote tumor growth via the GARP–TGF‐β axis. Moreover, it has been demonstrated that thrombin cleaves GARP, resulting in liberation of active TGF‐β1 from the GARP–LTGF‐β1 complex. TGF‐β1 has also been shown to downregulate the surface expression of NKp30, significantly inhibiting NK cell‐mediated killing of DCs, and reduce NK cell cytotoxicity by downregulating the activation receptor NKG2D,, leading to diminished cytotoxicity and IFN‐ production. Moreover, the expression of CD226 and CD96 on NK cells and their associated ligands on tumor cells can also be inhibited by platelet‐derived TGF‐β, thereby affecting the immune function of NK cells.
Platelets can also influence immune responses by affecting the efficiency of antigen presentation, thereby further promoting tumor metastasis. The endoplasmic reticulum‐resident heat shock protein Gp96 mediates the process of antigen presentation and pro‐inflammatory cytokine secretion by DCs. However, when platelets specifically bind to Gp96, they reduce the secretion of pro‐inflammatory cytokines and DC activation, impairing antigen presentation and subsequent T lymphocyte activation.
4.4 Platelet‐mediated tumor cell invasion and transendothelial migration
Platelets facilitate tumor cell migration to nearby vascular regions and promote tumor metastasis by enhancing EMT. In vitro experiments have shown that platelet treatment enhances the adhesion of colorectal adenocarcinoma cells to human umbilical vein endothelial cells (HUVECs). The interaction between platelets and the endothelium, particularly the P‐selectin‐mediated interaction, is considered a key factor in the adhesion of platelet‐tumor cell aggregates to the vascular wall. Additionally, ADAM15 expressed on vascular endothelial cells can bind to GPIIb‐IIIa on the platelet surface, further promoting platelet activation, secretion, and adhesion. Platelet‐derived TGF and direct platelet‐tumor cell connection can activate the TGF‐Smad and NF‐ B pathways in cancer cells, resulting in promoting the progression of EMT. Platelets can infiltrate from blood vessels to adjacent tumor tissues via FAK, and their expression of integrin α6β1 interacts with ADAM9 expressed by tumor cells to significantly promote tumor metastasis. In the colorectal cancer tumor microenvironment, platelets bind to TAMs through P‐selectin‐PSGL‐1, activating the JNK/STAT1 pathway, and facilitating CRC growth and metastasis.
The final step in hematogenous metastasis is distal colonization, in which platelets play an important role. Platelets directly contact circulating tumor cells in the vasculature and form an envelope on the surface of tumor cells, and the activated platelet receptor integrin αIIbβ3 mediates the adhesion of tumor cells to the vasculature. It has been shown that direct interaction between platelet‐derived lysophosphatidic acid (LPA) and the tumor antigen CD97 stimulates platelet activation and leads to granule secretion, ATP release, induces changes in vascular permeability and promotes transendothelial migration. Schumacher et al. found that activation of platelets by tumor cells causes ATP release, which activates endothelial P2Y2 receptors, which induces the opening of the endothelial barrier and disrupts endothelial cell junctions, facilitate tumor cells migration across the endothelium and promoting cancer cells extravasation.
In the local vasculature, platelets aid in recruiting immune cells that contribute to the formation of a pre‐metastatic microenvironment conducive to CTCs metastasis and promote tumor cell extravasation. Platelet‐derived cytokines may recruit monocytes and TAMs, further enhancing endothelial activation and indirectly promoting tumor extravasation. The direct interaction between circulating tumor cells, platelets and leukocytes activates endothelial cells, inducing the production of C‐C chemokine ligand 5 (CCL5), promoting extracellular matrix remodeling and tumor migration, with P‐selectin playing a crucial role in this process. In addition, Labelle et al. found that platelets assist CTCs in rapidly recruiting granulocytes to form an early metastatic microenvironment, and granulocytes secrete CXCL5 and CXCL7 upon platelet‐dependent contact with tumor cells to promote metastatic foci. Monocytes and macrophages further secrete vascular endothelial growth factor (VEGF) and cathepsins that can further help circulating tumor cells to cross the endothelial barrier and form early metastatic foci.
4.5 Platelets and tumor angiogenesis
The ‐granules released upon platelet activation contain high levels of proangiogenic factors such as VEGF, angiopoietin‐1 and anti‐vascular growth factors like endostatin. When platelets are activated, a large number of angiogenesis‐related factors are released into the tumor microenvironment including VEGF, platelet‐derived growth factor (PDGF), fibroblast growth factor (FGF), MMPs, and others (Figure 6). Platelets, as the major physiologic transporters of the most important proangiogenic factor VEGF, release VEGF upon activation and at sites of vascular injury to promote angiogenesis. Platelets are activated in malignant tumors, with ADP‐activated platelets observed to promote angiogenesis in HUVECs in vitro, and breast cancer cells could stimulate platelet VEGF secretion.
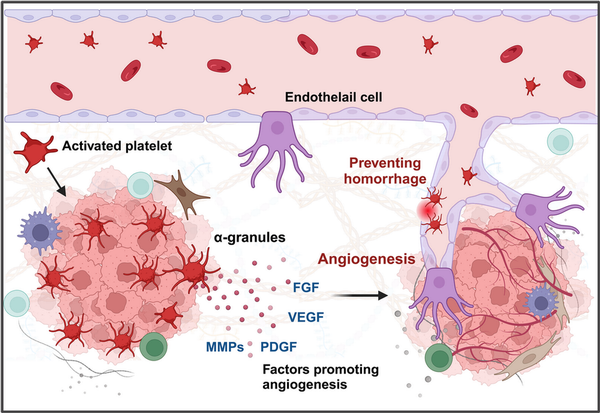
Figure 6
Platelets promote tumor angiogenesis and maintain intratumoral vascular stability. Platelets significantly enhance tumor angiogenesis, the formation of new blood vessels within the tumor, providing the tumor with essential nutrients and oxygen. Platelets are rich in proangiogenic factors, including VEGF, PDGF, and bFGF (left). These factors stimulate the proliferation and migration of endothelial cells, leading to the formation of new blood vessels in the tumor microenvironment. Additionally, platelets have been found to play a crucial role in preventing intratumoral hemorrhage (right). Tumors deficient in platelets exhibit bleeding within a short period, suggesting that platelets are essential for maintaining vascular homeostasis.,
Interestingly, it has been found that platelets in patients with malignant tumors are able to selectively take up and sequester certain angiogenic regulators. Joseph et al. found that proangiogenic and antiangiogenic proteins are segregated in different subpopulations of ‐granules secreted by platelets and macrophages. These different subpopulations of ‐granules may exert proangiogenic and antiangiogenic roles at different sites to exert proangiogenic and inhibitory angiogenic effects at different sites, which may be due to differential packaging of ‐granules. Additionally, Kuznetsov et al. found that certain luminal breast cancer (LBC) cells are able to recruit platelets to take up the cytokines they release and transport them to painless metastatic tumor foci, stimulating angiogenesis and promoting tumor growth.
Moreover, the presence of platelets not only stimulates angiogenesis but also plays a crucial role in preventing hemorrhage within newly formed blood vessels and maintaining vascular integrity, (Figure 6). Platelets help stabilize tumor vasculature. In mouse models of lung cancer and melanoma, inducing severe acute thrombocytopenia led to rapid destabilization of tumor blood vessels and intratumoral hemorrhage within 30 min, indicating that platelets help maintain tumor homeostasis. Inhibition of the GPIV function on the platelet surface further induced rapid intratumoral bleeding and increased the accumulation of chemotherapeutic drugs within the tumor, resulting in significant antitumor effects.
In summary, inhibiting angiogenesis within tumors and destabilizing tumor vasculature represent effective antitumor therapeutic strategies, with platelets serving as the most critical target.
4.6 Platelet‐mediated tumor cell metabolic reprogramming
Multiple studies have now shown that platelets can influence tumor cells by transferring lipids, proteins, and RNA, altering the expression profiles of tumor cells, and even leading to metabolic reprogramming. Platelets are rich in mitochondria, and recent studies have discovered that osteosarcoma cells can acquire platelet mitochondria through the PINK/Parkin‐Mfn2 pathway, reprogramming the cells into a metastatic state. Platelet mitochondria can also regulate the GSH/GSSG balance and ROS levels within tumor cells, thereby promoting tumor metastasis. Thrombin‐mediated hydrolysis is an important determinant of metastatic potential and tumor growth in colorectal cancer and found that PAR1 and fibrinogen or fibrin could promote tumor growth. A recent study found that medium‐sized extracellular vesicles (mEVs) released from thrombin‐activated platelets in patients with colorectal cancer promote prometastatic and prothrombotic phenotypes of tumor cells. Platelets shed extracellular vesicles known as PMPs into the extracellular matrix, which contain a wealth of bioactive substances, including RNA, transcription factors, and proteins., , , When chronic lymphocytic leukemia (CLL) cells are cocultured with PMPs in vitro, it induces metabolic reprogramming in the CLL cells, leading to increased oxygen consumption rate (OCR), ATP levels, and ROS production. Additionally, a newly discovered subpopulation of PMPs, termed mitoMPs, contains functional mitochondria. Tumor cells cocultured with mitoMPs acquire these exogenous mitochondria, exhibiting enhanced migratory and invasive characteristics.
5 ROLE OF PLATELET IN CANCER DIAGNOSE
Platelets are the most abundant cells in the circulation, aside from erythrocytes, and are produced by megakaryocytes, which possess strong endocytic capabilities and can store various proteins. Conversely, they can transfer cellular contents to other cells, facilitating platelet‐cell exchanges.
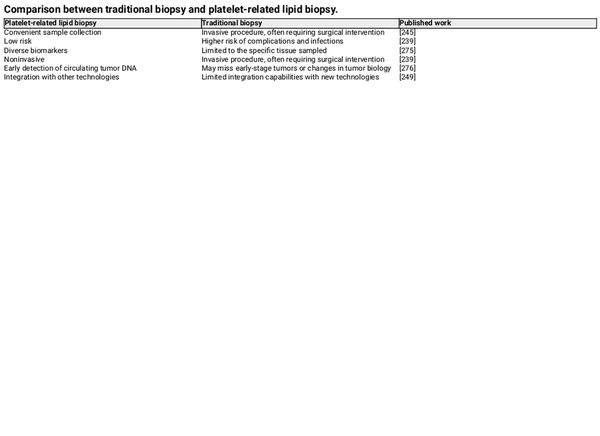
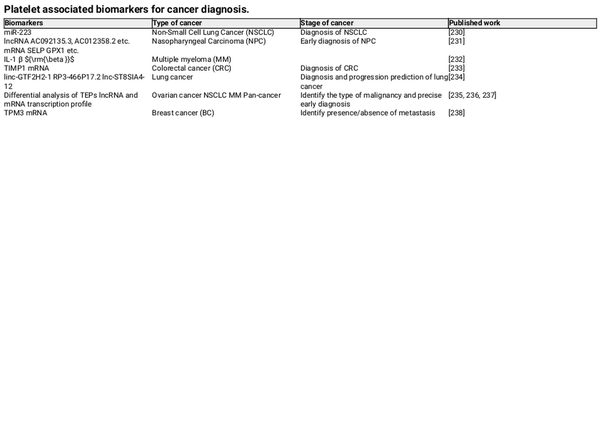
Kirschbaum et al. found that platelets and platelet‐like particles (PLPs) of the megakaryocyte lineage can be internalized by HepG2 cells, promoting their proliferation. Moreover, it was proved that the kinetic uptake of platelets by cancer cells, with the platelet‐specific protein CD42a integrating into the plasma membrane of cancer cells. Additionally, RNA sequencing revealed that after coculturing platelets with melanoma and triple‐negative breast cancer cells, the TGF‐β/Smad pathway was activated in the cancer cells, inducing the expression of SERPINE1 encoding Plasminogen Activator Inhibitor 1 (PAI‐1), thereby enhancing the migration and invasion of tumor cells. These findings suggest that platelets are capable of exchanging their own proteins and substances, such as RNA, to other cells through internalization, altering the gene expression of the recipient cells. In addition, PMPs, released by platelets, which function as bio‐vectors for intercellular communication, can activate receptors on the surface of target cells or transfer their contents to the interior of cells. Therefore, platelets are becoming increasingly important in cancer diagnosis. Compared to traditional biopsies, the noninvasive and low‐risk characteristics of platelet‐related liquid biopsies offer many benefits, and current research has identified various biomarkers, summarized in Tables 1 and 2. For instance, patients with NSCLC have higher levels of miR‐223 in both platelets and PMPs, which are delivered to cancer cells via PMPs to promote tumor invasion. Liquid biopsy has many advantages over surgical tumor biopsy, which is considered the “gold standard” for diagnosis, including being less invasive, less risky, overcoming the challenges of sampling difficult‐to‐obtain tissues, detecting and responding to tumor heterogeneity in real time, obtaining results faster, and facilitating repeat sampling for longitudinal comparisons. Platelets, on the other hand, have many advantages as an important source of liquid biopsies. Current platelet extraction techniques have reached maturity in the clinical setting, and blood samples can be stored at 4°C for up to 8 days before analysis. Platelet extraction is simple and only requires a multistep centrifugation method. However, platelets are prone to activation and RNA degradation. Amisten et al. described a method to rapidly and efficiently isolate human circulating platelets and extract RNA from a small amount of whole blood by filtration, removing residual contaminating leukocytes and erythrocytes with magnetic beads. The abundant RNA in platelets is mainly derived from megakaryocytes, and its transcription is influenced by the bone marrow microenvironment. For example, increased expression of ITGA2B in bone marrow megakaryocytes and circulating platelets occurs within 24 h of the onset of sepsis in patients. Platelets contain functional spliceosomes that are capable of splicing precursor mRNAs in response to signals from activated platelets. Moreover, platelets contain a rich library of RNAs, including pre‐messenger RNAs, spliceosomal components of mRNAs, transfer RNAs for functional translation, ribosomal RNAs, noncoding RNAs, microRNAs, and long‐chain noncoding RNAs. TEPs play an important role in tumor development and metastasis by altering their own RNA profiles. Myron et al. performed mRNA sequencing on 283 platelet samples, differentiating between 228 patients with limited and metastatic tumors with an accuracy of 96%, indicating that platelets provide a convenient and noninvasive way to diagnose pan‐cancer. As an important source of noninvasive testing, platelets may improve clinical decision‐making based on imaging alone. Currently, blood tests based on TEPs can be used not only for hematologic cancers but also for solid tumors to predict early cancer development and suggest tumor progression. Altered expression profiles of RNAs in platelets have been shown to be predictive in hematologic cancers. Examining the expression profile of platelet transcripts can help us understand disease progression. Shen et al. used transcriptome sequencing to analyze platelets and observed that three chronic myeloproliferative neoplasms (MPN) subtypes preferentially express genes related to immunoinflammatory responses, interferon signaling, and cell proliferation, reflecting MPN progression. Anandi et al. performed RNA sequencing of platelets from MPN patients, revealing unique RNA‐based biological markers that could help differentiate patients based on subtypes, mutation status, or treatment modalities, aiding clinical decision‐making. Platelets from multiple myeloma (MM) patients show a highly activated state with disease progression, marked by significant upregulation of IL‐1β. Moreover, platelet RNA sequencing is highly applicable to solid tumors. Myron et al. detected spliced RNA profiles from TEPs for noninvasive detection of patients with early and advanced non‐small cell lung cancer (NSCLC). Xue et al. identified 20 potential RNA biomarkers of TEPs through bioinformatics. Mafalda et al. sequenced and analyzed TEPs from 466 NSCLC patients and 410 asymptomatic individuals, demonstrating that TEP‐derived spliced RNA profiles can serve as biomarkers for minimally invasive clinical blood tests, assisting in the management of lung cancer patients alongside imaging tests. Additionally, lncRNAs in TEPs can be used for lung cancer diagnosis and progression prediction. Platelet RNA can also serve as a biomarker in various other solid tumors, such as breast cancer, colorectal cancer, nasopharyngeal cancer, ovarian cancer, gastric cancer, glioblastoma, and prostate cancer. The research team at the Amsterdam Cancer Center in the Netherlands has demonstrated that RNA profiles derived from TEPs can potentially detect up to 18 different types of cancer with high specificity. Additionally, using the thromboSeq algorithm, the origins of five types of tumors can be determined. This indicates that RNA screening methods based on TEPs have the potential for precise early diagnosis and localization of tumors.
6 CONCLUSION AND FUTURE PERSPECTIVES
This review highlights the important role of platelets in cancer. Platelets play a critical role in tumor growth, development, and metastasis, and are also pivotal in cancer diagnosis, staging, and prognosis analysis. The interaction between platelets and CTCs is a complex process that significantly promotes tumor growth, metastasis, immune evasion, and metastatic colonization. The diagnostic potential of TEPs has been gaining attention. Platelets can capture and absorb RNA, proteins, and other molecules from tumor cells, altering their own characteristics. These changes can be detected and analyzed for diagnostic purposes. The RNA characteristics of TEPs can serve as biomarkers for minimally invasive blood tests, aiding in the highly specific detection of up to 18 different types of cancer, including lung cancer. This makes TEPs a valuable tool for early cancer detection and precise tumor localization, providing a noninvasive and efficient alternative to traditional biopsy methods.
The interaction between platelets and tumor cells presents a new target for preventing or inhibiting tumor metastasis, with promising prospects. For instance, inhibiting platelet activation or the adhesion of platelets to tumor cells can reduce the protection platelets offer to CTCs, making them more susceptible to immune system attacks and shear stress in the bloodstream, thereby reducing metastasis. Additionally, targeting platelet activation can further decrease the release of ‐granules containing VEGF and TGF‐β, which may inhibit tumor angiogenesis and EMT,, , thereby reducing metastasis. TGF‐β can inhibit the activation, proliferation, and effector differentiation of peripheral autoreactive T cells. Moreover, Th17 cell differentiation is dependent on TGF‐β1 [263]. Blocking TGF‐β signaling in B cells leads to enhanced B cell activation and proliferation, and a reduction in IgA production. Likewise, Erbin‐knockout in platelets/MKs have increasing mitochondrial oxidative phosphorylation and secret lipid metabolites like acyl‐carnitine (Acar), which enhance the activity of mitochondrial electron transport chain complex and mitochondrial oxidative phosphorylation in B cells, suppressing lung metastasis in mice. The dense granules released during platelet activation contain a large amount of serotonin (5‐HT). 5‐HT can upregulate PKM2 to promote glycolysis via the 5‐HTR2A/C‐mediated Jak1/STAT3 pathway and enhance mitochondrial biogenesis through the adenylyl cyclase/PKC pathway, thereby promoting tumor cell proliferation. In a subcutaneous tumor mouse model, the injection of the TPH1 inhibitor telotristat or the inhibition of peripheral platelet‐released 5‐HT can suppress the growth of pancreatic and colorectal tumors in mice. Furthermore, blocking CLEC‐2 on platelets and PDPN on tumor cells can inhibit TCIPA formation, effectively reducing metastasis occurrence., In addition to the use of certain antithrombotic drugs, such as aspirin and platelet P2Y12 receptor antagonists, which have been shown in both in vivo and in vitro studies to suppress tumor progression and metastasis, antiplatelet therapy combined with PD1 antibodies has already entered clinical trials. Recent research has provided new strategies for targeting platelets in antitumor immunotherapy. For instance, anti‐PDL1 blocking antibodies (aPDL1) have been conjugated to platelets and encapsulated in hydrogels, leveraging the characteristic of platelet activation during postoperative inflammation to release PMPs that bind to tumors and block PD‐L1, thereby eliminating residual tumor cells after surgery.
Interestingly, some studies have modified platelets with granzyme B and perforin nanocomplexes, essentially “arming” the platelets to mimic T cells. In vivo experiments have shown that, upon systemic administration, platelet‐drug conjugates (PDCs) can automatically capture CTCs and, upon activation, simulate cytotoxic lymphocytes by producing PMPs that target tumor cells. Additionally, platelet decoy materials represent a novel approach in targeting platelets for antitumor therapy. For example, researchers have developed a human‐engineered platelet, known as a platelet decoy, which retains the binding functions of platelets but inhibits their aggregation and adhesion on thrombus surfaces, thereby preventing arterial thrombosis and platelet‐mediated tumor cell aggregation and growth.
As circulating cells, platelets play essential roles in hemostasis, vascular repair, and thrombosis, making them widely utilized in the development of innovative biomimetic drug delivery systems. Leveraging their unique physiological functions, platelet‐derived drug delivery systems (DDSs) show promising prospects in treating cancer, cardiovascular diseases, and infectious diseases. Nanoparticles coated with platelet membranes exhibit reduced phagocytosis by macrophage‐like cells and demonstrate selective adhesion to damaged human vasculature. Additionally, nanomaterials coated with platelet membranes exhibit enhanced biocompatibility and inherent targeting affinity. In vivo intratumoral injection of platelet membrane‐coated nanoparticles delivering the TLR agonist resiquimod (R848) has been shown to enhance local immune activation, leading to tumor regression. However, DDSs are still in the preclinical research stage due to numerous unresolved challenges in industrial production. These challenges include drug‐loading efficiency, stability, the source of platelets, variability among individual platelets, and the difficulty in controlling platelet activation.,
However, the translational process for antitumor therapies targeting platelets remains exceptionally challenging. For instance, there have been reports of the antitumor effects of platelets, attributed to the activation of CD8+ T lymphocytes via P2Y12‐dependent CD40L release, which inhibits tumor cell growth and metastasis. This indicates that platelets can influence the body's biological responses through complex regulatory mechanisms, necessitating a deeper research foundation to support their clinical application. Moreover, the heterogeneity of different cancer types and individual patient variations present significant challenges. For example, in elderly populations, platelet production is increased, and these platelets exhibit heightened reactivity with substantial alterations in differentiation pathways., Therefore, this therapeutic approach requires robust preclinical studies and may necessitate personalized treatment strategies.
Additionally, a critical challenge that remains is how to target platelets and leverage the interaction between platelets and tumors for antitumor therapy without compromising their essential role in coagulation.
In conclusion, platelets are becoming key players in cancer diagnosis, staging, and treatment. Their unique properties and interactions with tumor cells offer valuable opportunities for developing innovative diagnostic tools and therapeutic strategies. Continued research into these molecular interactions will pave the way for more effective and personalized cancer treatments.
AUTHOR CONTRIBUTIONS
Leyi Tang, Hubing Shi and Yong Luo contributed to conception and manuscript design. Leyi Tang wrote the manuscript. Leyi Tang, Hubing Shi and Yong Luo were involved in manuscript revision. The authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
Graphical abstract and Figures 1, 2, 3, 4, 5 and 6 in this review were created using BioRender.com (https://biorender.com). The study received no funding.
REFERENCES
- 1. van der Meijden PEJ, Heemskerk JWM, et al. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16:166–179.
- 2. Gerdes N, Zhu L, Ersoy M, et al. Platelets regulate CD4+ T‐cell differentiation via multiple chemokines in humans. Thromb Haemost. 2011;106(2):353–362.
- 3. Lisman T. Platelet‐neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res. 2018;371(3):567–576.
- 4. Maurer S, Ferrari de Andrade L. NK cell interaction with platelets and myeloid cells in the tumor milieu. Front Immunol. 2020;11(11):608849.
- 5. Langer HF, Daub K, Braun G, et al. Platelets recruit human dendritic cells via Mac‐1/JAM‐C interaction and modulate dendritic cell function in vitro. Arterioscler Thromb Vasc Biol. 2007;27(6):1463–1470.
- 6. Rolling CC, Sowa MA, Wang TT, et al. P2Y12 inhibition suppresses proinflammatory platelet‐monocyte interactions. Thromb Haemost. 2023;123(2):231–244.
- 7. Trousseau A, Bazire PV, Cormack JR. Lectures on Clinical Medicine, Delivered at the Hotel‐Dieu. New Sydenham Society; 1872.
- 8. DAVIS RB. Comparative studies of blood coagulation and platelet aggregation in patients with cancer and nonmalignant diseases. Ann Intern Med. 1969;71(1):67.
- 9. Gasic GJ, Gasic TB, Galanti N, Johnson T, Murphy S. Platelet‐tumor‐cell interactions in mice. The role of platelets in the spread of malignant disease. Int J Cancer. 1973;11(3):704–718.
- 10. Yun SH, Sim EH, Goh RY, Park JI, Han JY. Platelet activation: the mechanisms and potential biomarkers. Biomed Res Int. 2016;2016:1–5.
- 11. Malara A, Balduini A. Blood platelet production and morphology. Thromb Res. 2012;129(3):241–244.
- 12. Scridon A. Platelets and their role in hemostasis and thrombosis‐from physiology to pathophysiology and therapeutic implications. Int J Mol Sci. 2022;23(21):12772.
- 13. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemostasis. 2011;9(2):237–249.
- 14. Anvari S, Osei E, Maftoon N. Interactions of platelets with circulating tumor cells contribute to cancer metastasis. Sci Rep. 2021;11(1):15477.
- 15. Nieswandt B, Hafner M, Echtenacher B, Männel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295–1300.
- 16. Placke T, Örgel M, Schaller M, et al. Platelet‐derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 2012;72(2):440–448.
- 17. Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial‐mesenchymal‐like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–59090.
- 18. Marcolino E, Siddiqui YH, van den Bosch M, Poole AW, Jayaraman PS, Gaston K. Blood platelets stimulate cancer extravasation through TGFβ‐mediated downregulation of PRH/HHEX. Oncogenesis. 2020;9(2):10.
- 19. Cho MS, Bottsford‐Miller J, Vasquez HG, et al. Platelets increase the proliferation of ovarian cancer cells. Blood. 2012;120(24):4869–4872.
- 20. Zhou L, Zhang Z, Tian Y, Li Z, Liu Z, Zhu S. The critical role of platelet in cancer progression and metastasis. Eur J Med Res. 2023;28(1):385.
- 21. Ren J, He J, Zhang H, et al. Platelet tlr4‐erk5 axis facilitates net‐mediated capturing of circulating tumor cells and distant metastasis after surgical stress. Cancer Res. 2021;81(9):2373–2385.
- 22. Levin J. Thrombocytosis associated with malignant disease. Arch Intern Med. 1964;114:497–500.
- 23. Haemmerle M, Stone RL, Menter DG, Afshar‐Kharghan V, Sood AK. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell. 2018;33(6):965–983.
- 24. Bailey SER, Ukoumunne OC, Shephard E, Hamilton W. How useful is thrombocytosis in predicting an underlying cancer in primary care? a systematic review. Fam Pract. 2017;34(1):4–10.
- 25. Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610–618.
- 26. Sasaki K, Kawai K, Tsuno NH, Sunami E, Kitayama J. Impact of preoperative thrombocytosis on the survival of patients with primary colorectal cancer. World J Surg. 2012;36(1):192–200.
- 27. Li H, Jiang W, Zhang SR, et al. The platelet pannexin 1‐IL‐1β axis orchestrates pancreatic ductal adenocarcinoma invasion and metastasis. Oncogene. 2023;42(18):1453–1465.
- 28. Roweth HG, Battinelli EM. Lessons to learn from tumor‐educated platelets. Blood. 2021;137(23):3174–3180.
- 29. Sabrkhany S, Kuijpers MJE, Oude Egbrink MGA, Griffioen AW. Platelets as messengers of early‐stage cancer. Cancer Metastasis Rev. 2021;40(2):563–573.
- 30. Gay LJ, Felding‐Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134.
- 31. Harker LA. Platelet production. N Engl J Med. 1970;282(9):492–494.
- 32. Guo K, Machlus KR, Camacho V. The many faces of the megakaryocytes and their biological implications. Curr Opin Hematol. 2024;31(1):1–5.
- 33. Williamson AE, Liyanage S, Hassanshahi M, et al. Discovery of an embryonically derived bipotent population of endothelial‐macrophage progenitor cells in postnatal aorta. Nat Commun. 2024;15(1):7097.
- 34. Machlus KR, Italiano Jr JE. The incredible journey: from megakaryocyte development to platelet formation. J Cell Biol. 2013;201(6):785–796.
- 35. Randi AM, Laffan MA. Von Willebrand factor and angiogenesis: basic and applied issues. J Thromb Haemostasis. 2017;15(1):13–20.
- 36. Andrews R, Shen Y, Gardiner E, Dong J, López J, Berndt M. The glycoprotein Ib‐IX‐V complex in platelet adhesion and signaling. Thromb Haemost. 1999;82(2):357–364.
- 37. De Candia E, Hall SW, Rutella S, Landolfi R, Andrews RK, De Cristofaro R. Binding of thrombin to glycoprotein Ib accelerates the hydrolysis of Par‐1 on intact platelets. J Biol Chem. 2001;276(7):4692–4698.
- 38. Tsuji M, Ezumi Y, Arai M, Takayama H. A novel association of Fc receptor γ‐chain with glycoprotein VI and their co‐expression as a collagen receptor in human platelets. J Biol Chem. 1997;272(38):23528–23531.
- 39. Nieswandt B. Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen. EMBO J. 2001;20(9):2120–2130.
- 40. Egan K, Crowley D, Smyth P, et al. Platelet adhesion and degranulation induce pro‐survival and pro‐angiogenic signalling in ovarian cancer cells. PLoS ONE. 2011;6(10):e26125.
- 41. Saboor M, Ayub Q, Ilyas S, Moinuddin S. Platelet receptors: an instrumental of platelet physiology. Pak J Med Sci. 2013;29(3):891–896.
- 42. Ofosu FA, Nyarko KA. Human platelet thrombin receptors. Hematol Oncol Clin North Am. 2000;14(5):1185–1198, x.
- 43. Kahn ML, Zheng YW, Huang W, et al. A dual thrombin receptor system for platelet activation. Nature. 1998;394(6694):690–694.
- 44. Soslau G, Class R, Morgan DA, et al. Unique pathway of thrombin‐induced platelet aggregation mediated by glycoprotein Ib. J Biol Chem. 2001;276(24):21173–21183.
- 45. Dörmann D, Clemetson KJ, Kehrel BE. The GPIb thrombin‐binding site is essential for thrombin‐induced platelet procoagulant activity. Blood. 2000;96(7):2469–2478.
- 46. Adam F, Guillin MC, Jandrot‐Perrus M. Glycoprotein Ib‐mediated platelet activation. A signalling pathway triggered by thrombin. Eur J Biochem. 2003;270(14):2959–2970.
- 47. Thon JN, Montalvo A, Patel‐Hett S, et al. Cytoskeletal mechanics of proplatelet maturation and platelet release. J Cell Biol. 2010;191(4):861–874.
- 48. Patel SR, Richardson JL, Schulze H, et al. Differential roles of microtubule assembly and sliding in proplatelet formation by megakaryocytes. Blood. 2005;106(13):4076–4085.
- 49. Yadav S, Storrie B. The cellular basis of platelet secretion: emerging structure/function relationships. Platelets. 2017;28(2):108–118.
- 50. Pokrovskaya ID, Yadav S, Rao A, et al. 3D ultrastructural analysis of α‐granule, dense granule, mitochondria, and canalicular system arrangement in resting human platelets. Res Pract Thromb Haemost. 2020;4(1):72–85.
- 51. Grichine A, Jacob S, Eckly A, et al. The fate of mitochondria during platelet activation. Blood Adv. 2023;7(20):6290–6302.
- 52. Ding Y, Gui X, Chu X, et al. MTH1 protects platelet mitochondria from oxidative damage and regulates platelet function and thrombosis. Nat Commun. 2023;14(1):4829.
- 53. Chung J, Jeong D, Kim G, et al. Super‐resolution imaging of platelet‐activation process and its quantitative analysis. Sci Rep. 2021;11(1):10511.
- 54. Sabrkhany S, Kuijpers MJE, Oude Egbrink MGA, Griffioen AW. Platelets as messengers of early‐stage cancer. Cancer Metastasis Rev. 2021;40(2):563–573.
- 55. Gharib E, Veilleux V, Boudreau LH, Pichaud N, Robichaud GA. Platelet‐derived microparticles provoke chronic lymphocytic leukemia malignancy through metabolic reprogramming. Front Immunol. 2023;14:1207631.
- 56. Nieswandt B, Watson SP. Platelet‐collagen interaction: is GPVI the central receptor? Blood. 2003;102(2):449–461.
- 57. Jung SM, Tsuji K, Moroi M. Glycoprotein (GP) VI dimer as a major collagen‐binding site of native platelets: direct evidence obtained with dimeric GPVI‐specific Fabs. J Thromb Haemostasis. 2009;7(8):1347–1355.
- 58. Cho MJ, Liu J, Pestina TI, et al. The roles of αIIbβ3‐mediated outside‐in signal transduction, thromboxane A2, and adenosine diphosphate in collagen‐induced platelet aggregation. Blood. 2003;101(7):2646–2651.
- 59. Meyers KM, Holmsen H, Seachord CL. Comparative study of platelet dense granule constituents. Am J Physiol Regulat Integrat Compar Physiol. 1982;243(3):R454–R461.
- 60. BORN GVR. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–929.
- 61. Jin J, Kunapuli SP. Coactivation of two different G protein‐coupled receptors is essential for ADP‐induced platelet aggregation. Proc Natl Acad Sci. 1998;95(14):8070–8074.
- 62. Hardy AR, Jones ML, Mundell SJ, Poole AW. Reciprocal cross‐talk between P2Y1 and P2Y12 receptors at the level of calcium signaling in human platelets. Blood. 2004;104(6):1745–1752.
- 63. Hollopeter G, Jantzen HM, Vincent D, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409(6817):202–207.
- 64. Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP‐induced platelet activation. J Biol Chem. 1998;273(4):2030–2034.
- 65. Rahman SM, Hlady V. Downstream platelet adhesion and activation under highly elevated upstream shear forces. Acta Biomater. 2019;91:135–143.
- 66. Gremmel T, Frelinger 3rd A, Michelson A. Platelet physiology. Semin Thromb Hemost. 2016;42(3):191–204.
- 67. Matsui H, Sugimoto M, Mizuno T, et al. Distinct and concerted functions of von Willebrand factor and fibrinogen in mural thrombus growth under high shear flow. Blood. 2002;100(10):3604–3610.
- 68. Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nature Med. 2007;13(4):463–469.
- 69. Blair P, Rex S, Vitseva O, et al. Stimulation of Toll‐like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3‐kinase. Circ Res. 2009;104(3):346–354.
- 70. Wong CHY, Jenne CN, Petri B, Chrobok NL, Kubes P. Nucleation of platelets with blood‐borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nature Immunol. 2013;14(8):785–792.
- 71. Semple JW, Italiano JE, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–274.
- 72. Yu G, Rux AH, Ma P, Bdeir K, Sachais BS. Endothelial expression of E‐selectin is induced by the platelet‐specific chemokine platelet factor 4 through LRP in an NF‐κB–dependent manner. Blood. 2005;105(9):3545–3551.
- 73. Zhou H, Deng M, Liu Y, et al. Platelet HMGB1 is required for efficient bacterial clearance in intra‐abdominal bacterial sepsis in mice. Blood Adv. 2018;2(6):638–648.
- 74. Ngamsri KC, Putri RA, Jans C, et al. CXCR4 and CXCR7 inhibition ameliorates the formation of Platelet‐neutrophil complexes and neutrophil extracellular traps through Adora2b signaling. Int J Mol Sci. 2021;22(24):13576.
- 75. Tokoro T, Makino I, Harada S, et al. Interactions between neutrophils and platelets in the progression of acute pancreatitis. Pancreas. 2020;49(6):830–836.
- 76. Gaertner F, Ahmad Z, Rosenberger G, et al. Migrating platelets are mechano‐scavengers that collect and bundle bacteria. Cell. 2017;171(6):1368–1382.e23.
- 77. Silvestre‐Roig C, Braster Q, Ortega‐Gomez A, Soehnlein O. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol. 2020;17(6):327–340.
- 78. Etulain J, Martinod K, Wong SL, Cifuni SM, Schattner M, Wagner DD. P‐selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015;126(2):242–246.
- 79. Rinder H, Bonan J, Rinder C, Ault K, Smith B. Dynamics of leukocyte‐platelet adhesion in whole blood. Blood. 1991;78(7):1730–1737.
- 80. Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci. 2010;107(36):15880–15885.
- 81. Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nature Med. 2017;23(3):279–287.
- 82. Zarbock A, Müller H, Kuwano Y, Ley K. PSGL‐1‐dependent myeloid leukocyte activation. J Leukoc Biol. 2009;86(5):1119–1124.
- 83. Ivanov II, Apta BHR, Bonna AM, Harper MT. Platelet p‐selectin triggers rapid surface exposure of tissue factor in monocytes. Sci Rep. 2019;9(1):13397.
- 84. Weyrich AS, Elstad MR, McEver RP, et al. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97(6):1525–1534.
- 85. Moore KL, Stults NL, Diaz S, et al. Identification of a specific glycoprotein ligand for P‐selectin (CD62) on myeloid cells. J Cell Biol. 1992;118(2):445–456.
- 86. Wang Y, Gao H, Shi C, et al. Leukocyte integrin Mac‐1 regulates thrombosis via interaction with platelet GPIbα. Nat Commun. 2017;8:15559.
- 87. Silverstein RL, Asch AS, Nachman RL. Glycoprotein IV mediates thrombospondin‐dependent platelet‐monocyte and platelet‐U937 cell adhesion. J Clin Invest. 1989;84(2):546–552.
- 88. Wright SD, Weitz JI, Huang AJ, Levin SM, Silverstein SC, Loike JD. Complement receptor type three (CD11b/CD18) of human polymorphonuclear leukocytes recognizes fibrinogen. Proc Natl Acad Sci. 1988;85(20):7734–7738.
- 89. Diacovo TG, deFougerolles AR, Bainton DF, Springer TA. A functional integrin ligand on the surface of platelets: intercellular adhesion molecule‐2. J Clin Invest. 1994;94(3):1243–1251.
- 90. Santoso S, Sachs UJH, Kroll H, et al. The junctional adhesion molecule 3 (JAM‐3) on human platelets is a counterreceptor for the leukocyte integrin Mac‐1. J Exp Med. 2002;196(5):679–691.
- 91. Phillips JH, Chang C, Lanier LL. Platelet‐induced expression of FcγRIII (CD16) on human monocytes. Eur J Immunol. 1991;21(4):895–899.
- 92. Lee SJ, Yoon BR, Kim HY, Yoo SJ, Kang SW, Lee WW. Activated platelets convert CD14+CD16‐ into CD14+CD16+ monocytes with enhanced FcγR‐mediated phagocytosis and skewed M2 polarization. Front Immunol. 2021;11:611133.
- 93. Lishko VK, Yakubenko VP, Ugarova TP, Podolnikova NP. Leukocyte integrin Mac‐1 (CD11b/CD18, αMβ2, CR3) acts as a functional receptor for platelet factor 4. J Biol Chem. 2018;293(18):6869–6882.
- 94. Pervushina O, Scheuerer B, Reiling N, et al. Platelet factor 4/CXCL4 induces phagocytosis and the generation of reactive oxygen metabolites in mononuclear phagocytes independently of Gi protein activation or intracellular calcium transients. J Immunol. 2004;173(3):2060–2067.
- 95. Chatterjee M, von Ungern‐Sternberg SNI, Seizer P, et al. Platelet‐derived CXCL12 regulates monocyte function, survival, differentiation into macrophages and foam cells through differential involvement of CXCR4‐CXCR7. Cell Death Dis. 2015;6(11):e1989.
- 96. Laffont B, Corduan A, Rousseau M, et al. Platelet microparticles reprogram macrophage gene expression and function. Thromb Haemost. 2016;115(2):311–323.
- 97. Barrett TJ, Schlegel M, Zhou F, et al. Platelet regulation of myeloid suppressor of cytokine signaling 3 accelerates atherosclerosis. Sci Transl Med. 2019;11(517):eaax0481.
- 98. von Hundelshausen P, Koenen RR, Sack M, et al. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood. 2005;105(3):924–930.
- 99. Williams MWY, Guiffre AK, Fletcher JP. Platelets and smooth muscle cells affecting the differentiation of monocytes. PLoS ONE. 2014;9(2):e88172.
- 100. Marx C, Novotny J, Salbeck D, et al. Eosinophil‐platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood. 2019;134(21):1859–1872.
- 101. Pitchford SC, Momi S, Giannini S, et al. Platelet P‐selectin is required for pulmonary eosinophil and lymphocyte recruitment in a murine model of allergic inflammation. Blood. 2005;105(5):2074–2081.
- 102. Diacovo TG, Puri KD, Warnock RA, Springer TA, von Andrian UH. Platelet‐mediated lymphocyte delivery to high endothelial venules. Science. 1996;273(5272):252–255.
- 103. Koh CH, Lee S, Kwak M, Kim BS, Chung Y. CD8 T‐cell subsets: heterogeneity, functions, and therapeutic potential. Exp Mol Med. 2023;55(11):2287–2299.
- 104. Speiser DE, Chijioke O, Schaeuble K, Münz C. CD4+ T cells in cancer. Nature Cancer. 2023;4(3):317–329.
- 105. Shi G, Field DJ, Ko K, et al. Platelet factor 4 limits Th17 differentiation and cardiac allograft rejection. J Clin Invest. 2014;124(2):543–552.
- 106. Gerdes N, Zhu L, Ersoy M, et al. Platelets regulate CD4+ T‐cell differentiation via multiple chemokines in humans. Thromb Haemost. 2011;106(2):353–362.
- 107. Rossaint J, Thomas K, Mersmann S, et al. Platelets orchestrate the resolution of pulmonary inflammation in mice by T reg cell repositioning and macrophage education. J Exp Med. 2021;218(7):e20201353.
- 108. Cognasse F, Hamzeh‐Cognasse H, Lafarge S, et al. Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Exp Hematol. 2007;35(9):1376–1387.
- 109. Duffau P, Seneschal J, Nicco C, et al. Platelet CD154 potentiates Interferon‐α secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci Transl Med. 2010;2(47):47ra63.
- 110. Han P, Hanlon D, Arshad N, et al. Platelet P‐selectin initiates cross‐presentation and dendritic cell differentiation in blood monocytes. Sci Adv. 2020;6(11):eaaz1580.
- 111. Bock M, Bergmann CB, Jung S, Biberthaler P, Heimann L, Hanschen M. Platelets differentially modulate CD4+ Treg activation via GPIIa/IIIb‐, fibrinogen‐, and PAR4‐dependent pathways. Immunol Res. 2022;70(2):185–196.
- 112. Guo L, Shen S, Rowley JW, et al. Platelet MHC class I mediates CD8+ t‐cell suppression during sepsis. Blood. 2021;138(5):401–416.
- 113. Bénézech C, Nayar S, Finney BA, et al. CLEC‐2 is required for development and maintenance of lymph nodes. Blood. 2014;123(20):3200–3207.
- 114. Zhang Z, Xu X, Zhang D, et al. Targeting erbin‐mitochondria axis in platelets/megakaryocytes promotes B cell‐mediated antitumor immunity. Cell Metab. 2024;36(3):541–556.e9.
- 115. Unruh D, Horbinski C. Beyond thrombosis: the impact of tissue factor signaling in cancer. J Hematol Oncol. 2020;13(1):93.
- 116. Matsui Y, Amano H, Ito Y, et al. Thromboxane A2 receptor signaling facilitates tumor colonization through P‐selectin‐mediated interaction of tumor cells with platelets and endothelial cells. Cancer Sci. 2012;103(4):700–707.
- 117. Egan K, Crowley D, Smyth P, et al. Platelet adhesion and degranulation induce pro‐survival and pro‐angiogenic signalling in ovarian cancer cells. PLoS ONE. 2011;6(10):e26125.
- 118. Haemmerle M, Bottsford‐Miller J, Pradeep S, et al. FAK regulates platelet extravasation and tumor growth after antiangiogenic therapy withdrawal. J Clin Invest. 2016;126(5):1885–1896.
- 119. Mitrugno A, Tassi Yunga S, Sylman JL, et al. The role of coagulation and platelets in colon cancer‐associated thrombosis. Am J Physiol Cell Physiol. 2019;316(2):C264–C273.
- 120. Shaker H, Bundred NJ, Landberg G, et al. Breast cancer stromal clotting activation (Tissue Factor and thrombin): a pre‐invasive phenomena that is prognostic in invasion. Cancer Med. 2020;9(5):1768–1778.
- 121. De Candia E. Mechanisms of platelet activation by thrombin: a short history. Thromb Res. 2012;129(3):250–256.
- 122. Adams GN, Rosenfeldt L, Frederick M, et al. Colon cancer growth and dissemination relies upon thrombin, stromal PAR‐1, and fibrinogen. Cancer Res. 2015;75(19):4235–4243.
- 123. Rondon AMR, Kroone C, Kapteijn MY, Versteeg HH, Buijs JT. Role of tissue factor in tumor progression and Cancer‐associated thrombosis. Semin Thromb Hemost. 2019;45(4):396–412.
- 124. Kim YJ, Borsig L, Varki NM, Varki A. P‐selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci. 1998;95(16):9325–9330.
- 125. Polgar J, Matuskova J, Wagner DD. The P‐selectin, tissue factor, coagulation triad. J Thromb Haemostasis. 2005;3(8):1590–1596.
- 126. Furie B, Furie BC. Role of platelet P‐selectin and microparticle PSGL‐1 in thrombus formation. Trends Mol Med. 2004;10(4):171–178.
- 127. Ay C, Simanek R, Vormittag R, et al. High plasma levels of soluble P‐selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna cancer and thrombosis study (CATS). Blood. 2008;112(7):2703–2708.
- 128. Mammadova‐Bach E, Gil‐Pulido J, Sarukhanyan E, et al. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell‐derived galectin‐3. Blood. 2020;135(14):1146–1160.
- 129. Mammadova‐Bach E, Zigrino P, Brucker C, et al. Platelet integrin α6β1 controls lung metastasis through direct binding to cancer cell‐derived ADAM9. JCI Insight. 2016;1(14):e88245.
- 130. Qu M, Zou X, Fang F, et al. Platelet‐derived microparticles enhance megakaryocyte differentiation and platelet generation via miR‐1915‐3p. Nat Commun. 2020;11(1):4964.
- 131. Sadallah S, Schmied L, Eken C, Charoudeh HN, Amicarella F, Schifferli JA. Platelet‐derived ectosomes reduce NK cell function. J Immunol 2016;197(5):1663–1671.
- 132. Aggarwal A, Jennings CL, Manning E, Cameron SJ. Platelets at the vessel wall in Non‐thrombotic disease. Circ Res. 2023;132(6):775–790.
- 133. Li X, Wang Q. Platelet‐derived microparticles and autoimmune diseases. Int J Mol Sci. 2023;24(12):10275.
- 134. Qi C, Wei B, Zhou W, et al. P‐selectin‐mediated platelet adhesion promotes tumor growth. Oncotarget. 2015;6(9):6584–6596.
- 135. Krishnan H, Rayes J, Miyashita T, et al. Podoplanin: an emerging cancer biomarker and therapeutic target. Cancer Sci. 2018;109(5):1292–1299.
- 136. Riedl J, Preusser M, Nazari PMS, et al. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood. 2017;129(13):1831–1839.
- 137. Rayes J, Watson SP, Nieswandt B. Functional significance of the platelet immune receptors GPVI and CLEC‐2. J Clin Invest. 2019;129(1):12–23.
- 138. Tsukiji N, Osada M, Sasaki T, et al. Cobalt hematoporphyrin inhibits CLEC‐2‐podoplanin interaction, tumor metastasis, and arterial/venous thrombosis in mice. Blood Adv. 2018;2(17):2214–2225.
- 139. Shirai T, Inoue O, Tamura S, et al. C‐type lectin‐like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor‐bearing mice. J Thromb Haemostasis. 2017;15(3):513–525.
- 140. Hwang BO, Park SY, Cho ES, et al. Platelet CLEC2‐podoplanin axis as a promising target for oral cancer treatment. Front Immunol. 2021;12:807600.
- 141. Xu M, Wang X, Pan Y, et al. Blocking podoplanin suppresses growth and pulmonary metastasis of human malignant melanoma. BMC Cancer. 2019;19(1):599.
- 142. Alonso‐Escolano D, Strongin AY, Chung AW, Deryugina EI, Radomski MW. Membrane type‐1 matrix metalloproteinase stimulates tumour cell‐induced platelet aggregation: role of receptor glycoproteins. Br J Pharmacol. 2004;141(2):241–252.
- 143. Yu LX, Yan L, Yang W, et al. Platelets promote tumour metastasis via interaction between TLR4 and tumour cell‐released high‐mobility group box 1 protein. Nat Commun. 2014;5:5256.
- 144. Dudiki T, Veleeparambil M, Zhevlakova I, et al. Mechanism of tumor‐platelet communications in cancer. Circ Res. 2023;132(11):1447–1461.
- 145. Nilsson RJA, Balaj L, Hulleman E, et al. Blood platelets contain tumor‐derived RNA biomarkers. Blood. 2011;118(13):3680–3683.
- 146. Sabrkhany S, Kuijpers MJE, Knol JC, et al. Exploration of the platelet proteome in patients with early‐stage cancer. J Proteomics. 2018;177:65–74.
- 147. Gao Y, Liu CJ, Li HY, et al. Platelet RNA enables accurate detection of ovarian cancer: an intercontinental, biomarker identification study. Protein Cell. 2023;14(6):579–590.
- 148. Bombeli T, Schwartz BR, Harlan JM. Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa‐dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM‐1), αvβ3 integrin, and GPIbα. J Exp Med. 1998;187(3):329–339.
- 149. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer‐associated venous thrombosis. Blood. 2013;122(10):1712–1723.
- 150. Hisada Y, Mackman N. Cancer‐associated pathways and biomarkers of venous thrombosis. Blood. 2017;130(13):1499–1506.
- 151. Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(29):4839–4847.
- 152. Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11(1):125.
- 153. Heinmöller E, Weinel RJ, Heidtmann HH, et al. Studies on tumor‐cell‐induced platelet aggregation in human lung cancer cell lines. J Cancer Res Clin Oncol. 1996;122(12):735–744.
- 154. Sun L, Li Q, Guo Y, et al. Extract of caulis spatholobi, a novel platelet inhibitor, efficiently suppresses metastasis of colorectal cancer by targeting tumor cell‐induced platelet aggregation. Biomed Pharmacother. 2020;123:109718.
- 155. Chen X, Li Q, Kan XX, et al. Extract of caulis spatholobi, a novel blocker targeting tumor cell-induced platelet aggregation, inhibits breast cancer metastasis. Oncol Rep. 2016;36(6):3215–3224.
- 156. Garcia‐Leon MJ, Liboni C, Mittelheisser V, et al. Platelets favor the outgrowth of established metastases. Nat Commun. 2024;15(1):3297.
- 157. Sheng M, Sun R, Fu J, Lu G. The podoplanin‐CLEC‐2 interaction promotes platelet‐mediated melanoma pulmonary metastasis. BMC Cancer. 2024;24(1):399.
- 158. Oria VO, Lopatta P, Schilling O. The pleiotropic roles of ADAM9 in the biology of solid tumors. Cell Mol Life Sci. 2018;75(13):2291–2301.
- 159. Weber MR, Zuka M, Lorger M, et al. Activated tumor cell integrin αvβ3 cooperates with platelets to promote extravasation and metastasis from the blood stream. Thromb Res. 2016;140:S27–S36.
- 160. Trikha M, Zhou Z, Timar J, et al. Multiple roles for platelet GPIIb/IIIa and alphavbeta3 integrins in tumor growth, angiogenesis, and metastasis. Cancer Res. 2002;62(10):2824–2833.
- 161. Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P‐selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci. 2001;98(6):3352–3357.
- 162. Ali AS, Ajaz A. The role of mucin‐educated platelet activation in tumor invasiveness: an unfolding concern in the realm of cancer biology. Biomedicine. 2017;7(4):21.
- 163. Wahrenbrock M, Borsig L, Le D, Varki N, Varki A. Selectin‐mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest. 2003;112(6):853–862.
- 164. Pereira‐Veiga T, Schneegans S, Pantel K, Wikman H. Circulating tumor cell‐blood cell crosstalk: biology and clinical relevance. Cell Rep. 2022;40(9):111298.
- 165. Ganesh K, Massagué J. Targeting metastatic cancer. Nature Med. 2021;27(1):34–44.
- 166. Läubli H, Spanaus KS, Borsig L. Selectin‐mediated activation of endothelial cells induces expression of CCL5 and promotes metastasis through recruitment of monocytes. Blood. 2009;114(20):4583–4591.
- 167. Chen Y, Zhou J, Liu Z, et al. Tumor cell‐induced platelet aggregation accelerates hematogenous metastasis of malignant melanoma by triggering macrophage recruitment. J Exp Clin Cancer Res. 2023;42(1):277.
- 168. Marrella A, Fedi A, Varani G, et al. High blood flow shear stress values are associated with circulating tumor cells cluster disaggregation in a multi‐channel microfluidic device. PLoS ONE. 2021;16(1):e0245536.
- 169. Zamora C, Cantó E, Nieto JC, et al. Binding of platelets to lymphocytes: a potential Anti‐inflammatory therapy in rheumatoid arthritis. J Immunol. 2017;198(8):3099–3108.
- 170. Kopp HG, Placke T, Salih HR. Platelet‐derived transforming growth factor‐β down‐regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009;69(19):7775–7783.
- 171. Castriconi R, Cantoneti C, Della Chiesa M, et al. Transforming growth factor β1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK‐mediated killing of dendritic cells. Proc Natl Acad Sci. 2003;100(7):4120–4125.
- 172. Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF‐β1 secretion and down‐modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172(12):7335–7340.
- 173. Liu X, Song J, Zhang H, et al. Immune checkpoint HLA‐E:CD94‐NKG2A mediates evasion of circulating tumor cells from NK cell surveillance. Cancer Cell. 2023;41(23):272–287.e9.
- 174. Hinterleitner C, Strähle J, Malenke E, et al. Platelet PD‐L1 reflects collective intratumoral PD‐L1 expression and predicts immunotherapy response in non‐small cell lung cancer. Nat Commun. 2021;12(1):7005.
- 175. Cho MS, Li J, Gonzalez‐Delgado R, et al. The effect of platelet G proteins on platelet extravasation and tumor growth in the murine model of ovarian cancer. Blood Adv. 2021;5(7):1947–1951d.
- 176. Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell‐mediated elimination of tumor cells. Blood. 2005;105(1):178–185.
- 177. Palumbo JS, Talmage KE, Massari JV, et al. Tumor cell‐associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell‐dependent and‐independent mechanisms. Blood. 2007;110(1):133–141.
- 178. Clar KL, Hinterleitner C, Schneider P, Salih HR, Maurer S. Inhibition of NK reactivity against solid tumors by Platelet‐derived RANKL. Cancers. 2019;11(3):277.
- 179. Placke T, Salih HR, Kopp HG. GITR ligand provided by thrombopoietic cells inhibits NK cell antitumor activity. J Immunol. 2012;189(1):154–160.
- 180. Zhou Y, Heitmann JS, Clar KL, et al. Platelet‐expressed immune checkpoint regulator GITRL in breast cancer. Cancer Immunol Immunother. 2021;70(9):2483–2496.
- 181. Cluxton CD, Spillane C, O'Toole SA, Sheils O, Gardiner CM, O'Leary JJ. Suppression of natural killer cell NKG2D and CD226 anti‐tumour cascades by platelet cloaked cancer cells: implications for the metastatic cascade. PLoS ONE. 2019;14(3):e0211538.
- 182. Maurer S, Kropp KN, Klein G, et al. Platelet‐mediated shedding of NKG2D ligands impairs NK cell immune‐surveillance of tumor cells. Oncoimmunology. 2017;7(2):e1364827.
- 183. Miyama Y, Morikawa T, Miyakawa J, et al. The prognostic value of PD‐L1 expression in upper tract urothelial carcinoma varies according to platelet count. Cancer Med. 2018;7(9):4330–4338.
- 184. Rolfes V, Idel C, Pries R, et al. PD‐L1 is expressed on human platelets and is affected by immune checkpoint therapy. Oncotarget. 2018;9(44):27460–27470.
- 185. Zaslavsky AB, Adams MP, Cao X, et al. Platelet PD‐L1 suppresses anti‐cancer immune cell activity in PD‐L1 negative tumors. Sci Rep. 2020;10(1):19296.
- 186. Cho MS, Lee H, Gonzalez‐Delgado R, et al. Platelets increase the expression of PD‐L1 in ovarian cancer. Cancers. 2022;14(10):2498.
- 187. Shi G, Field DJ, Ko K, et al. Platelet factor 4 limits Th17 differentiation and cardiac allograft rejection. J Clin Invest. 2014;124(2):543–552.
- 188. Gerdes N, Zhu L, Ersoy M, et al. Platelets regulate CD4+ T‐cell differentiation via multiple chemokines in humans. Thromb Haemost. 2011;106(2):353–362.
- 189. Bock M, Bergmann CB, Jung S, Biberthaler P, Heimann L, Hanschen M. Platelets differentially modulate CD4+ Treg activation via GPIIa/IIIb‐, fibrinogen‐, and PAR4‐dependent pathways. Immunol Res. 2022;70(2):185–196.
- 190. Ahamed J, Burg N, Yoshinaga K, Janczak CA, Rifkin DB, Coller BS. In vitro and in vivo evidence for shear‐induced activation of latent transforming growth factor‐β1. Blood. 2008;112(9):3650–3660.
- 191. Rachidi S, Metelli A, Riesenberg B, et al. Platelets subvert T cell immunity against cancer via GARP‐TGFβ axis. Sci Immunol. 2017;2(11):eaai7911.
- 192. Metelli A, Wu BX, Riesenberg B, et al. Thrombin contributes to cancer immune evasion via proteolysis of platelet‐bound GARP to activate LTGF‐β. Sci Transl Med. 2020;12(525):eaay4860.
- 193. Köhler S, Ullrich S, Richter U, Schumacher U. E‐/P‐selectins and colon carcinoma metastasis: first in vivo evidence for their crucial role in a clinically relevant model of spontaneous metastasis formation in the lung. Br J Cancer. 2010;102(3):602–609.
- 194. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168(4):670–691.
- 195. Langer H, May AE, Bültmann A, Gawaz M. ADAM 15 is an adhesion receptor for platelet GPIIb‐IIIa and induces platelet activation. Thromb Haemost. 2005;94(3):555–561.
- 196. Haemmerle M, Bottsford‐Miller J, Pradeep S, et al. FAK regulates platelet extravasation and tumor growth after antiangiogenic therapy withdrawal. J Clin Invest. 2016;126(5):1885–1896.
- 197. Mammadova‐Bach E, Zigrino P, Brucker C, et al. Platelet integrin α6β1 controls lung metastasis through direct binding to cancer cell‐derived ADAM9. JCI Insight. 2016;1(14):e88245.
- 198. Li X, Chen X, Gong S, et al. Platelets promote CRC by activating the C5a/C5aR1 axis via PSGL‐1/JNK/STAT1 signaling in tumor‐associated macrophages. Theranostics. 2023;13(6):2040–2056.
- 199. Lonsdorf AS, Krämer BF, Fahrleitner M, et al. Engagement of αIIbβ3 (GPIIb/IIIa) with ανβ3 integrin mediates interaction of melanoma cells with platelets. J Biol Chem. 2012;287(3):2168–2178.
- 200. Ward Y, Lake R, Faraji F, et al. Platelets promote metastasis via binding tumor CD97 leading to bidirectional signaling that coordinates transendothelial migration. Cell Rep. 2018;23(3):808–822.
- 201. Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet‐derived nucleotides promote tumor‐cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24(1):130–137.
- 202. Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast‐tumour metastasis. Nature. 2011;475(7355):222–225.
- 203. Läubli H, Spanaus KS, Borsig L. Selectin‐mediated activation of endothelial cells induces expression of CCL5 and promotes metastasis through recruitment of monocytes. Blood. 2009;114(20):4583–4591.
- 204. Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci. 2014; 111(30):E3053–E3061.
- 205. Jiang L, Luan Y, Miao X, et al. Platelet releasate promotes breast cancer growth and angiogenesis via VEGF‐integrin cooperative signalling. Br J Cancer. 2017;117(5):695–703.
- 206. Wartiovaara U, Salven P, Mikkola H, et al. Peripheral blood platelets express VEGF‐C and VEGF which are released during platelet activation. Thromb Haemost. 1998;80(1):171–175.
- 207. Kisucka J, Butterfield CE, Duda DG, et al. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc Natl Acad Sci. 2006;103(4):855–860.
- 208. Kaiser R, Escaig R, Kranich J, et al. Procoagulant platelet sentinels prevent inflammatory bleeding through GPIIBIIIA and GPVI. Blood. 2022;140(2):121–139.
- 209. Möhle R, Green D, Moore MAS, Nachman RL, Rafii S. Constitutive production and thrombin‐induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci. 1997;94(2):663–668.
- 210. Verheul HM, Hoekman K, Lupu F, et al. Platelet and coagulation activation with vascular endothelial growth factor generation in soft tissue sarcomas. Clin Cancer Res 2000;6(1):166–171.
- 211. Wojtukiewicz MZ, Sierko E, Hempel D, Tucker SC, Honn KV. Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev. 2017;36(2):249–262.
- 212. Battinelli EM, Markens BA, Italiano Jr JE. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118(5):1359–1369.
- 213. Lakka Klement G, Yip TT, Cassiola F, et al. Platelets actively sequester angiogenesis regulators. Blood. 2009;113(12):2835–2842.
- 214. Italiano JE, Richardson JL, Patel‐Hett S, et al. Angiogenesis is regulated by a novel mechanism: pro‐ and antiangiogenic proteins are organized into separate platelet α granules and differentially released. Blood. 2008;111(3):1227–1233.
- 215. Sehgal S, Storrie B. Evidence that differential packaging of the major platelet granule proteins von Willebrand factor and fibrinogen can support their differential release. J Thromb Haemostasis. 2007;5(10):2009–2016.
- 216. Kuznetsov HS, Marsh T, Markens BA, et al. Identification of luminal breast cancers that establish a tumor‐supportive macroenvironment defined by proangiogenic platelets and bone marrow‐derived cells. Cancer Discovery. 2012;2(12):1150–1165.
- 217. Ho‐Tin‐Noé B, Goerge T, Cifuni SM, Duerschmied D, Wagner DD. Platelet granule secretion continuously prevents intratumor hemorrhage. Cancer Res. 2008;68(16):6851–6858.
- 218. Volz J, Mammadova‐Bach E, Gil‐Pulido J, et al. Inhibition of platelet GPVI induces intratumor hemorrhage and increases efficacy of chemotherapy in mice. Blood. 2019;133(25):2696–2706.
- 219. Rodriguez‐Martinez A, Simon‐Saez I, Perales S, et al. Exchange of cellular components between platelets and tumor cells: impact on tumor cells behavior. Theranostics. 2022;12(5):2150–2161.
- 220. Zhang W, Zhou H, Li H, et al. Cancer cells reprogram to metastatic state through the acquisition of platelet mitochondria. Cell Rep. 2023;42(9):113147.
- 221. Contursi A, Fullone R, Szklanna‐Koszalinska P, et al. Tumor‐educated platelet extracellular vesicles: proteomic profiling and crosstalk with colorectal cancer cells. Cancers. 2023;15(2):350.
- 222. Wojtukiewicz MZ, Tang DG, Nelson KK, et al. Thrombin enhances tumor cell adhesive and metastatic properties via increased alpha IIb beta 3 expression on the cell surface. Thromb Res. 1992;68(3):233–245.
- 223. Gharib E, Veilleux V, Boudreau LH, Pichaud N, Robichaud GA. Platelet‐derived microparticles provoke chronic lymphocytic leukemia malignancy through metabolic reprogramming. Front Immunol. 2023;14:1207631.
- 224. Veilleux V, Pichaud N, Boudreau LH, Robichaud GA. Mitochondria transfer by Platelet‐derived microparticles regulates breast cancer bioenergetic states and malignant features. Mol Cancer Res. 2024;22(3):268–281.
- 225. Kirschbaum M, Karimian G, Adelmeijer J, Giepmans BNG, Porte RJ, Lisman T. Horizontal RNA transfer mediates platelet‐induced hepatocyte proliferation. Blood. 2015;126(6):798–806.
- 226. Martins Castanheira N, Spanhofer AK, Wiener S, Bobe S, Schillers H. Uptake of platelets by cancer cells and recycling of the platelet protein CD42a. J Thromb Haemostasis. 2022;20(1):170–181.
- 227. Collinson RJ, Boey D, Wilson L, et al. PlateletSeq: a novel method for discovery of blood‐based biomarkers. Methods. 2023;219:139–149.
- 228. Tong H, Li K, Zhou M, et al. Coculture of cancer cells with platelets increases their survival and metastasis by activating the TGFβ/Smad/PAI‐1 and PI3K/AKT pathways. Int J Biol Sci. 2023;19(13):4259–4277.
- 229. Mezouar S, Mege D, Darbousset R, et al. Involvement of platelet‐derived microparticles in tumor progression and thrombosis. Semin Oncol. 2014;41(3):346–358.
- 230. Liang H, Yan X, Pan Y, et al. MicroRNA‐223 delivered by platelet‐derived microvesicles promotes lung cancer cell invasion via targeting tumor suppressor EPB41L3. Mol Cancer. 2015;14:58.
- 231. Xu Y, Chen L, Chen Y, et al. Prediction of potential biomarkers in early‐stage nasopharyngeal carcinoma based on platelet RNA sequencing. Mol Biotechnol. 2023;65(7):1096–1108.
- 232. Takagi S, Tsukamoto S, Park J, et al. Platelets enhance multiple myeloma progression via IL‐1β upregulation. Clin Cancer Res. 2018;24(10):2430–2439.
- 233. Yang L, Jiang Q, Li DZ, Zhou X, Yu DS, Zhong J. TIMP1 mRNA in tumor‐educated platelets is diagnostic biomarker for colorectal cancer. Aging. 2019;11(20):8998–9012.
- 234. Li X, Liu L, Song X, et al. TEP linc‐GTF2H2‐1, RP3‐466P17.2, and lnc‐ST8SIA4‐12 as novel biomarkers for lung cancer diagnosis and progression prediction. J Cancer Res Clin Oncol. 2021;147(6):1609–1622.
- 235. Liu CJ, Li HY, Gao Y, et al. Platelet RNA signature independently predicts ovarian cancer prognosis by deep learning neural network model. Protein Cell. 2023;14(8):618–622.
- 236. Best MG, Sol N, Kooi I, et al. RNA‐Seq of tumor‐educated platelets enables blood‐based pan‐cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell. 2015;28(5):666–676.
- 237. Shen Z, Du W, Perkins C, et al. Platelet transcriptome identifies progressive markers and potential therapeutic targets in chronic myeloproliferative neoplasms. Cell Rep Med. 2021;2(10):100425.
- 238. Yao B, Qu S, Hu R, et al. Delivery of platelet TPM3 mRNA into breast cancer cells via microvesicles enhances metastasis. FEBS Open Bio. 2019;9(12):2159–2169.
- 239. In 't Veld SGJG, Arkani M, Post E, et al. Detection and localization of early‐ and late‐stage cancers using platelet RNA. Cancer Cell. 2022;40(9):999–1009.
- 240. Amisten S. A rapid and efficient platelet purification protocol for platelet gene expression studies. Methods Mol Biol. 2012;788:155–172.
- 241. Middleton EA, Rowley JW, Campbell RA, et al. Sepsis alters the transcriptional and translational landscape of human and murine platelets. Blood. 2019;134(12):911–923.
- 242. Denis MM, Tolley ND, Bunting M, et al. Escaping the nuclear confines: signal‐dependent pre‐mRNA splicing in anucleate platelets. Cell. 2005;122(3):379–391.
- 243. D'Ambrosi S, Nilsson RJ, Wurdinger T. Platelets and tumor‐associated RNA transfer. Blood. 2021;137(23):3181–3191.
- 244. Nilsson RJA, Karachaliou N, Berenguer J, et al. Rearranged EML4‐ALK fusion transcripts sequester in circulating blood platelets and enable blood‐based crizotinib response monitoring in non‐small‐cell lung cancer. Oncotarget. 2016;7(1):1066–1075.
- 245. Rowley JW, Schwertz H, Weyrich AS. Platelet mRNA: the meaning behind the message. Curr Opin Hematol. 2012;19(5):385–391.
- 246. Krishnan Anandi, Zhang Yue, Perkins Cecelia, Gotlib Jason, Zehnder JamesL. Platelet transcriptomic signatures in myeloproliferative neoplasms. Blood. 2017;130:5288.
- 247. Best MG, Sol N, In ‘t Veld SGJG, et al. Swarm intelligence‐enhanced detection of non‐small‐cell lung cancer using tumor‐educated platelets. Cancer Cell. 2017;32(2):238–252.e9.
- 248. Xue L, Xie L, Song X, Song X. Identification of potential tumor‐educated platelets RNA biomarkers in non‐small‐cell lung cancer by integrated bioinformatical analysis. J Clin Lab Anal. 2018;32(7):e22450.
- 249. Antunes‐Ferreira M, D'Ambrosi S, Arkani M, et al. Tumor‐educated platelet blood tests for non‐small cell lung cancer detection and management. Sci Rep. 2023;13(1):9359.
- 250. Saito R, Shoda K, Maruyama S, et al. Platelets enhance malignant behaviours of gastric cancer cells via direct contacts. Br J Cancer. 2021;124(3):570–573.
- 251. Sol N, In ‘t Veld SGJG, Vancura A, et al. Tumor‐educated platelet RNA for the detection and (pseudo)progression monitoring of glioblastoma. Cell Rep Med. 2020;1(7):100101.
- 252. Nilsson RJA, Balaj L, Hulleman E, et al. Blood platelets contain tumor‐derived RNA biomarkers. Blood. 2011;118(13):3680–3683.
- 253. Qi ZH, Xu HX, Zhang SR, et al. The significance of liquid biopsy in pancreatic cancer. J Cancer. 2018;9(18):3417–3426.
- 254. Shi Q, Xu J, Chen C, et al. Direct contact between tumor cells and platelets initiates a FAK‐dependent F3/TGF‐β positive feedback loop that promotes tumor progression and EMT in osteosarcoma. Cancer Lett. 2024;591:216902.
- 255. Takemoto A, Okitaka M, Takagi S, et al. A critical role of platelet TGF‐β release in podoplanin‐mediated tumour invasion and metastasis. Sci Rep. 2017;7:42186.
- 256. Oh SA, Liu M, Nixon BG, et al. Foxp3‐independent mechanism by which TGF‐β controls peripheral T cell tolerance. Proc Natl Acad Sci. 2017;114(36):E7536–E7544.
- 257. Sola‐Penna M, Paixão LP, Branco JR, et al. Serotonin activates glycolysis and mitochondria biogenesis in human breast cancer cells through activation of the Jak1/STAT3/ERK1/2 and adenylate cyclase/PKA, respectively. Br J Cancer. 2020;122(2):194–208.
- 258. Zhang Z, Xu X, Zhang D, et al. Targeting erbin‐mitochondria axis in platelets/megakaryocytes promotes B cell‐mediated antitumor immunity. Cell Metab. 2024;36(3):541–556.
- 259. Schneider MA, Heeb L, Beffinger MM, et al. Attenuation of peripheral serotonin inhibits tumor growth and enhances immune checkpoint blockade therapy in murine tumor models. Sci Transl Med. 2021;13(611):eabc8188.
- 260. Ichikawa J, Ando T, Kawasaki T, et al. Role of platelet C‐type lectin‐like receptor 2 in promoting lung metastasis in osteosarcoma. J Bone Miner Res. 2020;35(9):1738–1750.
- 261. Sasaki T, Shirai T, Tsukiji N, et al. Functional characterization of recombinant snake venom rhodocytin: rhodocytin mutant blocks CLEC‐2/podoplanin‐dependent platelet aggregation and lung metastasis. J Thromb Haemostasis. 2018;16(5):960–972.
- 262. Gebremeskel S, LeVatte T, Liwski RS, Johnston B, Bezuhly M. The reversible P2Y12 inhibitor ticagrelor inhibits metastasis and improves survival in mouse models of cancer. Int J Cancer. 2015;136(1):234–240.
- 263. US National Library of Medicine . ClinicalTrials.gov, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT03245489
- 264. Hu Q, Li H, Archibong E, et al. Inhibition of post‐surgery tumour recurrence via a hydrogel releasing CAR‐T cells and anti‐PDL1‐conjugated platelets. Nat Biomed Eng. 2021;5(9):1038–1047.
- 265. Yang Y, Wang Y, Yao Y, et al. T cell‐mimicking platelet‐drug conjugates. Matter. 2023;6(7):2340–2355.
- 266. Papa AL, Jiang A, Korin N, et al. Platelet decoys inhibit thrombosis and prevent metastatic tumor formation in preclinical models. Sci Transl Med. 2019;11(479):eaau5898.
- 267. Fang M, Liu R, Fang Y, Zhang D, Kong B. Emerging platelet‐based drug delivery systems. Biomed Pharmacother. 2024;177:117131.
- 268. Hu CMJ, Fang RH, Wang KC, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–121.
- 269. Bahmani B, Gong H, Luk BT, et al. Intratumoral immunotherapy using platelet‐cloaked nanoparticles enhances antitumor immunity in solid tumors. Nat Commun. 2021;12(1):1999.
- 270. Zhu Y, Xu L, Kang Y, Cheng Q, He Y, Ji X. Platelet‐derived drug delivery systems: pioneering treatment for cancer, cardiovascular diseases, infectious diseases, and beyond. Biomaterials. 2024;306:122478.
- 271. Fang RH, Gao W, Zhang L. Targeting drugs to tumours using cell membrane‐coated nanoparticles. Nat Rev Clin Oncol. 2023;20(1):33–48.
- 272. Ma C, Fu Q, Diggs LP, et al. Platelets control liver tumor growth through P2Y12‐dependent CD40L release in NAFLD. Cancer Cell. 2022;40(9):986–998.
- 273. Iyer KS, Dayal S. Modulators of platelet function in aging. Platelets. 2020;31(4):474–482.
- 274. Poscablo DM, Worthington AK, Smith‐Berdan S, et al. An age‐progressive platelet differentiation path from hematopoietic stem cells causes exacerbated thrombosis. Cell. 2024;187(12):3090–3107.e21.
- 275. Krishnan A, Thomas S. Toward platelet transcriptomics in cancer diagnosis, prognosis and therapy. Br J Cancer. 2022;126(3):316–322.
- 276. Best MG, Wesseling P, Wurdinger T. Tumor‐Educated platelets as a noninvasive biomarker source for cancer detection and progression monitoring. Cancer Res. 2018;78(13):3407–3412.