INTRODUCTION
From 2000 to 2023 (Q4) over 499,852 service members (SMs) reportedly sustained initial traumatic brain injuries (TBIs), with over 82% categorized as mild. It is generally accepted that despite the mechanism of initial insult, dizziness and sensory impairment are common symptoms among those with TBIs,, further supported within the military population with complaints of persistent dizziness and vestibular disorders. A correlation between post-concussive symptoms and persistent complaints of dizziness has also been noted among SMs with comorbid TBIs and psychological health concerns. Consequently, it is likely that the evaluation of SMs referred for TBIs will include a vestibular assessment with eye-tracking.
The focus on identification, treatment, and prevention of TBIs in SMs mandated by recent policies, including the Warfighter Brain Health Initiative and certain sections of the National Defense Authorization Acts, will ultimately increase referrals for evaluation and treatment of TBIs. At the National Intrepid Center of Excellence (NICoE), active duty SMs with TBIs and comorbid PH conditions are evaluated and treated for their chronic symptoms, including a comprehensive vestibular evaluation. Of note, this patient population is considerably diverse in terms of the number of historical TBI events and duration since their last TBI event.
Central vestibulopathy (CV), one potential feature of TBIs, is a dysfunction between the neural pathways and the visual, vestibular, and somatosensory systems, including the vestibular nuclei, cerebellum, thalamus, midbrain, and higher centers of cortical function such as the parieto-insular vestibular cortex. CV is typically assessed via optokinetic, gaze stabilization, smooth pursuit (SP), saccade, and positional testing. Abnormalities found on these oculomotor tests may be indicative of CV and further investigation is often warranted to rule out other pathologies such as visual dysfunction, migraines, head trauma, stroke, and cerebellar degeneration. At present, SP performance, particularly in the vertical plane, appears to be a good means to determine if further assessments are warranted when TBI sequela are present.,
The SP test (SPT) is a component of a much larger vestibular evaluation but can also be used independently as a limited oculomotor screening for mild TBI (mTBI) regardless of length of time since injury., SP may be evaluated either subjectively by bedside assessment or objectively with video-nystagmography (VNG); however, there is no standardized method for evaluation. Arguably, VNG is the preferred option given the ability to assess eye movements objectively while facilitating subjective clinical evaluation via recorded eye-movement video; however, VNG equipment cost and space requirements often prevent availability for many disciplines that evaluate oculomotor function. Limitations with bedside examination of SP include variations in both execution and performance with regard to excursions, speeds, and repetitions. There are differing opinions on what qualifies performance as normal or abnormal, with some looking at quality of eye movement,, while others look at symptom reproduction.,, Given the issues with subjective evaluations and the lack of widely accepted formal guidelines for standardized SP performance and assessment, increased inter-rater variability is a concern.
At NICoE, we addressed these concerns by combining objective VNG-generated metrics with clinical subjective ratings of the eye-tracking video for the evaluation of SP eye movements. Our adopted SPT protocol evaluated 3 frequencies for each plane (horizontal and vertical) and required at least 2 frequencies to be abnormal for an overall abnormal clinical interpretation for a specific plane. This reduced false positives by preventing a single test frequency from dictating the overall interpretation; however, there was concern that the subjective component of the analyses might be introducing some degree of undesired variability via clinician bias. Specifically, our clinicians utilized a 4-choice rating classification system for subjective ratings of SP and 2 of the mid-rating classifications were suspected as the source of most clinician disagreements. A simpler 3-choice classification system was hypothesized to produce greater clinical agreement and consistency and thus was evaluated as a possible solution.
Additionally, our VNG system manufacturer introduced a novel metric, percent saccade (PS) that calculates the degree of saccadic pursuit present during SPT. This metric is of interest since it provides an independent objective measure for a common clinical sign that we evaluate in the subjective evaluation of SP for TBI subjects. As such, the PS metric potentially offers a means of improving SPT by reducing common subjective evaluation variance.
Thus, this study was designed to examine the consistency among our clinicians using the original 4-choice classification system. The second purpose was to evaluate if a simpler 3-choice classification would result in greater clinical agreement and consistency. The final objective was to evaluate the sensitivity and specificity of the PS metric in classifying SP when compared to clinician ratings.
MATERIALS AND METHODS
Participants
Between January 2017 and May 2019, 78 SMs underwent vestibular testing during their stay at the NICoE Intensive Outpatient Program. All vestibular tests were performed by an audiologist and 1 of 2 vestibular physical therapists (PTs). From these, participants were then randomly selected and counterbalanced by PTs to reduce interpretation bias for a total of 70 (i.e., 35 seen by one PT and 35 by the other). One subject was excluded due to the SM’s request to not have their data used and the remaining were excluded to prevent an unbalanced dataset (one PT saw more patients than the other). All participants were on active duty representing all branches of service, predominately males (68), and ranged in age from 29 to 51 years (x[Combining Macron] =39.7; s =5.06).
Procedures
Horizontal and vertical SPTs were performed with Neuro Kinetics I-PORTAL Neuro-Otologic Test Center (NOTC) using version 3.2.1 of I-PORTAL Portable Assessment Software (with a frame-capture rate of 100 frames/s) and version 7.5.2 of the VEST software for data collection (NKI). The test parameters used were specific to NICoE vestibular testing at the time and, as such, may vary from other clinics; therefore, they are provided in detail: SP (both planes) were performed clinically using frequencies of 0.1 Hz (2 cycles), 0.3 Hz (4 cycles), and 0.5 Hz (6 cycles) with maximum deviations at ±10°.
Clinical SPT videos and objective VNG test data for the selected subjects were downloaded from the NOTC rotational chair system. A research assistant with no experience in oculomotor assessment then relabeled all video files and VNG test data (including time plots of SP eye-position data) with unique subject identification numbers after removing all traces of identifying data. The net result of this step was the development of de-identified SPT data and video for each subject for all test frequencies (0.1, 0.3, and 0.5 Hz) in both the horizontal and vertical planes, totaling 6 tests per subject.
The 3 vestibular clinicians (1 audiologist and 2 PTs), each with over 15 years of experience, then independently analyzed the de-identified test results of all 70 subjects in a blinded manner without any collaboration or prior discussion of criteria with the others. These clinicians were tasked with rating each subject’s SP performance on the 3 individual test frequencies as normal (N), grossly normal (GN), mildly abnormal (MA), or abnormal (AB) for a total of 6 ratings (3 horizontal and 3 vertical). The GN and MA ratings were defined as follows: GN was used when the rater felt that the SP was not quite normal, but insufficient evidence was available to justify an MA or AB rating; the MA rating was used to indicate that abnormalities were present but were insufficient to rate a clear AB rating. The raters also provided an overall rating of N, GN, MA, or AB for each plane (a composite of all 3 frequencies per plane, 1 for horizontal and 1 for vertical). SPTs were considered overall AB if the results were abnormal for 2 or more frequencies tested for a given plane of movement. Fewer than 2 abnormal results resulted in an overall rating of MA, GN, or N, at the clinician’s discretion.
Development of Area Under the Curves
The clinicians’ consistency and reliability were determined by calculating the probability that each clinician correctly evaluated each of the SPT for a specific plane (horizontal or vertical) by comparing their independent reanalysis decision to the original clinical decision, which was used as the gold standard for all comparisons. The area under the curve (AUC) analysis was performed to determine how close each clinician was to achieving perfect consistency and reliability; an AUC value of 1.000 is perfect, and scores closer to 1.000 are ideal. A mathematical explanation of AUC and its use here is provided (Supplementary S1).
Statistical Analyses
The consistency and reliability of all 3 clinicians when using all 4 diagnostic categories were determined by calculating the probability that each clinician correctly evaluated each of the 70 horizontal and vertical SPTs by comparing each of their independent decisions to the original clinical diagnosis using the AB, MA, GN, and N categories.
To determine their impact on agreement, the GN and MA categories were combined into a single subclinical (SUBC) category, and Kappa values were calculated for this new combined category. The consistency and reliability of all 3 clinicians when using only 3 diagnostic categories were determined by calculating the probability that each clinician correctly evaluated each of the 70 SPTs by comparing their decision to the original clinical diagnosis using a 3-category rating system (AB, SUBC, and N). For this, MA and GN were merged into a single SUBC category, and the AUC analysis was repeated to determine the impact of simplifying the decision criteria to only 3 categories on rater consistency and reliability. For these analyses, the original clinical diagnosis was treated as the “gold standard” as it reflected a decision made jointly by 2 clinicians at the time of testing and was felt to serve as a good reference for comparisons.
Cut-off values for the PS metric at each frequency for horizontal SP (HSP) and vertical SP (VSP) were developed with both receiver operation characteristic (ROC) analysis and discrimination analysis, which determined optimal classification with the highest accuracy. These cut-off values were applied to the saccadic percent value for the SP results of all 70 subjects. When the respective result exceeded the cut-off value, the SP was labeled AB whereas all others were labeled NOR. From this, sensitivity and specificity were calculated for each frequency for both HSP and VSP.
RESULTS
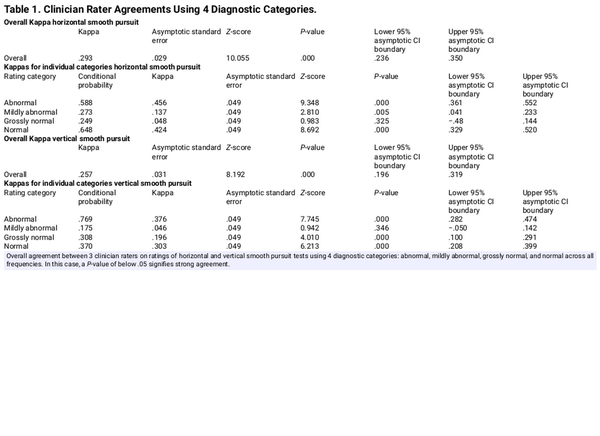
Clinician agreement in interpretation of SP was evaluated by having each clinician independently analyze and interpret de-identified SP videos and associated time plots of eye-tracking positional data. These re-analysis results were then compared to the original clinical interpretations. When the clinicians were asked to perform this task for SP responses using 4 clinical categories, their overall agreement was fair (Kappa = .293, P = .000). Table 1 shows that clinicians exhibited moderate agreement when judging a horizontal pursuit as being either N (Kappa = .424, P = .000) or AB (Kappa = .456, P = .000). Interestingly, only slight agreement was noted when the clinicians judged a horizontal pursuit as MA (Kappa = .137, P = .005) or GN (Kappa = .048, P = .325). Similar findings were present when clinicians were asked to reanalyze and rate VSP responses using the same 4 clinical categories, with fair overall agreement (Kappa = .257, P = .000). As observed for HSP, clinicians exhibited moderate agreement when judging a vertical pursuit as being either N (Kappa = .303, P = .000) or AB (Kappa = .376, P = .000). Again, only slight agreement was noted when judging vertical pursuit as MA (Kappa = .046, P = .346) or GN (Kappa = .196, P = .325).
To examine consistency and reliability, AUCs were generated for each clinician when independently reanalyzing SP results when using a 4-choice criterion (AB, MA, GN, and N). As can be seen, all clinicians demonstrated consistency and reliability above 50% (chance probability) in their evaluations of HSP at each of the 3 test frequencies, which ranged from .750 to.851 for clinician 1, from .732 to .875 for clinician 2, and from .686 to .905 for clinician 3 (Figure 1A). All 3 clinicians demonstrated similar consistency and reliability when evaluating VSP as seen in the respective AUCs, which ranged from .697 to .820 for clinician 1, from .651 to .808 for clinician 2, and from .678 to .880 for clinician 3. It should be noted that AUC values are smaller for VSP than HSP evaluations, suggesting that the clinicians demonstrate less consistency and reliability when judging VSP versus HSP.
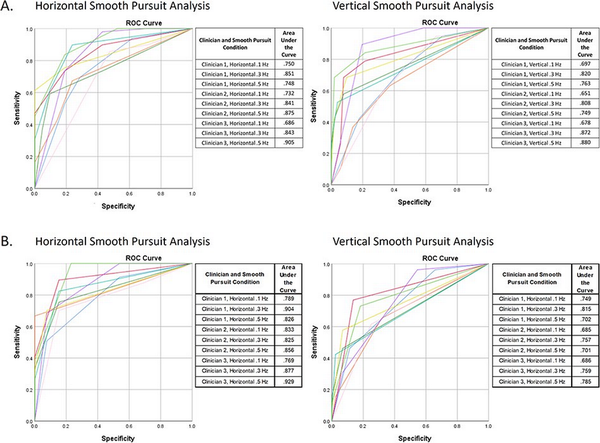
Figure 1
Receiver operating characteristics (ROC) curves and accompanying areas under the curve (AUCs) demonstrating consistency and reliability of all 3 clinician raters in subjective clinical interpretation of horizontal and vertical smooth pursuit tests for 3 frequencies. (A) Clinicians instructed to rate test results using 4 diagnostic categories: AB, MA, GN, and N. ROC curves were developed by combining the AB and MA categories into a single AB category and combining the GN and N categories into a single N category. The original clinical interpretation was defined as the correct response. (B) Clinicians instructed to rate test results using 3 diagnostic categories: AB, SUBC, and N.
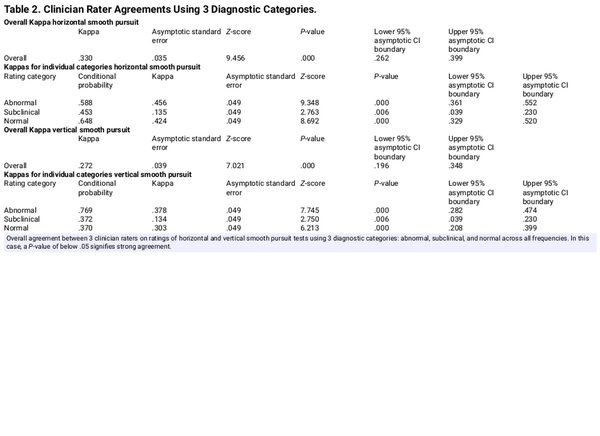
When the response categories for MA and GN categories were combined into a single SUBC category to address concerns regarding ambiguity between the MA and GN categories, the overall agreement for the 3 clinicians improved slightly when evaluating HSP as seen in Table 2 (Kappa = .330, P = .000). For the new combined subclinical category, agreement continued to be slight but significant (Kappa = .135, P = .006). When the response categories for MA and GN categories were combined into a single SUBC category for VSP, the overall agreement for the 3 clinicians again improved slightly (Kappa = .272, P = .000). For the new combined SUBC category, agreement continued to be slight but significant (Kappa = .134, P = .006).
Figure 1B shows the ROC curve generated by each clinician when independently reanalyzing SP using a 3-category rating system—NOR, SUBC, and AB. As can be seen, all clinicians continued to demonstrate consistency and reliability above 50% (chance probability) in their evaluations of both horizontal and vertical SPTs at each of the 3 test frequencies. The calculated AUCs for each clinician when evaluating HSP ranged from .789 to .901 for clinician 1, from .825 to .856 for clinician 2, and from .768 to .929 for clinician 3. All 3 clinicians demonstrated similar consistency and reliability when evaluating VSP as seen in the respective AUCs, which ranged from .702 to .815 for clinician 1, from .685 to .757 for clinician 2, and from .686 to .785 for clinician 3. It should be noted that AUC values for both the 3- and 4-category rating systems are smaller for VSP versus HSP, again suggesting that the clinicians demonstrate less consistency and reliability when judging VSP compared to HSP.
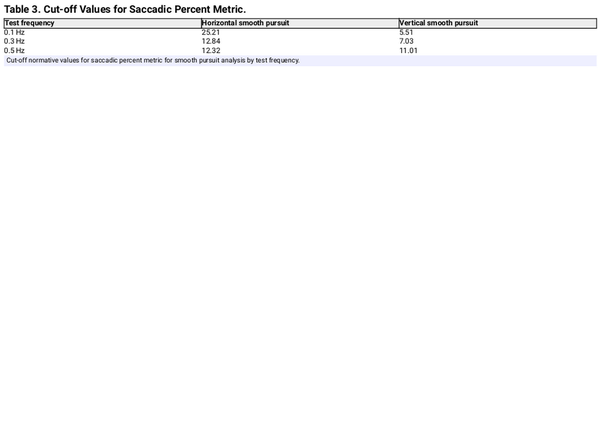
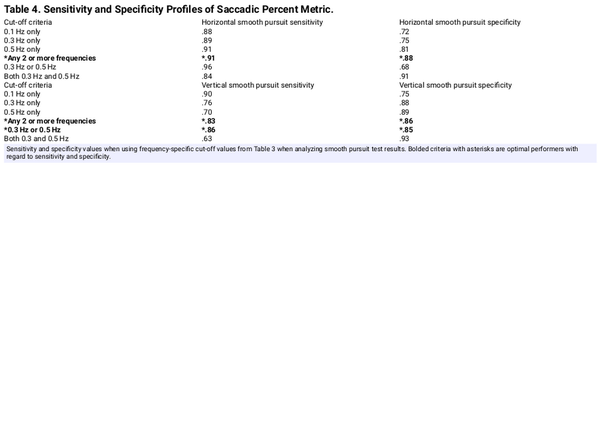
Table 3 shows the cut-off PS values generated for both HSP and VSP by frequency. To use this table, SP with PS metrics above these cut-off values would be classified as AB. These cut-off values were then used to yield N versus AB classification results that were then used to calculate sensitivity and specificity for all SP by frequency and plane of movement, which are presented in Table 4. For the HSP, the best sensitivity and specificity values of .91 and .88, respectively, were obtained when the cut-off values were exceeded for any 2 SPT frequencies. For VSP, the best sensitivity and specificity values of .83 and .86, respectively, were obtained when any 2 frequencies exceeded the cut-off values; however, when the values for 0.3 and 0.5 Hz were exceeded the cut-off thresholds, the sensitivity and specificity values were 0.86 and 0.85 Hz, respectively.
DISCUSSION
This study arose from a discussion among the vestibular clinicians at NICoE on the merits of using qualitative assessments during oculomotor assessment when computerized eye-tracking is arguably more objective and sensitive. While qualitative measures appear less sensitive, sometimes bedside oculomotor examination is the only option for clinicians and there is value in looking beyond subjective complaints and considering qualitative findings on testing. Despite technological advances, VNG systems are unable to detect or accurately measure some aspects of eye movements, and thus combining objective and subjective evaluations provides a more complete picture for the clinician.
The primary focus was the consistency of subjective rating of SPT among clinicians. Table 1 shows that all 3 clinicians were in good agreement when subjectively rating SPT as N or AB for both HSP and VSP. Disagreement was present for the GN or MA classifications, suggesting that the boundary between these 2 is sometimes unclear. In terms of clinical impact, this disagreement has no bearing on the overall diagnosis as both the GN and MA categories are subclinical and thus are not considered when determining if the overall SPT is normal or abnormal.
The secondary purpose was to address concerns of decreased agreements when including GN and MA in clinician ratings. Combining these 2 problematic classifications into a single SUBC category was proposed as this would greatly simplify clinical decision-making, increase overall agreement, and have no negative impact on the overall interpretation of SP. Table 2 confirms that shifting from a 4- to 3-category system increased clinician agreement, supporting a revision of the original 4-choice rating system with a simpler and more effective 3-choice rating. Of interest, this evaluation of an existing rating system can be used for any type of clinical evaluation that is dependent on subjective observations. While simply eliminating both the GN and MA categories and going to a binary categorical system with only N and AB as choices would appear to be a simpler option, it would likely come at the cost of reduced agreement among clinicians as a binary system would force clinicians to choose between N and AB when SP presentations do not meet either criterion.
It is interesting that good agreement was observed between all 3 clinicians on overall SP ratings when using qualitative data. This may reflect the clinical experience level of the raters and may not be the case if performed by less experienced clinicians who may demonstrate larger decision-making variances. In fact, this analysis approach could be useful to determine if clinicians, especially those with less experience, differ from their colleagues in their clinical decision-making. The decision to use the original clinical interpretation as the “gold standard” stemmed from their cooperative origin as there currently is no such widely accepted standard for these assessments. Figure 1 shows very good consistency and reliability for all 3 clinicians when comparing the outcomes of their independent evaluation to the original clinical diagnosis, especially for HSP. This observed good consistency using mostly qualitative clinical observation suggests that raters are visually identifying the same features of concerns in eye movements. In turn, this provides strong evidence that, especially when evaluating SP performance for indication of central signs, the qualitative aspect of a patient’s SP performance can provide important indications of whether central insults, including mTBI, are present.
The PS metric reflects an effort to use technology to capture and measure SP performance previously limited to subjective assessments. Cut-off values were generated for the PS metric and were shown to have clinically acceptable sensitivity and specificity values when applied to both the 0.3- and 0.5-Hz SP tests for a specific plane of movement (Tables 3 and 4). Including 0.1 Hz as a cut-off criterion for determining normal versus abnormal results resulted in a decrease for both sensitivity and specificity. Additionally, the use of only one test frequency increased the false positive rate, thereby decreasing sensitivity and specificity of the PS metric. Interestingly, these findings appear to validate our adopted clinical criteria that require at least 2 frequencies to be abnormal before judging SPT in a specific plane as abnormal.
When looking at Table 3, it is interesting to note that the cut-off values for the PS metric decrease with increasing frequency for HSP. Clinically, we have observed that minor degrees of saccadic intrusions and/or square wave jerks can be present at 0.1 Hz for HSP and disappear for 0.3 and 0.5 Hz, consistent with the PS cut-off trend. The opposite effect for VSP was observed, as cut-off values increased with increasing frequency. This is also consistent with our clinical findings, as we often see increasing degrees of extraocular intrusions at higher test frequencies. These differences in HSP versus VSP are understandable as the HSP primarily relies on the lateral and medial rectus muscles, which act upon the eye in a relatively uniform plane, whereas the VSP involves the inferior and superior oblique muscles, which act upon the eye in a non-uniform plane, and requires a more complex interaction of the oculomotor muscles to maintain smooth movement. These observations are important as they indicate that HSP and VSP should not be considered equivalent, supporting the need to perform both horizontal and vertical components when performing SPT.
Overall, the sensitivity and specificity values achieved when using cut-off values appear acceptable for clinic use. The caveat is that the cut-off values reported here may not generalize to other patient populations. Thus, it is recommended that clinicians obtain their own normative data or validate the data reported here before using them in their clinic. It is likely that the use of cut-off values will complement clinical decision-making and be useful for many clinics, but the validation process may indicate that different cut-off values or regression models may better fit their respective patient populations. Furthermore, it is the opinion of the authors that the cut-off values provided in Table 3 are intended as a tool to help the clinician and should not replace the expert qualitative evaluation of the SP performance by a trained clinician or with access to integrate advanced testing equipment.
Several limitations were observed for this study, most notably that the subjects were drawn from a unique patient population of service members with varying histories of mTBIs with both blast- and impact-induced injuries. As such, the PS data reported may be limited to patients with chronic mTBI and may not apply to civilian-only clinics. Likewise, our patient population was heavily weighted toward males, and thus gender effects cannot be discerned from this study. With regard to methodology, a limiting factor was the small number of clinicians utilized to perform ratings.
The aims of the study were met as clinician consistency was evaluated for both an existing 4-choice and a re-designed 3-choice classification system, of which the simpler 3-choice system demonstrated improved clinical agreement and consistency. Further, sensitivity and specificity of the PS metric was investigated and found to offer good clinical utility.
CONCLUSIONS
When clinical decision-making for SP involves 4 categories (AB, MA, GN, and N), there is ambiguity between MA and GN categories, evidenced by minimal agreement among clinicians for these 2 categories.
Clinician agreement improves when combining MA and GN into a single SUBC category.
The PS metric can be used to classify SP as N or AB using a cut-off threshold.
Results of this study demonstrated maximum sensitivity and specificity when requiring at least 2 of 3 frequencies to exceed the PS cut-off, which supports the assessment of more than one frequency for SP.
Results also demonstrated differences between HSP and VSP in terms of qualitative appearance at varying test frequencies, indicating that SPT should be performed in both horizontal and vertical planes.
ACKNOWLEDGMENTS
The authors would like to acknowledge the significant contributions of Dr. Paula Kodosky without whom this effort would not have been possible.
REFERENCES
- 1. System MH. DOD TBI worldwide numbers. Accessed 5 June, 2024. https://www.health.mil/Military-Health-Topics/Centers-of-Excellence/Traumatic-Brain-Injury-Center-of-Excellence/DOD-TBI-Worldwide-Numbers
- 2. Furman JM, Whitney SL. Central causes of dizziness. Phys Ther. 2000;80(2):179–87.doi: 10.1093/ptj/80.2.179
- 3. Dean S, Colantonio A, Ratcliff G, Chase S. Clients’ perspectives on problems many years after traumatic brain injury. Psychol Rep. 2000;86(2):653–8.doi: 10.2466/pr0.2000.86.2.653
- 4. Akin FW, Murnane OD. Head injury and blast exposure: vestibular consequences. Otolaryngol Clin North Am. 2011;44(2):323–34,viii.doi: 10.1016/j.otc.2011.01.005
- 5. Scherer MR, Schubert MC. Traumatic brain injury and vestibular pathology as a comorbidity after blast exposure. Phys Ther. 2009;89(9):980–92.doi: 10.2522/ptj.20080353
- 6. Baldassarre M, Smith B, Harp J, et al. Exploring the relationship between mild traumatic brain injury exposure and the presence and severity of postconcussive symptoms among veterans deployed to Iraq and Afghanistan. PM R. 2015;7(8):845–58.doi: 10.1016/j.pmrj.2015.03.003
- 7. MacGregor AJ, Dougherty AL, Tang JJ, Galarneau MR. Postconcussive symptom reporting among US combat veterans with mild traumatic brain injury from Operation Iraqi Freedom. J Head Trauma Rehabil. 2013;28(1):59–67.doi: 10.1097/HTR.0b013e3182596382
- 8. Scherer MR, Burrows H, Pinto R, et al. Evidence of central and peripheral vestibular pathology in blast-related traumatic brain injury. Otol Neurotol. 2011;32(4):571–80.doi: 10.1097/MAO.0b013e318210b8fa
- 9. Brenner LA, Ivins BJ, Schwab K, et al. Traumatic brain injury, posttraumatic stress disorder, and postconcussive symptom reporting among troops returning from Iraq. J Head Trauma Rehabil. 2010;25(5):307–12.doi: 10.1097/HTR.0b013e3181cada03
- 10. Cornwell K. Defense department taking action with warfighter brain health initiative. U.S. Department of Defense. December 20, 2023. Accessed June 18, 2024. https://www.defense.gov/News/News-Stories/Article/Article/3622388/defense-department-taking-action-with-warfighter-brain-health-initiative
- 11. Kirsch V, Keeser D, Hergenroeder T, et al. Structural and functional connectivity mapping of the vestibular circuitry from human brainstem to cortex. Brain Struct Funct. 2016;221(3):1291–308.doi: 10.1007/s00429-014-0971-x
- 12. Brandt T, Glasauer S, Stephan T, et al. Visual-vestibular and visuovisual cortical interaction: new insights from fMRI and pet. Ann N Y Acad Sci. 2002;956(1):230–41.doi: 10.1111/j.1749-6632.2002.tb02822.x
- 13. Ventre-Dominey J. Vestibular function in the temporal and parietal cortex: distinct velocity and inertial processing pathways. Front Integr Neurosci. 2014;4(8):53.doi: 10.3389/fnint.2014.00053
- 14. Alhilali LM, Yaeger K, Collins M, Fakhran S. Detection of central white matter injury underlying vestibulopathy after mild traumatic brain injury. Radiology. 2014;272(1):224–32.doi: 10.1148/radiol.14132670
- 15. Hunfalvay M, Roberts CM, Murray NP, et al. Vertical smooth pursuit as a diagnostic marker of traumatic brain injury. Concussion. 2020;5(1):Cnc69.doi: 10.2217/cnc-2019-0013
- 16. King JE, Pape MM, Kodosky PN. Vestibular test patterns in the NICoE intensive outpatient program patient population. Mil Med. 2018;183(suppl_1):237–44.doi: 10.1093/milmed/usx170
- 17. Mucha A, Collins MW, Elbin RJ, et al. A Brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42(10):2479–86.doi: 10.1177/0363546514543775
- 18. Corwin DJ, Arbogast KB, Swann C, et al. Reliability of the visio-vestibular examination for concussion among providers in a pediatric emergency department. Am J Emerg Med. 2020;38(9):1847–53.doi: 10.1016/j.ajem.2020.06.020
- 19. DiCesare CA, Kiefer AW, Nalepka P, Myer GD. Quantification and analysis of saccadic and smooth pursuit eye movements and fixations to detect oculomotor deficits. Behav Res Methods. 2017;49(1):258–66.doi: 10.3758/s13428-015-0693-x
- 20. Tirelli G, Rigo S, Bullo F, et al. Saccades and smooth pursuit eye movements in central vertigo. Acta Otorhinolaryngol Ital. 2011;31(2):96–102.
- 21. McDevitt J, Appiah-Kubi KO, Tierney R, Wright WG. Vestibular and oculomotor assessments may increase accuracy of subacute concussion assessment. Int J Sports Med. 2016;37(9):738–47.doi: 10.1055/s-0042-100470