Introduction
Immunoglobulin G4–related disease (IgG-RD) is a fibro-inflammatory condition presented with isolated or concurrent tumefactive lesions in multiple organs with indolent course. Characteristic clinical appearance with definitive histological evidence usually clinches the diagnosis. The classical histopathological pattern includes IgG4-positive plasma cell–predominant lymphoplasmacytic infiltration, storiform fibrosis, and obliterative phlebitis []. Serum IgG4 concentration is a valuable tool for supporting the diagnosis but is not permanently raised and has a significant overlap with other causes. It is hypothesised that CD4+ cytotoxic T cells orchestrate the disease and are sustained by continuous antigen presentation by cells of the B lymphocyte lineage, including but not limited to plasmablasts []. A T follicular helper cell response is likely to be responsible for the development of germinal centres within lymph nodes (and involved organs) and the cytokine production that leads to IgG4 class switch, leading to the formation of IgG4-secreting plasmablasts and long-lived plasma cells []. IgG4-RD can virtually affect any organ system like the salivary gland, pancreaticobiliary structure, lymph nodes, retroperitoneum, lung, and kidney with various clinical manifestations. Limited geographical presentation is also noted in Mikulicz and Kuttner’s variant. However, isolated renal or bladder involvement is infrequent. The exact prevalence of IgG4-RD is unknown; very few studies have been done, and its prevalence is growing. The average annual incidence rate of IgG4-RD in the USA is 1.20 per 100,000 person-years [].
Case report
A 39-year-old male presented at our facility (Department of Clinical Immunology and Rheumatology, King George’s Medical University, Lucknow, Uttar Pradesh, India) with complaints of dysuria, urgency, and hesitancy for the last 3 months. He was diagnosed with an uncomplicated urinary tract infection and treated with antibiotics from an outside physician with partial resolution of symptoms. He had a recurrence of symptoms thrice in the subsequent 2 months of symptom onset. However, there was no history of fever, haematuria, genital ulcer, or other constitutional symptoms. No history of prior comorbidity or family history of cancer. No history of addiction, substance abuse, or recent sexual exposure. He has no exposure to any chemicals or pesticides at his workplace. General as well as systemic examinations were unremarkable. Routine blood investigations were within normal limits (Table 1). Urine routine microscopy showed 10–15 red blood cells/high power field, but the culture was sterile. Ultrasound of the abdomen revealed a hyperechoic lesion protruding from the anterior wall of the urinary bladder with partial obstruction to bladder outflow, likely to be a pedunculated bladder mass with high suspicion for malignancy. Urine for malignant cytology was negative. Contrast-enhanced computed tomography (CT) of the abdomen and pelvis revealed a large irregular lobulated heterogeneously enhancing lesion (74 × 61 × 40 mm) involving the anteroinferior wall of the urinary bladder extending from mid-body up to the neck region with significant perivesical fat stranding, and multiple ill-defined perivesical deposits were also noticed in anterior and inferior perivesical space, largest measuring ∼38 × 23 mm (Figure 1). Contrast-enhanced CT also revealed a hypodense soft tissue lesion (3.5 × 3 cm) in the perigastric region at the level of the body of the stomach, and two other similar morphology satellite nodules were seen in its vicinity, larger one measuring 13 × 13 mm (Figure 2). The possibility of bladder carcinoma and infections like tuberculosis and schistosomiasis was kept as differential diagnoses. Interferon gamma–releasing assay for mycobacterium tuberculosis was negative. The patient denied for endoscopy and cystoscopy. CT-guided perigastric biopsy showed a core of fibrocollagenous tissue with dense lymphoplasmacytic infiltrate with mild eosinophilic infiltrate; immunohistochemistry of the perigastric tissue biopsy showed an elevated IgG4:IgG cell ratio of 60%; however, it showed the lack of obliterative phlebitis or storiform fibrosis, which are commonly seen in IgG4-RD (Figure 3). Ultrasound-guided urinary bladder biopsy done showed lymphoplasmacytic infiltration with eosinophils (image not available). Serum IgG4 levels were significantly higher (350 mg/dl) than the reference range (3–200 mg/dl). Treatment with 0.6 mg/kg prednisolone started with gradual tapering. Rituximab was given as a steroid-sparing agent. Ultrasound of the abdomen was done every 3 months, which showed a reduction in the size of the mass to 18 × 11 mm at the end of 1 year and repeat serum IgG4 of 28 mg/dl with no clinical complaints with the current steroid dose of 5 mg prednisolone.
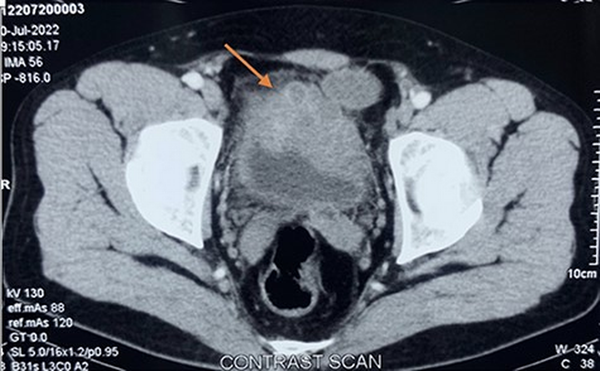
Figure 1
Contrast enhanced CT showing large irregular lobulated heterogenously enhancing lesion involving anteroinferior wall of urinary bladder (orange arrow).
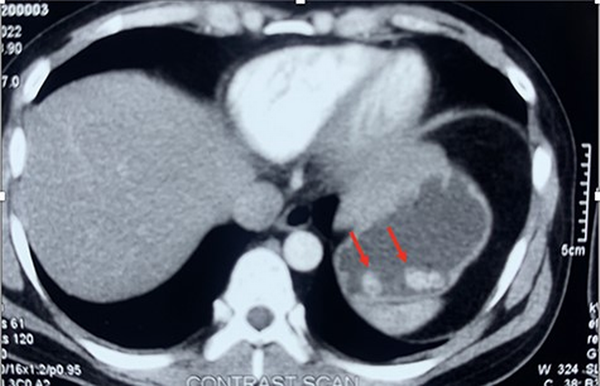
Figure 2
Contrast-enhanced CT showing hypodense soft tissue lesion in the perigastric region, and two other similar morphology satellite nodules(redarrow)
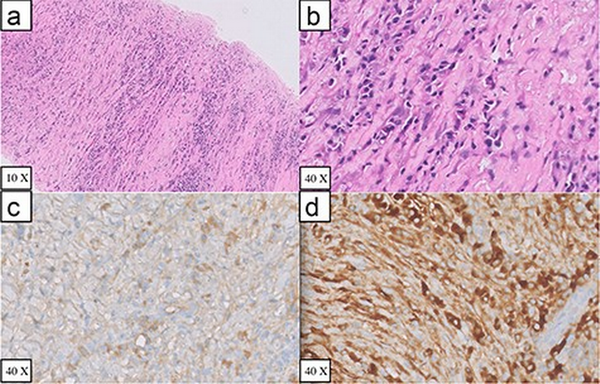
Figure 3
Perigastric biopsy specimen (A) Haematoxylin and eosin stained sections of core biopsy showing alternate areas of fibrosis and inflammation (B) Haematoxylin and eosin stained section showing plasma cell rich inflammation (C) Immunohistochemical IgG staining (D) Immunohistochemical IgG4 staining
Discussion
We described a case of IgG4-RD presenting as an isolated bladder mass with obstructive urinary symptoms and incidentally detected perigastric mass with no other organ involvement. The patient had high serum IgG4 levels, and biopsy showed lymphoplasmacytic infiltrate with immunohistochemistry showing an elevated IgG4:IgG cell ratio of 60%, but lacking the obliterative phlebitis or storiform fibrosis, which are commonly seen in IgG4-RD. This may be explained as needle biopsy sample are small and in some cases may not show complete histological picture. The patient responded effectively to glucocorticoids and rituximab therapy. Clinical and serological responses were observed without any relapse at the end of 12 months.
IgG4-RD is a well-known clinical entity which presents with pseudotumor in any organ, but evidence of urinary bladder involvement is minimal. PubMed and Google Scholar were searched for literature review regarding involvement of urinary bladder involvement in IgG4-RD, and a total of eight case reports [] have been published to date and summarised in Table 2. The mean age of presentation was 54 years (range 26–75 years). The male-to-female ratio was 1:1. Baseline serum IgG4 levels were documented in six out of eight patients (75%), which were significantly raised in all patients. Three patients [] underwent partial cystectomy, three patients [] underwent transurethral resection of the mass, and two patients [, ] were diagnosed with cystoscopy-guided biopsy. There was no prior history of autoimmune disorder or malignancy in 5/8 (62.5%) patients. However, Dropkin et al. [] described a known case of IgG4-related disease previously treated with steroids and rituximab along with a history of malignant melanoma, Kufukihara et al. [] described a patient who was a known case of autoimmune pancreatitis, and Chung et al. [] described a patient who had a history of high-grade uroepithelial carcinoma of the urinary bladder. Three patients (37.5%) had isolated urinary bladder involvement at presentation; one patient had involvement of sigmoid colon, one had regional lymphadenopathy, one had urethral involvement, one had pulmonary nodule with mediastinal lymphadenopathy, and one patient had concomitant renal and ureteral involvement. Of the three patients with isolated urinary bladder at presentation, two [, ] had a prior history of other IgG4-RD manifestations. Glucocorticoids were given to 7/8 patients (87.5%), while one patient had complete remission after surgical resection, even at a 2-year follow-up. Three patients received rituximab after oral steroids due to corticosteroid-related toxicity in one patient and disease relapse in two other patients.
Conclusion
Urinary bladder involvement is uncommon in IgG4-RD but has a significant impact on morbidity and quality of life. Varying degrees of presentation like incidentally discovered mass to gross haematuria and recurrent urinary tract infections can be observed depending on the anatomic area and extent of involvement. A high index of suspicion along with prompt initiation of therapy is required to minimise residual damage and the need for surgical intervention.
References
- [1]. Perugino CA, Stone JH. IgG4-related disease: an update on pathophysiology and implications for clinical care. Nat Rev Rheumatol 2020;16:702–14.
- [2]. Mattoo H, Mahajan VS, Maehara T et al. Clonal expansion of CD4+ cytotoxic T lymphocytes in patients with IgG4-related disease. J Allergy Clin Immunol 2016;138:825–38.
- [3]. Ito F, Kamekura R, Yamamoto M et al. IL-10+ T follicular regulatory cells are associated with the pathogenesis of IgG4-related disease. Immunol Lett 2019;207:56–63.
- [4]. Wallace ZS, Miles G, Smolkina E et al. Incidence, prevalence and mortality of IgG4-related disease in the USA: a claims-based analysis of commercially insured adults. Ann Rheum Dis 2023;82:957–62.
- [5]. Montironi R, Scarpelli M, Cheng L et al. Immunoglobulin G4–related disease in genitourinary organs: an emerging fibroinflammatory entity often misdiagnosed preoperatively as cancer. Eur Urol 2013;64:865–72.
- [6]. Park S, Ro JY, Lee DH et al. Immunoglobulin G4–associated inflammatory pseudotumor of urinary bladder: a case report. Ann Diagn Pathol 2013;17:540–3.
- [7]. Dum TW, Zhang D, Lee EK. IgG4-related disease in a urachal tumour. Case Rep Urol 2014;2014:1–3.
- [8]. Dropkin BM, Ingimarsson JP, Jones JD et al. Immunoglobulin G4‐related disease in the urinary bladder. Int J Urol 2015;22:605–7.
- [9]. Kufukihara R, Niwa N, Mizuno R et al. Immunoglobulin G4-related disease arising from the bladder wall. Urol Int 2018;103:488–90.
- [10]. Zhang Z, Yu W, Guan W et al. Immunoglobulin G4-related disease involving the bladder wall and urethra. Int Urol Nephrol 2020;52:1701–2.
- [11]. Gehring C, Starkebaum GA, Voelzke BB et al. Immunoglobulin G4–related disease of the urinary bladder. Rheumatology 2019;59:907–8.
- [12]. Chung R, Movassaghi M, Moran G et al. Genitourinary involvement of immunoglobulin G4–related disease. Rheumatology 2021;60:e444–6.