Introduction
Diabetes mellitus (DM) is a critical global public health problem due to its steadily increasing prevalence and huge impact on both patients and society. The prevalence of DM among adults in 2015 was estimated to be 8.8% globally ().
Optimizing glycemic control is fundamental to reducing and delaying complications in DM patients. The American Diabetes Association recommends that pharmacologic and nonpharmacologic treatment and personalized medical nutrition therapy should be given to both complex and chronic DM patients for better outcomes (; ; ).
Meal replacement (MR) is one of the effective tools for weight loss and weight control. A meta-analysis of six randomized controlled trials with 487 participants over 18 years of age, with and without DM, demonstrated that participants receiving MR shakes (beverages) had greater weight loss than to those who followed conventional reduced-calorie diets (). reported that overweight or obese adults receiving soy-based MR shakes showed significant weight loss and more positive changes in total cholesterol and low-density lipoprotein cholesterol (LDL-C) levels than the control group in a 12-week prospective randomized controlled trail. Furthermore, 120 overweight or obese individuals with type 2 diabetes (T2DM) who consumed MRs had greater HbA1 and weight reduction than those with a normal diet in a 6-month randomized controlled trial (). The consumption of MR products, as part of a comprehensive behavioral strategy, was also shown to improve weight loss and metabolic outcomes in participants with T2DM who were overweight or obese in Look AHEAD (Action for Health in Diabetes) ().
Several studies examining the efficacy of MRs in DM patients’ outcomes were improved by using diet variation; however, a low glycemic index (GI) diet has never been observed. Low-GI diets have shown to promote health and wellness in patients with DM (; ; ; ), such as controlling glucose levels and reducing the risk of coronary heart disease (). A MR diet in DM patients with a randomized controlled trial has never been studied in Thailand: metabolic responses are varying depending on nationality and the study setting.
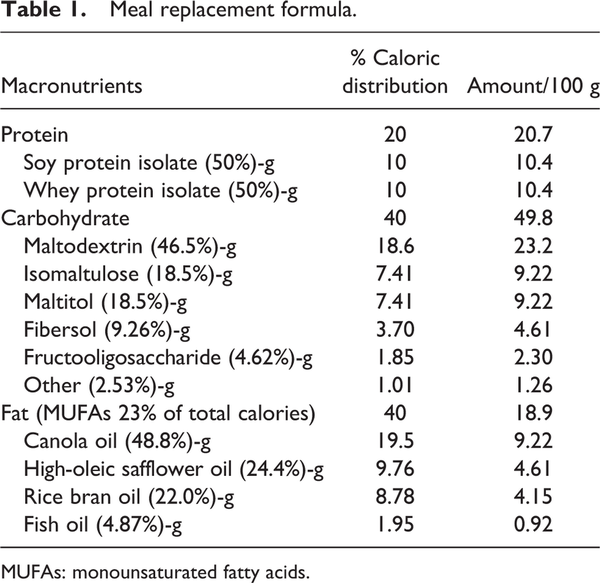
The MR formula was developed as a suitable macronutrient proportion for glycemic and metabolic control with a GI of 27.99 (, ). The ratio of carbohydrate:protein:fat is 40:20:40, respectively. Sources of carbohydrate were maltodextrin, isomaltulose and maltitol. Isomaltulose effects include slowing glucose absorption and stabilizing the glucose level and insulin level to avoid blood sugar spikes (; ). Therefore, the body stores a more balanced and prolonged energy source in glucose format. Being low-insulinemic, isomaltulose also supports the improvement of fat oxidation during physical activity, as high insulin levels hinder the use of lipids as an energy source. Moreover, soluble fiber was added in the formula for its benefits in blood glucose level stabilization, insulin sensitivity and lowering cholesterol (; ; ). Fat composition was selected from highly polyunsaturated fatty acids; eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). As recommended by the European Association for the study of Diabetes (EASD), canola oil, high oleic-safflower oil, rice bran oil and fish oil were opted as qualified fat sources (). Monounsaturated fatty acid (MUFA)-rich oil sources, such as canola oil, high oleic-safflower oil and rice bran oil were also recommended in a healthy diet. Several studies reported that MUFAs promote glycemic control and cardiovascular disease prevention, owing to better insulin sensitivity and diminishing metabolic syndrome risk factors (, ; ; ; ). also state that MUFAs can be used to improve insulin sensitivity in T2DM patients. Whey protein isolate (WPI) and soy protein isolate were selected as qualified protein sources. WPI can reduce the postprandial blood glucose level and is considered a suitable ingredient for patients with DM (; ), whereas soy protein isolate can control the cholesterol level ().
The purpose of this study was to compare metabolic outcomes, glycemic levels (fasting plasma glucose (FPG), hemoglobin A1C (HbA1c) and 2-hour postprandial glucose (2-hour PPG)), lipid profiles, body weight (BW), body mass index (BMI) and waist circumference (WC), between a group of patients receiving therapy with the new MR formula (ONCE PRO) and a group of patients receiving a normal controlled diet.
Materials and methods
Study design
This multicenter, open-label, randomized controlled study was conducted among patients with T2DM recruited from the outpatient departments of six university hospitals in Thailand (Phramongkutklao Hospital, Ramathibodi Hospital, Siriraj Hospital, Maharaj Nakorn Chiang Mai Hospital, Srinagarind Hospital and Songklanagarind Hospital) from December 2014 to October 2015. The study was reviewed and approved by relevant Institutional Review Boards or Independent Ethics Committees then conducted according to Good Clinical Practices, the Declaration of Helsinki and its subsequent amendments and national regulations. The study was registered with http://www.clinicaltrials.in.th, primary site ID: TCTR20131206002.
Study participants
Individuals were enrolled in the study if they met the following four criteria: an age of 18 years or older; a HbA1c level of 7–9%, a diagnosis of T2DM at least 6 months prior to enrollment; and a history of taking the same antihyperglycemic agents and lipid-lowering agents (if required) at stable doses for at least 3 months prior to enrollment. Certain individuals who met the study’s exclusion criteria were excluded from the study for the following reasons: a history of severe hypoglycemia (neuroglycopenia); severe complications (e.g., amputation or diabetic coma), gastrointestinal dysfunction (e.g., short bowel syndrome or colectomy); cardiovascular diseases (e.g., ischemic heart disease, stroke or history of heart failure up to 6 weeks before enrollment); abnormal laboratory values, including hemoglobin <10 g/dL, a white blood cell count <3 × 106/μL, a platelet count <100 × 106/μL, aspartate aminotransferase or alanine aminotransferase >3 times the upper limit of normal, total bilirubin >2 times the upper limit of normal or a glomerular filtration rate <60 mL/min/1.73m2 according to the modification of diet in the renal disease formula), psychiatric disorders; alcoholism; drug addiction; active thyroid dysfunction; malignancy within 5 years before enrollment; pregnancy or lactation or refusal to use an effective birth control method; the use of herbal products; taking corticosteroids (except topical steroids) for at least seven consecutive days or other investigational medication up to 4 weeks before the date of enrollment; and allergy to WPI, soy protein isolate or fish oil.
Study procedure
After informed consent was received, the participants were assigned a number in ascending order that corresponded to their admission in the study. The research team assigned the participants to be randomized in balanced blocks, generated by a statistician, with an equal probability of being assigned to the group that received MR for one meal per day plus normal controlled diets for their other meals (the MR group) or a group that ate only a normal controlled diet (the control group). The two groups followed the prescribed diets for 90 days. The participants in the MR group were given advice on how to prepare the MR, which contained 30% of the total daily energy requirement: mix the dry powder with drinking water and consume it as a replacement for one meal per day.
The MR product (called ONCE PRO) is a novel formula developed by the Thai Otsuka Pharmaceutical Company Limited (, ). ONCE PRO is a water soluble powder with a low GI (27.3) and the appropriate caloric distribution of carbohydrate, protein and fat (40%, 20% and 40%, respectively), improving glycemic control in DM patients (; ; , ) (Table 1). The intention of particular fat type and content, in this formula, is specifically to delay gastric emptying, which contributes to limitation of blood glucose fluctuations().
All participants received a dietary record and individual dietary counseling sessions from trained nutritionists/dieticians of each hospital at baseline. The nutritional goal was to achieve 25–30 kcal/kg/day based on their ideal BW and physical activity (), with a caloric distribution of protein, fat and carbohydrate of 20%, 30% and 50%, respectively, due to expert doctors’ suggestions and recommendations from the Canadian Diabetes Association (). The ideal BW was calculated using the following equations from the Hamwi formula (): (1) 48 + 2.7 kg for each 2.5 cm over 150 cm for men and (2) 45.5 + 2.2 kg for each 2.5 cm over 150 cm for women.
During the study period, the participants periodically filled in a dietary record along with their individual dietary counseling sessions and dietary reviews for validating information. All participants were required to come to their respective hospitals at the baseline and at the end or during the study period to assess their FPG, HbA1c and lipid profiles, measured by each hospital’s central laboratory using automated analyzers of each center as well as their BW, BMI and WC. In a separate period of the study, to obtain information about the different glycemic responses to the MR and the reference formula, 2-hour PPG tests with 75 g of carbohydrate were performed in the MR group after the intake of the MR (148.9 g, 633 kcal) and reference formula (125 g, 563 kcal). Each formula was provided once on different days; the reference formula was an enteral product generally used in a hospital.
Efficacy assessment
The primary outcomes of this study were the differences in HbA1c levels between the baseline and end point (day 91) in each group and between groups. The secondary outcomes were the differences in FPG, lipid profiles, BW, BMI and WC between the baseline and end point in each group and between groups. The outcome assessments in the MR group were performed only on the participants who had followed the study’s dietary guidelines for 90 days.
Data analysis and statistical methods
The data were analyzed using per protocol analyses. All analyses were performed with SPSS version 18.0 (SPSS Inc., Chicago, IL, USA), using a two-sided test and a significance level of 0.05. The data distribution was evaluated using the Kolmogorov–Smirnov test and did not differ significantly from a normal distribution. The participants’ characteristics and study outcomes of the two groups were analyzed with a repeated-measures analysis of variance (ANOVA) for within-group comparisons and Student’s t-test and chi-square test for between-group comparisons.
The sample size was obtained using the formula developed by Rosner (). A total sample size of at least 66 (33per group) achieved 80% power to detect a mean difference of 1.3% in HbA1c. This assumed a SD of 1.88 and correlation between measures of 0.8, with the significance level of 0.05 based on a study by . To allow for a dropout rate of 20%, the total sample size for this study was 120 (60 per group).
Results
Participant baseline characteristics
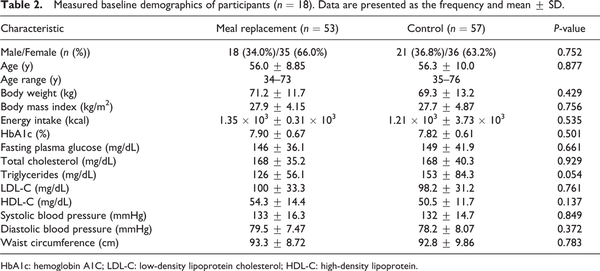
A total of 152 individuals were screened, and only 119 were eligible. Among them, nine participants discontinued participation for the following reasons: three violated the protocol; two were unwilling to continue; two had hypoglycemia, one had diarrhea; and one suffered a loss of appetite and wished to drop out. Therefore, only 110 participants completed the study, 53 and 57 participants in the MR and control groups, respectively (Figure 1). The mean age was 56 years, and the average BMI was 27.8 kg/m2. There was no statistically significantly difference in BMI, energy intake or metabolic profiles, including the HbA1c, FPG and lipid profiles, between the two groups (Table 2).
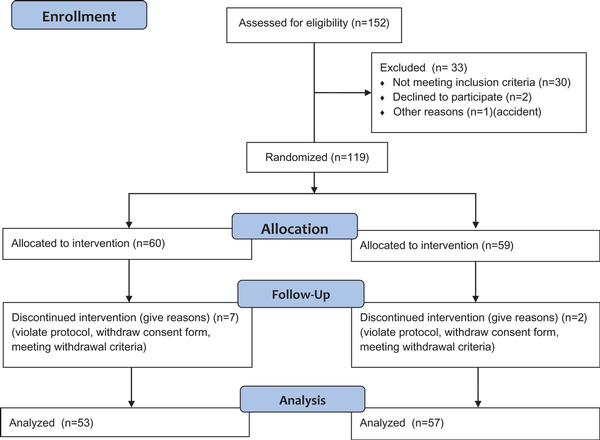
Figure 1
Study flow from recruitment and screening to the final analysis.
Changes in HbA1c level
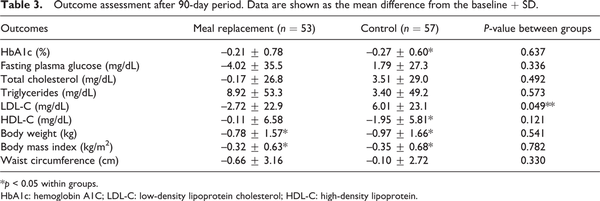
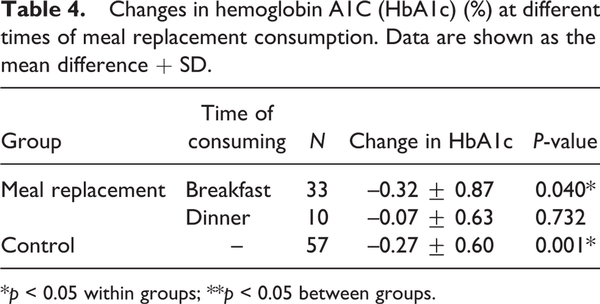
The mean HbA1c level decreased gradually from baseline in both groups; however, HbA1c reduction at 3-month treatment was statistically significant only in the control group from 7.82 ± 0.61 to 7.55 ± 0.79, p = 0.001. In the MR group, the HbA1c level also decreased from 7.90 ± 0.67 to 7.69 ± 0.74, but it was not significantly different. Likewise, there was no significant difference in HbA1c levels between the two groups (p = 0.637) (Table 3). In the subgroup analysis, we found that only participants who consumed a MR for breakfast had a significant reduction in HbA1c compared with the baseline (p = 0.040) (Table 4).
Changes in 2-hour PPG
A statistically significant difference (p < 0.001) in 2-hour PPG levels between the intake of the MR and the reference formula was analyzed. Two-hour PPG levels after consumption of the reference formula increased by 111 mg/dL from 147 ± 33.3 to 258 ± 64.0 mg/dL, whereas there was a lower increase of 2-hour PPG of 71.5 mg/dL from 150 ± 34.2 to 222 ± 50.8 mg/dL after consumption of the MR (Figure 2).
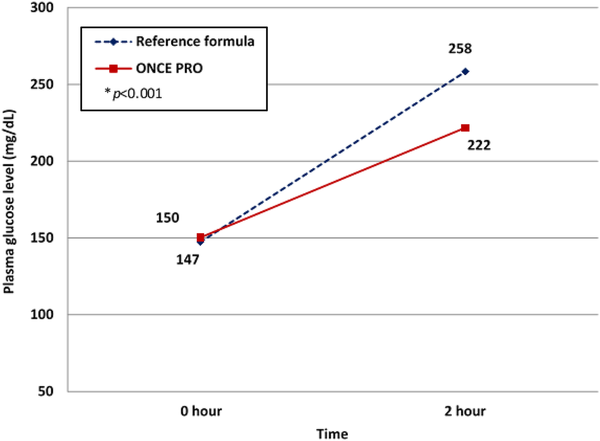
Figure 2
Two-hour postprandial plasma glucose level after consumption of 75 g of glucose through the reference formula (blue) and the meal replacement (red). (Color online only.)
Changes in BW and BMI
BW and BMI decreased significantly at 3-month treatment in both groups: 0.78 and 0.97 kg for BW and 0.32 and 0.35 kg/m2 for BMI in the MR and control groups, respectively. However, there were no statistically significant differences in BW and BMI between the two groups after the treatment (Table 3).
Changes in lipid profiles
LDL-C level decreased (–2.72 mg/dL) in the MR group but increased (+6.01 mg/dL) in the control group. In addition, the difference in LDL-C between groups was marked as significant (p = 0.049) (Table 3). High-density lipoprotein (HDL-C) decreased in both the MR and control groups, but only in the control group was this shown to be statistically significant.
In this study, total cholesterol decreased in the MR group, while in comparison it increased in the control group. Triglycerides were reported to have increased but did not reach statistical significance either within or between groups.
Discussion
Achieving an optimal HbA1c level is fundamental to T2DM management because it is associated with microvascular complication reduction (). Moreover, weight loss from lifestyle modification has been shown to result in significant improvement in glycemic control, blood pressure and lipid profiles (. ONCE PRO is effective to lose weight and is beneficial for HbA1c reduction in those having a MR for breakfast.
This study demonstrated that a diet, either MR therapy or a controlled meal plan, with the nutritional goal of 25–30 kcal/kg/day based on ideal BW and physical activity, and a balanced caloric distribution of protein, fat and carbohydrate of 20%, 30% and 50%, respectively, could significantly reduce participants’ BW and BMI. Satisfactory HbA1c control and WC reduction were shown in both groups, yet the change of HbA1c was statistically significant only in the control group. The mean reduction of FPG was approximately 4 mg/dL in the MR group; on the contrary, it increased by 1.79 mg/dL in the control group. Reduction in BW, glucose, insulin and HbA1c levels in the MR group compared with the control group was in accordance with the study of . In general, patients with DM are advised to have a smaller portion during dinners, compared with the others meals (). Consequently, subgroup analysis demonstrated that only the MR for breakfast group was significantly effective in HbA1c reduction.
The PPG level is another crucial outcome in T2DM management due to its relation with risks of cardiovascular diseases and other DM-related complications (; ). In the present study, the MR group showed greater capacity to control 2-hour PPG, compared with the reference formula, even though the MR provided higher caloric value. It can be concluded that low-GI carbohydrates (i.e., isomaltulose, maltitol, Fibersol and fructooligosaccharide (FOS)) in the formula benefit glycemic control. Further studies of maltodextrin and isomaltulose show similar results of reducing the glucose-uptake rate, stabilizing glucose and insulin levels and avoiding glucose spikes (). Likewise, maltitol gives lower glycemic and blood insulin responses (). Isomaltulose also promotes fat-provided energy, hence enhancing performance endurance (; ). Soluble fibers, Fibersol and fructooligosaccharides in the formula, affected gastric emptying prolongation, blood glucose fluctuation prevention, the glucagon-like peptide-1 secretion inducer and gut microbes and lipid metabolism improvement (; ; ; ). Several studies have exhibited extensive positive effects of this MR formula on glycemic levels and insulin sensitivity (; ; ; ).
Most participants have been taking lipid-lowering agents (statin) in proportions of 94.3% in the control group and 93.0% in the MR group; therefore, their LDL-C levels at baseline were in the normal range and there were no other beneficial effects, as expected, after daily intake and at the end of the study. Nevertheless, the MR group showed a statistically significant larger LDL-C reduction compared to the control group. According to , similar results were reported that MRs gave significant LDL-C reduction. Even though both groups received the same dietary counseling from nutritionists or dieticians, the results were still doubtful: an increase in LDL-C in the control group and triglyceride in both groups, in contrast to a decrease in HDL-C in the control group. These questionable results should be investigated in further studies. In the previous studies, MUFAs were mentioned to be of benefit to T2DM patients. To illustrate this, MUFAs promote insulin sensitivity, which can prevent cardiovascular disease in patients by reducing their risk factors. As a result, MUFAs in the formula present positive outcomes on total cholesterol and LDL-C lowering, while maintain the HDL-C level. WPI is a suitable protein source for DM patients due to its high content of branched-chain amino acids, which helps stabilize insulinotropic response and insulin secretion (incretin effect), resulting in PPG reduction (; ; ).
The limitation of this study is the 3-month period, which is not considered as a long-term study. In additional, a nutrition counselor should be monitored strictly in both groups in order to obtain better compliance. Nevertheless this multi-center prospective study should be considered ONCE PRO as an option in treating T2DM and the factors that are altered by MR diet should be studied in the future trial.
Conclusion
The control of the plasma glucose level in diabetic patients can be achieved by using MRs as dietary control. Three-month consumption of a low-GI MR provided significant improvement in glucose control, lipid profile, BW and BMI. In conclusion, ONCE PRO can be considered as a practical and convenient MR to help DM patients with their diet.
Acknowledgements
The authors thank the DM nurses and dietitians of Phramongkutklao Hospital, Ramathibodi Hospital, Siriraj Hospital, Maharaj Nakorn Chiang Mai Hospital, Srinagarind Hospital and Songklanagarind Hospital for data collection and patient education.
Author contributions All authors contributed equally to this work; Apussanee Boonyavarakul, Rattana Leelawattana, Chatlert Pongchaiyakul, Supawan Buranapin, Parinya Phanachet and Pornpoj Pramyothin performed the research; Apussanee Boonyavarakul contributed to editing, reviewing and final approval of article.
Availability of data and materials All data generated or analyzed during this study are included in this published article.
Consent for publication Detail as attached file.
Ethical approval The study was reviewed and approved by Ethic Committee of the Faculty of Medicine, Phramongkutklao Hospital, Price of Songkla University, The Khon Kaen University, Chiang Mai University, Ramathibodi Hospital and Siriraj Hospital Ethics Committee for Human Research.
Declaration of conflicting interests The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was sponsored by the Thai Otsuka Pharmaceutical Co., Ltd. This included study products, patient compensation and laboratory fee.
Apussanee Boonyavarakul
http://orcid.org/0000-0002-6125-8510
References
- Abbott (2014) Glucerna® 1.2 CAL. Available at: https://abbottnutrition.com/glucerna-1_2-cal (accessed 23 September).
- Allison DB, Gadbury G, Schwartz LG, et al. (2003) A novel soy-based meal replacement formula for weight loss among obese individuals: a randomized controlled clinical trial. European Journal of Clinical Nutrition 57: 514.
- American Diabetes Association (2014) Standards of medical care in diabetes—2014. Diabetes care 37(S1): S14–S80.
- American Diabetes Association (2015) 4. Foundations of care: education, nutrition, physical activity, smoking cessation, psychosocial care, and immunization. Diabetes care 38(S1): S20–S30.
- Aston LM (2006) Glycaemic index and metabolic disease risk. Proceedings of the Nutrition Society 65: 125–134.
- Bernard R (2000) Fundamentals of Biostatistics. Boston, MA: PWS Publishers, 2, pp.140–246.
- Blaak E, Antoine JM, Benton D, et al. (2012) Impact of postprandial glycaemia on health and prevention of disease. Obesity Reviews 13: 923–984.
- Boonyavarakul A (2013) The study of glycemic index of Gen-Premium. Journal of the Medical Association of Thailand= Chotmaihet thangphaet 96: 911–916.
- Brand JC, Colagiuri S, Crossman S, et al. (1991) Low-glycemic index foods improve long-term glycemic control in NIDDM. Diabetes Care 14: 95–101.
- Brown B, Roehl K, Betz M (2015) Enteral nutrition formula selection: current evidence and implications for practice. Nutrition in Clinical Practice 30: 72–85.
- Campbell SM (2006) An anthology of advances in enteral tube feeding formulations. Nutrition in Clinical Practice 21: 411–415.
- Ceriello A (2010) Point: postprandial glucose levels are a clinically important treatment target. United State of America: American Diabetes Association.
- Dworatzek PD, Arcudi K, Gougeon R, et al. (2013) Nutrition therapy. Canadian Journal of Diabetes 37: S45–S55.
- EFSA Panel on Dietetic Products and Allergies (2011) Scientific opinion on the substantiation of health claims related to resistant maltodextrin and reduction of post prandial glycaemic responses (ID 796), maintenance of normal blood LDL cholesterol concentrations (ID 2927), maintenance of normal (fasting) blood concentrations of triglycerides (ID 2927) and changes in bowel function (ID 797) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA Journal 9: 2070.
- Espeland M (2007) Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 30: 1374–1383.
- Festi D, Schiumerini R, Eusebi LH, et al. (2014) Gut microbiota and metabolic syndrome. World Journal of Gastroenterology: WJG 20: 16079.
- Floyd J, Fajans SS, Conn JW, et al. (1966) Stimulation of insulin secretion by amino acids. The Journal of Clinical Investigation 45: 1487–1502.
- Frid AH, Nilsson M, Holst JJ, et al. (2005) Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. The American Journal of Clinical Nutrition 82: 69–75.
- Hamwi G, Danowski T (1964) Diabetes Mellitus: Diagnosis and Treatment, Vol. 1. New York: American Diabetes Association.
- Heymsfield S, Van Mierlo C, Van Der Knaap H, et al. (2003) Weight management using a meal replacement strategy: Meta and pooling analysis from six studies. International Journal of Obesity 27: 537.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. (2012) Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 55: 1577–1596.
- Kamoi M, Shimizu Y, Kawauchi M, et al. (1972) Clinical experiment on maltitol metabolism. The Japanese Journal of Nutrition and Dietetics 30: 153–158.
- Katarzyna Slizewska JK, Barczynska R, Jochym K (2012) Resistant dextrins as prebiotic. Carbohydrates–Comprehensive Studies on Glycobiology and Glycotechnology 261–268.
- Keogh JB, Clifton PM (2012) Meal replacements for weight loss in type 2 diabetes in a community setting. Journal of Nutrition and Metabolism 1–7.
- Kishimoto Y, Yoshikawa Y, Miyazato S, et al. (2009) Effect of resistant maltodextrin on digestion and absorption of lipids. Journal of Health Science 55: 838–844.
- Kobyliak N, Virchenko O, Falalyeyeva T (2015) Pathophysiological role of host microbiota in the development of obesity. Nutrition Journal 15: 43.
- König D, Theis S, Kozianowski G, et al. (2012) Postprandial substrate use in overweight subjects with the metabolic syndrome after isomaltulose (Palatinose™) ingestion. Nutrition 28: 651–656.
- Kotake J, Tanaka Y, Umehara N, et al. (2004) Effects of a high-monounsaturated fat diet on glucose and lipid metabolisms in normal and diabetic mice. Journal of Nutritional Science and Vitaminology 50: 106–113.
- Kris-Etherton PM (1999) Monounsaturated fatty acids and risk of cardiovascular disease. Circulation 100: 1253–1258.
- Liljeberg H, Granfeldt Y, Björck I (1992) Metabolic responses to starch in bread containing intact kernels versus milled flour. European Journal of Clinical Nutrition 46: 561–575.
- Lina B, Jonker D, Kozianowski G (2002) Isomaltulose (Palatinose®): A review of biological and toxicological studies. Food and Chemical Toxicology 40: 1375–1381.
- Livesey G, Taylor R, Hulshof T, et al. (2008) Glycemic response and health—A systematic review and meta-analysis: The database, study characteristics, and macronutrient intakes–. The American Journal of Clinical Nutrition 87: 223S–236S.
- Ludwig DS (2002) The glycemic index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 287: 2414–2423.
- Newton L, Garvey W (2012) Nutritional and Medical Management of Diabetes Mellitus in Hospitalized Patients. The ASPEN Adult Nutrition Support Core Curriculum. 2nd ed. Silver Spring: American Society for Parenteral and Enteral Nutrition, pp. 580–602.
- Ogurtsova K, Da Rocha Fernandes J, Huang Y, et al. (2017) IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Research and Clinical Practice 128: 40–50.
- Perera PK, Li Y (2012) Functional herbal food ingredients used in type 2 diabetes mellitus. Pharmacognosy Reviews 6: 37.
- Petersen BL, Ward LS, Bastian ED, et al. (2009) A whey protein supplement decreases post-prandial glycemia. Nutrition Journal 8: 47.
- Pi-Sunyer X (2014) The Look AHEAD trial: A review and discussion of its outcomes. Current Nutrition Reports 3: 387–391.
- Ramdath DD, Padhi EM, Sarfaraz S, et al. (2017) Beyond the cholesterol-lowering effect of soy protein: A review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients 9: 324.
- Shi L, Ye X, Lu M, et al. (2013) Clinical and economic benefits associated with the achievement of both HbA1c and LDL cholesterol goals in veterans with type 2 diabetes. Diabetes Care 36: 3297–3304.
- Spence C (2017) Breakfast: The most important meal of the day? International Journal of Gastronomy and Food Science 8: 1–6.
- Tahrani AA, Piya MK, Kennedy A, et al. (2010) Glycaemic control in type 2 diabetes: Targets and new therapies. Pharmacology & Therapeutics 125: 328–361.
- Tatti P, Di Mauro P, Neri M, et al. (2010) Effect of a low-calorie high nutritional value formula on weight loss in type 2 diabetes mellitus. Mediterranean Journal of Nutrition and Metabolism 3: 65–69.
- Tetens I (2011) EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Scientific Opinion on the substantiation of a health claim related to “Appl’In® polyphenolic apple extract powder (Malus domestica)” and reduction of post-prandial glycaemic responses pursuant to Article 13 (5) of Regulation (EC) No 1924/2006. EFSA Journal, 9(10): 2383.
- Thai Otsuka Pharmaceutical Co. (2016a) ONCE PRO. Available at: https://www.thaiotsukanutrition.club/product/once-pro/ (accessed 14 October 2016).
- Thai Otsuka Pharmaceutical Co. (2016b) ONCE PRO low glycemic index medical food (accesssed 14 October 2016).
- Van Can JG, Van Loon LJ, Brouns F, et al. (2012) Reduced glycaemic and insulinaemic responses following trehalose and isomaltulose ingestion: implications for postprandial substrate use in impaired glucose-tolerant subjects. British Journal of Nutrition 108: 1210–1217.
- Vannice G, Rasmussen H (2014) Position of the academy of nutrition and dietetics: dietary fatty acids for healthy adults. Journal of the Academy of Nutrition and Dietetics 114: 136–153.
- WHO Western Pacific Region (2015) Chapter 4: Thai country case study. Available at: http://www.wpro.who.int/asia_pacific_observatory/country_comparative_studies/apo-ccs-ageing5b_chapter04.pdf
- Yang Z-H, Miyahara H, Mori T, et al. (2011) Beneficial effects of dietary fish-oil-derived monounsaturated fatty acids on metabolic syndrome risk factors and insulin resistance in mice. Journal of Agricultural and Food Chemistry 59: 7482–7489.
- Yip I, Go VLW, Deshields S, et al. (2001) Liquid meal replacements and glycemic control in obese type 2 diabetes patients. Obesity 9(S11): 341S–347S.