Staphylococcus aureus is a common cause of community- and hospital-acquired bacteremia. The estimated incidence of S. aureus bacteremia (SAB) is between 20 and 30 cases per 100 000 persons per year []. SAB is associated with a myriad of clinical syndromes, ranging from uncomplicated central line–associated bacteremia to fulminant endocarditis.
The local epidemiology and management of SAB depends on demography, health care usage, prevalence of intravenous drug use (IVDU), and prevalence of antibiotic resistance []. Use of diagnostic modalities such as echocardiography and positron emission tomography (PET)/computed tomography (CT) and infectious diseases consultation may also differ between hospitals, potentially leading to differences in identification of endocarditis and other foci of infection. All of these may vary between geographic regions and over time. As such, frequent updates are needed to inform clinicians on the manifestations and complications of SAB. However, many studies on SAB are single-center and tertiary care–based, which may reduce the external validity of epidemiological and prognostic findings [].
The reported 90-day mortality of SAB is around 30%, although recent studies have suggested that mortality is declining, possibly due to improved standards of care [, ]. As SAB often occurs in hospitalized and frail patients, part of the all-cause mortality may result from underlying comorbidities and advanced age. A recent systematic review concluded that infection-related deaths mostly occur in the first month after SAB, but they also concluded that there is a lack of rigorously conducted large studies on this subject [].
In this multicenter prospective cohort study, we describe the current epidemiology and clinical features of SAB in the Netherlands and risk factors for all-cause and infection-related mortality.
METHODS
Study Design and Setting
The Improved Diagnostic Strategies in Staphylococcus aureus bacteremia (IDISA) study was a prospective, multicenter cohort study conducted from July 1, 2017, through September 30, 2019, in 7 hospitals in the Randstad metropolitan region of the Netherlands, 2 university and 5 nonuniversity teaching hospitals. All sites had an Antimicrobial Stewardship Team providing bedside consultations for patients with SAB. The Medical Ethics Committee of the Academic Medical Centre Amsterdam approved this study (METC2017_094). This study is registered in the Netherlands Trial Register under trial code 6669.
Participants
Consecutive patients aged 18 years and older with 1 or more blood cultures positive for Staphylococcus aureus were eligible for inclusion. Patients were identified through the local hospital's microbiology service, which notified the study team after blood cultures became positive. All cases of SAB were considered clinically relevant and eligible for inclusion.
Patient Consent
A dedicated member of the study team approached patients or their legal representatives for written informed consent. Patients who died before informed consent could be obtained were included in the study as appropriate under Dutch law. Patients could enter the study only once, and subsequent episodes of SAB within the 90-day follow-up period were recorded as relapse infection.
Study Procedures
Patients were followed for up to 90 days after the first day of SAB. From the hospital electronic health records (EHRs), we collected demographic data, signs and symptoms, and microbiology, laboratory, and imaging data. No additional imaging or microbiology studies were required for the study. Status at day 90 (dead or alive) was determined through telephone follow-up, general practitioner consultation, EHR, or consultation of municipal death records.
Data extraction was performed by the study research physician (T.v.d.V.) and entered in the electronic case record file (Research Online) by trained junior researchers. The study research physician checked all data entered by the junior researchers.
Definitions
Comorbidities were classified using the Charlson comorbidity index []. Place of acquisition (community, hospital, or health care associated) was classified based on the criteria set by Friedman []. Infectious disease (ID) consultation was recorded only if an internal medicine or ID physician performed a bedside consultation. Patients who received only telephone consultation were judged not to have had a bedside consultation.
Presumed port of entry of S. aureus was the port of entry identified by the attending or consulting physician. Presumed focus of infection was the working diagnosis within 2 days after collection of the first positive blood culture. Definite focus of infection was the diagnosis according to the treating physician at the time of hospital discharge. A patient could have multiple presumed points of entry and infectious foci. For comparing infectious foci, we used the ranked approach of classifying focus introduced by Kaasch []. The presence of infective endocarditis was defined using the modified Duke Criteria [].
Sepsis and septic shock were defined according to the Sepsis-3 criteria []. For determination of the presence of sepsis and septic shock at presentation, we used the most extreme value (highest or lowest when appropriate) for temperature, systolic blood pressure, heart rate, respiratory rate, and Glasgow Coma Scale recorded within 24 hours of blood culture collection. Fever was defined as a rectal or auricular temperature >38.0°C. Persistent fever was fever >72 hours after the start of effective antimicrobial therapy.
We recorded antibiotic use for all study participants. Antimicrobial agents started before culture results were known were classified as empirical therapy, and agents started thereafter as definitive therapy. Empiric therapy that was continued after culture results became known was thereafter classified as definitive therapy. For empiric therapy, all agents are reported, and for definitive therapy we report the agent used for the largest part of the treatment.
Infection-related mortality at 90 days was scored using a 3-tier system. Mortality was considered infection-related if patients died from direct complications of infection (eg, septic brain hemorrhage, death following infection control surgery) or in case of persistent signs of infection (ongoing fever, persistent positive blood cultures, leucocytosis, elevated CRP) at the time of death. Mortality was considered non-infection-related if patients had survived to the full length of antibiotic treatment and died from a definite other cause, without signs or symptoms of relapse. Mortality events that did not fit the definitions of infection-related or non-infection-related were considered possibly infection-related.
Infection-related mortality was determined by an adjudication committee of 2 independent infectious disease specialists (J.M., A.G., B.L., K.St., K.Si., V.S.). Discrepancies between 2 panel members were discussed with the study research physician (T.v.d.V.) until consensus was reached; if no consensus could be reached, a third infectious disease specialist was consulted.
Analysis
Clinical features of SAB were reported using the appropriate descriptive statistics for normally and non–normally distributed variables, for the total cohort and for community-acquired, health care–associated, and hospital-acquired bacteremia separately.
For all-cause and infection-related mortality, we constructed Kaplan-Meier curves for each dominant focus of infection [] and determined the predictive value of known risk factors for mortality []. The relationship between risk factors and all-cause and infection-related mortality was examined using univariate and multivariate logistic regression. For the multivariate models, only variables with a univariate P value <.1 were entered into the model. Because of the prospective nature of the data collection, there were few missing data, and for variables with missing data (mostly laboratory values), we assumed missing values were normal. No imputation of missing data was performed.
This study is reported using the STROBE guidelines for reporting of observational studies [].
Statistical significance was tested at a 2-sided P value of .05, and 95% CIs are reported for all inferential statistics. All statistical analyses were done in R, version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Inclusion
Between August 2017 and September 2019, 636 patients with SAB were screened, and 490 (77%) were included in the study. Reasons for exclusion were: patient discharged home before consent was possible (46/146), refusal to provide informed consent (29/146), and incapacitated patients without a legal representative (22/146). There were no significant differences in the median age or sex of the nonincluded patients. Detailed follow-up data were available for 489 (99%) patients, and mortality data were available for all.
Demographics
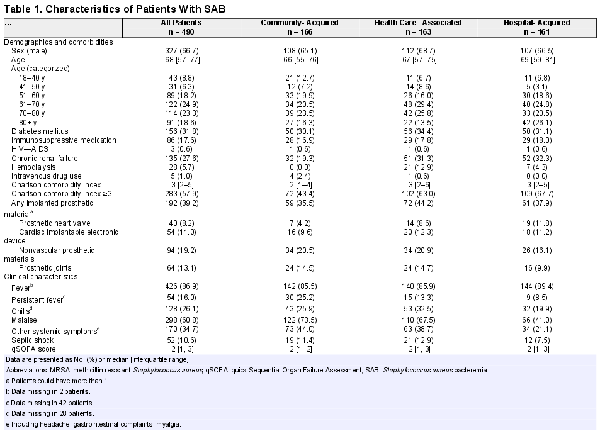
The median patient age (interquartile range [IQR]) was 68 (57–77) years, and 327 (66.7%) were male. Community-acquired, health care–associated, and hospital-acquired bacteremia were equally prevalent. Intravenous drug use and methicillin-resistant Staphylococcus aureus (MRSA) bacteremia were rare (1% and 2%, respectively). Permanent implanted prosthetic material (eg, prosthetic joint, heart valve, pacemaker) was present in 192 patients (39.2%). An overview of demographic and clinical characteristics is shown in Table 1.
Clinical Characteristics
Fever was present in 426 patients (86.9%). The median duration of fever before collection of the first blood culture (IQR) was 0 (0–1) days. Overall, patients with community-acquired SAB had a longer duration of symptoms before blood cultures were collected compared with hospital- and health care–associated SAB (median, 2 days vs 0 days and 1 day; P < .001) (Supplementary Table 1). Septic shock at presentation was present in 52 patients (10.6%). A presumed port of entry was found in 344 patients (70.2%), but in only 82 of 166 patients (49.4%) with community-acquired SAB. The most common port of entry was the skin (peripheral or central venous catheter or skin or soft tissue infection [SSTI] and surgical site infection): 244 patients (49.8%). At least 1 presumed diagnosis (presumed focus of infection) could be identified in the first 2 days after the first blood culture was taken in 391 patients (79.8%) (Supplementary Table 1).
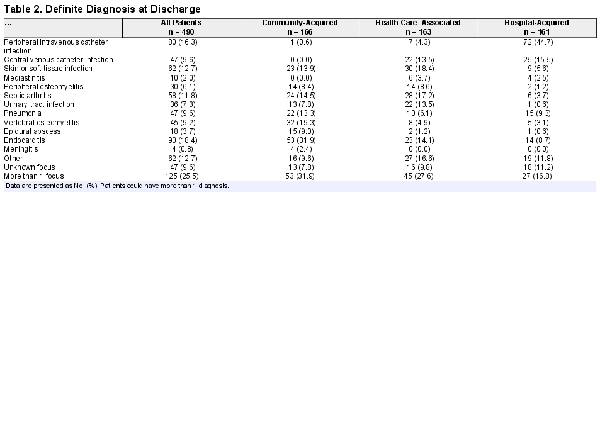
At discharge, at least 1 diagnosis (focus of infection) was reported in 443 patients (90.4%). Table 2 provides the final diagnoses reported at discharge. Endocarditis (n = 90, 18.4%) was the most frequent definite diagnosis, followed by peripheral intravenous catheter infection (n = 80, 16.3%), SSTI (n = 62, 12.7%), and septic arthritis (n = 58, 11.8%). In 125 patients (25.5%), 2 or more infectious foci were identified, while in 47 patients (9.6%) no focus of infection could be determined. In 216 patients (44.1%), S. aureus was also cultured from body sites, such as the respiratory tract (n = 50, 10.2%) and urine (n = 65, 13.3%). Of the 36 patients (7.3%) deemed by the treating physician to have S. aureus UTI, 31 had a urine culture positive for S. aureus, while the remaining 5 had an invasive procedure involving the urinary tract in the days preceding bacteremia.
Endocarditis (53/166, 31.9%), vertebral osteomyelitis (32/166, 19.3%), and septic arthritis (24/166, 14.5%) were the most frequent diagnoses in patients with community-acquired SAB. In health care–associated SAB, SSTIs (30/163, 18.4%) and septic arthritis (28/163, 17.2%) were most frequent, while in hospital-acquired SAB peripheral intravenous catheter infection (72/161, 44.7%) and central venous catheter (CVC) infection (25/161, 15.5%) were most common.
In the 125 patients who acquired SAB from a peripheral intravenous (IV) or CVC, 8 (6.4%) had endocarditis, 5 of whom had a predisposing heart condition for endocarditis.
Other bacteria apart from S. aureus were found in blood cultures from 63 patients (12.8%), the majority of which were coagulase-negative staphylococci (n = 23) and other skin contaminants (n = 15).
Management of Patients With SAB
Bedside infectious diseases consultation was performed in 385 patients (78.6%) and withheld in 18 (3.7%) patients who died or were on palliative care before consultation could be performed. TTE and TEE were performed in 409 (83.5%) and 201 patients (41.0%), respectively, and 188 (38.3%) had both a TTE and a TEE. 18-FDG-PET/CT was performed in 178 patients (36.3%). Empirical treatment, before culture results were known, consisted most frequently of a cephalosporin (n = 294, 60.0%) or flucloxacillin (n = 65, 13.3%), and 77 patients (15.7%) received adjunctive aminoglycosides. Definitive therapy consisted of flucloxacillin (396 patients, 80.8%), cefazolin (40 patients, 8.2%) vancomycin (19 patients, 3.9%), and other (35 patients, 7.1%).
Mortality and Risk Factors for Mortality
All-cause mortality was 9.8%, 15.3%, 23.7%, and 32.9% at days 7, 14, 30, and 90 days since SAB, respectively. At 90 days after SAB, 162 patients had died, and death was adjudicated as infection-related in 97 (59.9%), as non-infection-related in 29 (17.9%), and as possibly infection-related in 36 patients (22.2%). Infection-related mortality mostly occurred early in the disease phase, while the majority of non-infection-related or possibly infection-related deaths happened after 4 weeks (Figure 1). The majority of non-infection-related deaths were due to multimorbidity (24 patients) malignancy (14 patients), and other infections (11 patients). Inter-rater agreement for adjudicating infection-related mortality was good (Cohen's Kappa 0.64 for identifying infection-related mortality and 0.82 for identifying non-infection-related mortality). Mortality rates for infection-related, possibly infection-related, and non-infection-related mortality at various time points and for different foci of infection are presented in Supplementary Tables 2 and 3. Infection-related mortality plateaued after 30 days for all foci except for endocarditis and was highest for patients with endocarditis (43.3%) and lowest for patients with a CVC-related infection (7.5%) (Figure 2).
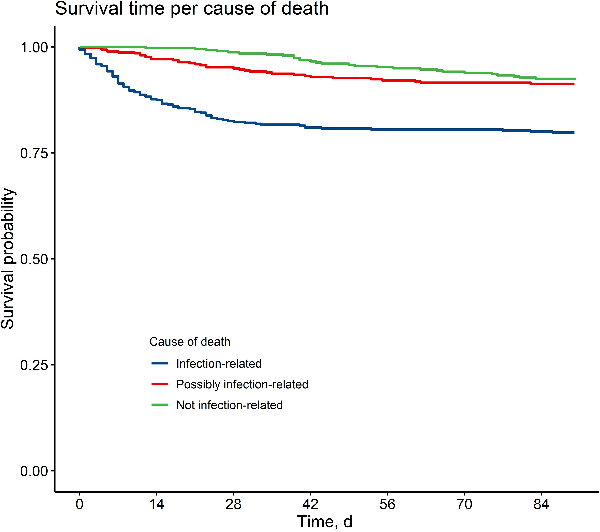
Figure 1
Time to death per cause of death.
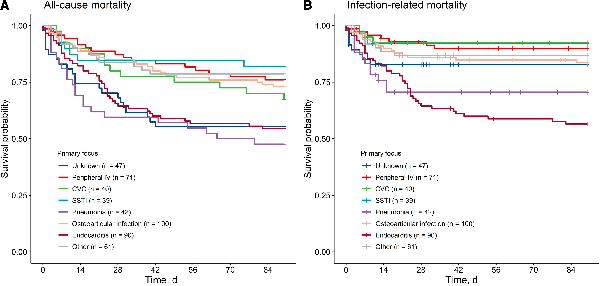
Figure 2
Kaplan-Meier curves for all-cause (A) and infection-related (B) mortality, stratified by dominant focus of infection. Abbreviations: CVC, central venous catheter; SSTI, skin and soft tissue infection.
Risk Factors for Mortality
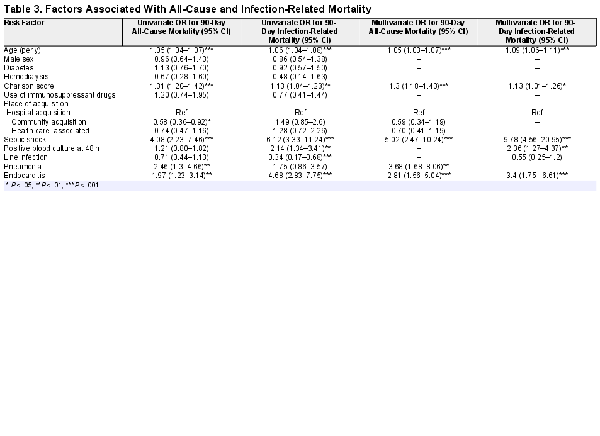
Age, comorbidity, presence of endocarditis, and septic shock were all independently predictive of both 90-day all-cause and infection-related mortality (Table 3). Persistent SAB at 48 hours was predictive of infection-related mortality. Despite being associated with more severe manifestations of SAB, such as endocarditis and vertebral osteomyelitis, community acquisition was not associated with higher all-cause or infection-related mortality. Pneumonia as the dominant focus of infection was associated with worse all-cause mortality, but not with increased infection-related mortality. Associations between other determinants tested, such as gender, diabetes, hemodialysis dependence, and use of immunosuppressants, and 90-day mortality did not reach statistical significance.
DISCUSSION
Key Findings
In this prospective cohort study, we describe the current epidemiology and outcomes of SAB in the Netherlands. Permanent implanted prosthetic material (eg, prosthetic joint, heart valve, pacemaker) was present in 39% of patients, and one-third of cases were hospital-acquired. Metastatic infection (eg, endocarditis) was more common in the patients with non-hospital-acquired SAB. All-cause mortality 90 days after the first day of SAB was 33%; 60% of these deaths were adjudicated as directly related to SAB, and almost all these deaths occurred in the first 30 days after SAB. Age, comorbidities, presence of endocarditis, and septic shock were independently predictive of 90-day all-cause and infection-related mortality.
Study in Context
In general, our cohort matches the epidemiological profile of other recently published cohorts of patients with SAB from high-income countries [, , ], though regional differences exist. For example, the proportion of patients with nosocomial acquisition of SAB was 33% in our cohort, similar to the 32% reported in a recent Swedish study (32%), which was lower than the reported 54% in the French VIRSTA cohort [, ]. Moreover, proportions of patients with health care–associated SAB appear to be higher in recent studies from the United States [, ]. The prevalence of both MRSA and IV drug use is low in the Netherlands and Nordic countries [, , ], but higher elsewhere, which influences antibiotic treatment options and potentially patient outcomes. Other demographic and clinical factors are remarkably consistent across different cohorts, such as the high median age, predominance of males, the dominance of skin lesions or intravenous catheters as the source of infection, and the frequent occurrence of metastatic complications such as endocarditis and vertebral osteomyelitis [, , ]. Apart from regional differences, the epidemiology of SAB changes over time. In a recent single-center study from a university hospital in the United States, prevalence of implanted prosthetic materials increased over time, as did the proportion of patients with health care–associated SAB []. Also in our cohort, 39% of patients had permanently implanted prosthetic material, which is considerably higher than reported in studies from earlier time periods [, ].
The prevalence of endocarditis was 18.3%, which may result from the high proportion of patients who underwent echocardiography (>80%) and from some selection bias, as 7% of patients eligible for inclusion were discharged before informed consent could be obtained. Such patients are less likely to have endocarditis. Although one-fourth of SAB episodes were associated with the presence of peripheral IV or CVC, infection-related mortality in these patients was low, at 10% and 8%, respectively. However, as these infections are potentially preventable, the presence of infection-related mortality, albeit lower than in SAB episodes originating from other sources, underscores the importance of infection control measures []. A notable 7.3% of patients with SAB were also diagnosed with urinary tract infection, a prevalence comparable to CVC infections (9.6%), peripheral osteomyelitis (6.1%), and vertebral osteomyelitis (9.2%), all of which are widely considered important foci of S. aureus infection. S. aureus UTI is associated with structural defects or prosthetic material in the urinary tract, but bacteriuria can in some cases also result from hematogenic seeding []. The optimal management of S. aureus UTI deserves further study.
At 90 days, overall mortality was 33%, which is consistent with other recent studies on SAB mortality [, , ]. Infection-related mortality ranged between 46% and 80% in other studies, but definitions applied also differed between studies [, , , ]. In our cohort, 60% of deaths were considered infection-related. In some patients, the role of SAB in causing death may be ambiguous, which is a possible explanation for the varying proportions of infection-related mortality reported. We have quantified this uncertainty by adding a possibly infection-related mortality classification, which was applicable to 20% of all patients who died from SAB within 90 days. The majority of infection-related deaths occurred early in the disease: At 30 days, 74% of deaths were infection-related. After 30 days, infection-related deaths were rare and occurred almost exclusively in patients with endocarditis. The majority of deaths after day 30 were unrelated to infection. Our findings, therefore, strongly support those from a systematic review on attributable mortality in SAB []. That analysis also indicated that the majority of deaths >1 month after SAB were not infection-related and likely not preventable by an intervention that improves the outcome of SAB. Treatment studies could, therefore, limit follow-up for all-cause mortality to 1 month, thereby reducing noise and loss of statistical power []. Yet, an important caveat is endocarditis, in which 18% (7/39) of infection-related deaths occurred after 30 days. Therefore, for endocarditis, longer follow-up is needed if the intervention continues after 4 weeks, as is the case in trials of treatment duration or switch to oral therapy. A 30-day follow-up period for mortality, however, may be considered when determining the effect of short-term interventions, such as choice of empirical therapy, short-term adjunctive (antimicrobial) treatment, and other interventions in the early phase of disease.
We confirmed known risk factors such as age, comorbidities, septic shock, and endocarditis on all-cause mortality and demonstrated that these risk factors were likewise risk factors for infection-related mortality, although the effects of septic shock and presence of endocarditis appeared more pronounced on infection-related mortality.
Strengths and Limitations
The strengths of this study are the prospective multicenter design with few missing data. We recruited patients in both university and nonuniversity hospitals, which increases the external validity of our data. The main limitation of this study is that not all patients with SAB were included, as 5% of eligible patients refused informed consent and 7% were discharged home before informed consent could be obtained. The latter group may introduce some bias, as these patients were more likely to have an uncomplicated disease course. As such, it is possible that the mortality rates mortality we found are slightly overestimated, but since this was only a small proportion of eligible patients, this is unlikely to be a large source of bias. Finally, adjudication of infection-related mortality is difficult, and we cannot rule out that in some patients with infection-related death the final cause of death was not uncontrolled infection but the deterioration of the patients’ underlying comorbidities as a result of the infection.
CONCLUSIONS
Mortality of SAB remains high, and the majority of infection-related deaths occur within the first month, with the exception of endocarditis. Future studies may consider using 28-day or 30-day mortality as an end point for treatment interventions in patients with SAB without evidence of endocarditis.
Acknowledgments
We thank Abraham (Bram) Goorhuis, Bregtje Lemkes, Cornelis (Kees) Stijnis, Kim Sigaloff, and Veroniek (Niekie) Spoorenberg for their help in adjudicating infection-related mortality.
Financial support. This work was supported by the Universitair Medisch Centrum Utrecht and the Academic Medical Center Amsterdam.
References
- 1. van Cleef BA, van Benthem BH, Haenen AP, Bosch T, Monen J, Kluytmans JA. Low incidence of livestock-associated methicillin-resistant Staphylococcus aureus bacteraemia in the Netherlands in 2009. PLoS One2013; 8:e73096.
- 2. Jokinen E, Laine J, Huttunen R, et al Trends in incidence and resistance patterns of Staphylococcus aureus bacteremia. Infect Dis (Lond)2018; 50:52–8.
- 3. Thorlacius-Ussing L, Sandholdt H, Larsen AR, Petersen A, Benfield T. Age-dependent increase in incidence of Staphylococcus aureus bacteremia, Denmark, 2008–2015. Emerg Infect Dis2019; 25:875–82.
- 4. Kaasch AJ, Barlow G, Edgeworth JD, et al Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Infect2014; 68:242–51.
- 5. Bai AD, Lo CK, Komorowski AS, et al Staphylococcus aureus bacteremia mortality: a systematic review and meta-analysis. Clin Microbiol Infect2022; 28:1076–84.
- 6. Le Moing V, Alla F, Doco-Lecompte T, et al Staphylococcus aureus bloodstream infection and endocarditis—a prospective cohort study. PLoS One2015; 10:e0127385.
- 7. Austin ED, Sullivan SS, Macesic N, et al Reduced mortality of Staphylococcus aureus bacteremia in a retrospective cohort study of 2139 patients: 2007–2015. Clin Infect Dis2020; 70:1666–74.
- 8. Bai AD, Lo CKL, Komorowski AS, et al What is the optimal follow-up length for mortality in Staphylococcus aureus bacteremia? Observations from a systematic review of attributable mortality. Open Forum Infect Dis2022; 9:XXX–XX.
- 9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis1987; 40:373–83.
- 10. Friedman ND, Kaye KS, Stout JE, et al Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med2002; 137:791–7.
- 11. Li JS, Sexton DJ, Mick N, et al Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis2000; 30:633–8.
- 12. Singer M, Deutschman CS, Seymour CW, et al The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA2016; 315:801–10.
- 13. van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev2012; 25:362–86.
- 14. von Elm E, Altman DG, Egger M, et al Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ2007; 335:806–8.
- 15. Ariaans M, Roovers EA, Claassen MAA, Hassing RJ, Swanink CMA, Gisolf EH. Increased overall survival after introduction of structured bedside consultation in Staphylococcus aureus bacteraemia. Eur J Clin Microbiol Infect Dis2018; 37:1187–93.
- 16. Bai AD, Showler A, Burry L, et al Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis2015; 60:1451–61.
- 17. Kuehl R, Morata L, Boeing C, et al Defining persistent Staphylococcus aureus bacteraemia: secondary analysis of a prospective cohort study. Lancet Infect Dis2020; 20:1409–17.
- 18. Kahn F, Resman F, Bergmark S, et al Time to blood culture positivity in Staphylococcus aureus bacteraemia to determine risk of infective endocarditis. Clin Microbiol Infect2020; 27:1345.e7–12.
- 19. Palraj BR, Baddour LM, Hess EP, et al Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis2015; 61:18–28.
- 20. Souli M, Ruffin F, Choi SH, et al Changing characteristics of Staphylococcus aureus bacteremia: results from a 21-year, prospective, longitudinal study. Clin Infect Dis2019; 69:1868–77.
- 21. Rasmussen RV, Host U, Arpi M, et al Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: the value of screening with echocardiography. Eur J Echocardiogr2011; 12:414–20.
- 22. Cuijpers ML, Vos FJ, Bleeker-Rovers CP, et al Complicating infectious foci in patients with Staphylococcus aureus or Streptococcus species bacteraemia. Eur J Clin Microbiol Infect Dis2007; 26:105–13.
- 23. Fowler VGJ, Olsen MK, Corey GR, et al Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med2003; 163:2066–72.
- 24. Marschall J, Mermel LA, Fakih M, et al Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol2014; 35:753–71.
- 25. Mermel LA. Short-term peripheral venous catheter-related bloodstream infections: a systematic review. Clin Infect Dis2017; 65:1757–62.
- 26. Russell CD, Morris AK. Preventing peripheral venous catheter-related Staphylococcus aureus bacteraemia. Br J Hosp Med (Lond)2017; 78:666–7.
- 27. Al Mohajer M, Musher DM, Minard CG, Darouiche RO. Clinical significance of Staphylococcus aureus bacteriuria at a tertiary care hospital. Scand J Infect Dis2013; 45:688–95.
- 28. Grillo S, Cuervo G, Carratala J, et al Characteristics and outcomes of Staphylococcus aureus bloodstream infection originating from the urinary tract: a multicenter cohort study. Open Forum Infect Dis2020; 7:XXX–XX.
- 29. Ekkelenkamp MB, Verhoef J, Bonten MJ. Quantifying the relationship between Staphylococcus aureus bacteremia and S. aureus bacteriuria: a retrospective analysis in a tertiary care hospital. Clin Infect Dis2007; 44:1457–9.
- 30. Saunderson RB, Gouliouris T, Nickerson EK, et al Impact of routine bedside infectious disease consultation on clinical management and outcome of Staphylococcus aureus bacteraemia in adults. Clin Microbiol Infect2015; 21:779–85.
- 31. Lesens O, Methlin C, Hansmann Y, et al Role of comorbidity in mortality related to Staphylococcus aureus bacteremia: a prospective study using the Charlson weighted index of comorbidity. Infect Control Hosp Epidemiol2003; 24:890–6.