Urinary tract infection (UTI) refers to a plethora of clinical phenotypes, including cystitis, pyelonephritis, prostatitis, urosepsis, and catheter-associated UTI (CA-UTI) [, ]. In both clinical practice and in research, the diagnosis of UTI is based on a multitude of clinical signs and symptoms and diagnostic tests. Signs and symptoms can be further subdivided into (1) lower urinary tract symptoms, such as dysuria, frequency, and urgency; (2) systemic signs and symptoms, such as fever; and (3) nonspecific signs and symptoms, such as nausea and malaise. Commonly used diagnostic tests include urine dipstick for determining the presence of leukocyte esterase and nitrites, microscopy or flow cytometry for quantification of pyuria, and urine and blood cultures.
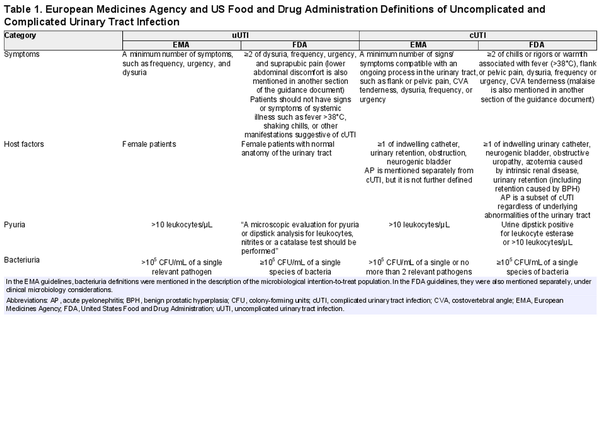
When defining and diagnosing UTI, numerous combinations of signs, symptoms, and outcomes of diagnostic tests are possible, and this diversity is reflected in various research guidelines. For drug development and approval purposes, the European Medicines Agency (EMA) [] and US Food and Drug Administration (FDA) [, ] have developed guidelines for clinical trials evaluating antimicrobials for the treatment of UTI, summarized in Table 1. These guidelines provide definitions for uncomplicated UTI, complicated UTI, and acute pyelonephritis. McGeer et al [] have developed research guidelines for studies in long-term care facilities (LTCFs). Clinical practice guidelines include the Infectious Diseases Society of America (currently being updated) [] and European Association of Urology [] guidelines. It is important to distinguish between research guidelines and clinical practice guidelines as the latter are meant for treatment recommendations, and the definitions in these clinical guidelines are generally based on often limited diagnostic information available when assessing a patient in the clinical, near-patient setting.
While the aforementioned research guidelines overlap in the sense that they all include a combination of symptoms and evidence of pyuria and/or bacteriuria in the definition of UTI, they also differ. For instance, none of these guidelines include the same set (or minimum number) of symptoms for the diagnosis of UTI. Moreover, the definition of complicated UTI is variable and based on either systemic signs and symptoms or the presence of host factors predisposing the patient to a complicated clinical course (eg, functional or anatomical abnormalities of the urinary tract).
It is probable that this wide range of possible definitions and different research guidelines pose problems for researchers conducting studies with patients with UTI. A uniform research definition increases homogeneity between studies, which is important for the interpretation, synthesis, and comparability of results, and mitigates the risk of misclassification bias. This is especially relevant in an era of rising antimicrobial resistance, in which novel antimicrobials are being investigated in large randomized controlled trials. The aim of this systematic review is to evaluate how UTI is defined in current studies, and to which extent these definitions differ between studies.
METHODS
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 guidelines [].
Eligibility Criteria
Studies published between January 2019 and May 2022, investigating any therapeutic or prophylactic intervention in adults with (recurrent) UTI, were eligible for inclusion. Given the fact that definitions tend to change over time, this time frame was chosen to reflect the most recent consensus. In addition, updated FDA and EMA guidelines were published in 2019. We excluded studies concerning only prostatitis, CA-UTI, pericatheter or perioperative prophylaxis, or asymptomatic bacteriuria. Studies investigating patients with spinal cord injury or neurogenic bladder were also excluded, because separate UTI definitions are mostly used for patients who are unable to experience (or have altered perception of) lower urinary tract symptoms. Finally, we excluded systematic reviews, meta-analyses, and studies published in non-English-language journals
Search Strategy
Multiple electronic databases (PubMed, Embase, Web of Science, and the Cochrane library) were searched on 16 May 2022. Our search strategy was constructed by a research librarian and was based on a population, intervention, comparison, outcome (PICO)–style approach. We applied language and publication year filters as described above and used an “article” type filter for clinical trials. The complete search strategy is provided in Supplementary Material 1.
Data Extraction and Analysis
Covidence software was used for screening and data extraction. References were imported and duplicates were removed. Title and abstract screening, full-text screening and data extraction were performed by 2 independent reviewers (M. P. B. and R. M. H. J.). In case of disagreement, a third researcher was consulted (M. M. C. L.) and a final decision was based on consensus.
For each study, the following data were collected: study design, setting, population, intervention, and the type of UTI under investigation. Criteria for the definition of UTI were subdivided into 3 categories: signs and symptoms, urinalysis, and urine culture. For each of these categories, we assessed whether they were required or conditionally required (ie, dependent on the presence of other categories) for the diagnosis of UTI. If categories were not mentioned, or if they were only required for a secondary outcome or definition, they were considered as not required. Definitions were derived from eligibility criteria unless definitions were explicitly stated elsewhere. For signs and symptoms, additional data were collected on minimum number of symptoms and symptom specification (eg, if fever and frequency were further defined). Moreover, we recorded which symptoms were part of the definition of acute cystitis, acute pyelonephritis, and UTI if a clinical phenotype was not mentioned (henceforth described as UTI–phenotype not specified). For the urinalysis category, we extracted which methods were used for determining pyuria, which cutoff values were applied, and whether nitrites were part of the UTI definition. Regarding the urine culture category, we recorded the cutoff value for colony-forming units (CFU)/mL and the maximum number of uropathogens. For all 3 categories, we assessed whether study definitions met FDA and EMA guideline requirements. Concerning complicated UTI, we collected the same components of the definition as described above, but we also assessed whether the definition was based on host factors, systemic involvement, or a combination of both. Finally, we compared definitions between studies, stratified per UTI type. No risk of bias assessment was performed as we studied definitions instead of outcomes. Data are summarized as proportions.
RESULTS
Study Selection and Study Characteristics
The study selection process is summarized in a PRISMA flowchart (Figure 1). We screened 348 reports published between January 2019 and May 2022. Studies that were excluded during title and abstract screening (n = 290) mainly involved patients with CA-UTI or conditions other than UTI (eg, interstitial cystitis), or investigated pericatheter or perioperative prophylaxis. During full-text screening, 7 non-English articles and secondary analyses of articles already included in the study using our search criteria were excluded. A total of 47 randomized controlled trials and cohort studies with a median of 145 participants were included [].
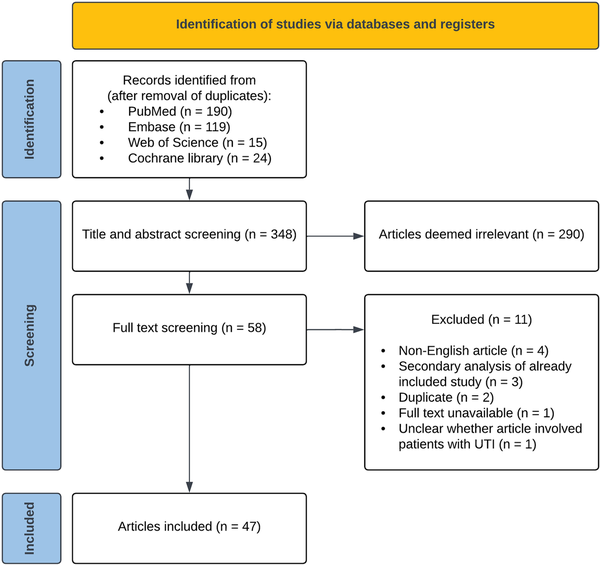
Figure 1
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of the study selection process. Abbreviation: UTI, urinary tract infection.
Thirty-one studies (66%) investigated antimicrobials for the treatment of UTI, and 15 (32%) evaluated antimicrobial prophylaxis for recurrent UTI. Sixteen studies (34%) only included women, 4 studies (9%) only included men, and 27 studies (57%) included both. Participants were hospitalized in 25 studies (53%) and treated through an outpatient or primary care clinic in 22 studies (47%). None of the included studies were conducted in LTCFs. Twelve studies (26%) included acute cystitis, 16 (34%) included acute pyelonephritis, and 13 (28%) included UTI–phenotype not specified. A table containing details of all included studies is provided in Supplementary Material 2.
UTI Definition and Heterogeneity
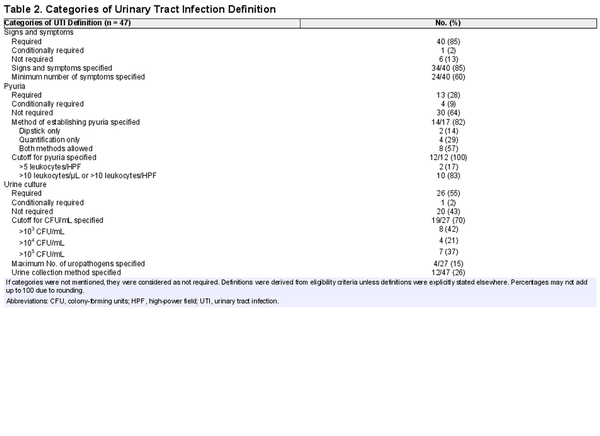
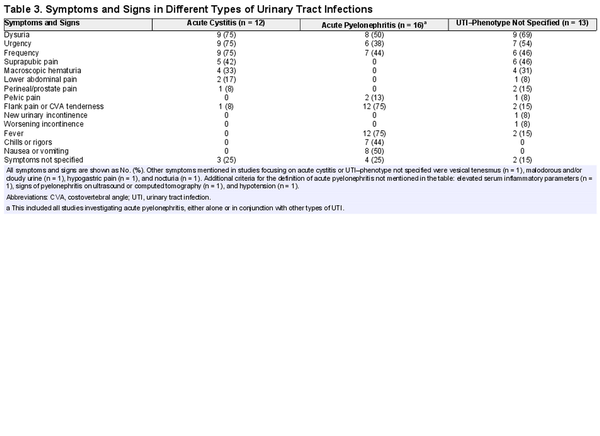
Table 2 shows how UTI was defined across the included studies. In 11 studies (23%) the definition consisted of only signs and symptoms, in 16 studies (34%) the definition consisted of both signs and symptoms and a positive urine culture, and in 5 studies (11%) all 3 components (signs and symptoms, the presence of pyuria, and a positive urine culture) were required for the diagnosis of UTI. None of the studies investigating acute cystitis (n = 12) or UTI–phenotype not specified (n = 13) included the same set of symptoms and diagnostic criteria in their definition. Of the studies defining acute pyelonephritis, 2 (17%) used identical definitions.
Signs and Symptoms
Signs and symptoms were required for the diagnosis of UTI in 40 studies (85%). Of these, 34 (85%) specified signs and symptoms in the definition. The different signs and symptoms that were included in the definition of acute cystitis, acute pyelonephritis, and UTI–phenotype not specified are highlighted in Table 3. FDA guidelines [] require a minimum of 2 of the following symptoms for patients with uncomplicated UTI: dysuria, urgency, frequency, and suprapubic pain. Two of 12 studies (17%) met these criteria. Flank pain and/or costovertebral angle tenderness, fever, nausea and/or vomiting, and dysuria were most often included in the definition of acute pyelonephritis. Frequency was not further specified in any study. Perineal and/or prostate pain was part of the definition in 3 of 31 (10%) studies involving men. A specific temperature cutoff for fever was defined in 7 of 17 (65%) studies that included fever in the definition of UTI.
Urinalysis and Urine Culture
The presence of pyuria was required for the diagnosis of UTI in 13 of 47 (28%) studies, while both FDA and EMA guidelines [] require pyuria in their definition of UTI. A cutoff for pyuria was specified in 12 studies, of which 10 (83%) applied a cutoff value of >10 leukocytes/µL or >10 leukocytes per high-power field (HPF). None of the included studies required the presence of nitrites for the diagnosis of UTI, although they were conditionally required in 3 studies (6%). A positive urine culture was mandatory for UTI diagnosis in 26 of 47 (55%) studies, of which 12 (55%) were conducted in the primary care or outpatient setting and 14 (56%) involved hospitalized patients. Of the 19 studies that mentioned a cutoff value for CFU/mL, 8 (42%) used a cutoff of 103 CFU/mL. Among all studies, 7 (15%) required a positive urine culture with at least 105 CFU/mL, complying with EMA and FDA guidelines [].
Complicated UTI
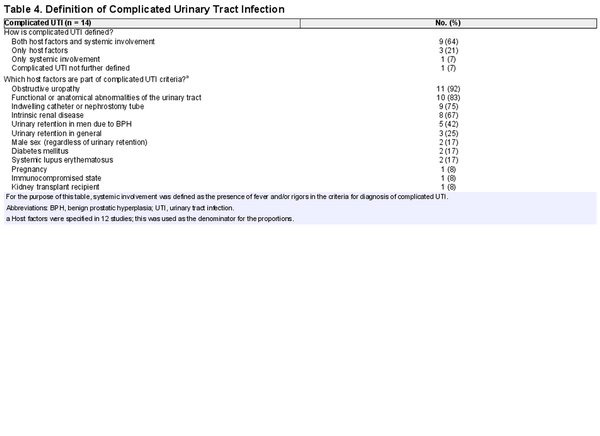
We included 14 studies that defined complicated UTI. Three (21%) based their definition on complicating host factors only, 1 (7%) on systemic involvement only, and 9 (64%) on both host factors and systemic involvement. The various host factors included in the definition are provided in Table 4. Male sex was considered a complicating factor in 2 studies (17%).
DISCUSSION
In this systematic review, we demonstrate that UTI definitions used in current research studies are highly heterogeneous in terms of clinical signs and diagnostic tests. In addition, few studies met symptom, pyuria, and urine culture criteria mentioned in existing research guidelines.
Signs and Symptoms
The presence of signs and symptoms was required in the majority of UTI definitions used in the included studies. As symptoms and signs remain the cornerstone of UTI diagnosis, it is noteworthy that 15% of studies did not require signs and symptoms for the diagnosis of UTI and an even greater number of studies did not specify which symptoms and signs needed to be present. Defining specific symptoms may help to mitigate the risk of misclassification. Symptom specification is especially relevant in studies involving older patients with UTI, given the high background prevalence of asymptomatic bacteriuria and pyuria []. Most of the studies that did clarify which symptoms were part of the UTI definition included classic UTI-associated symptoms such as dysuria, frequency, and urgency. However, we also found a broad variety of nonspecific manifestations, particularly in studies that did not define the UTI phenotype under investigation. Regardless of the unclear clinical relevance of nonspecific symptoms in UTI, this diversity of symptoms contributes to heterogeneity between studies, which is supported by our finding that few of the included studies used the same set of symptoms to define UTI. Furthermore, in over a third of the included reports, a minimum number of symptoms (for diagnosis) was not mentioned. Given the fact that even classic lower urinary tract symptoms are not 100% specific for UTI, and probability of UTI increases when a combination of symptoms is present, a minimum number of symptoms should be specified [].
Pyuria and Bacteriuria
Interestingly, less than a third of included studies required the presence of pyuria in the definition of UTI. With the exception of patients with absolute neutropenia and complete obstructive uropathy, pyuria is present in virtually all symptomatic patients with bacteriuria, and its absence has a high negative predictive value for UTI []. In the included studies, pyuria was rarely quantified and thresholds for significant pyuria were low. A recent study has shown that low pyuria cutoffs should be avoided in older women, as the specificity for UTI is very low in this population []. Moreover, studies used different units of measurement interchangeably (ie, identical thresholds were applied for cells/µL and HPF), while results are influenced by different (pre)analytical procedures and previous studies have shown a µL-to-HPF ratio of 5:1 []. Be that as it may, quantification of pyuria in UTI studies should be encouraged, and pyuria should be included in the definition of UTI to reduce the risk of misclassification.
As growth of a uropathogen supports the diagnosis of UTI in a symptomatic patient, it is surprising that a positive urine culture was not part of the UTI definition in approximately half of the included studies. Even though urine cultures are not always required in a clinical setting (eg, in primary care), we believe that culture confirmation should at least be encouraged in a research setting. Furthermore, we found that studies used varying cutoffs for significant bacteriuria, ranging from 103 to 105 CFU/mL, while EMA and FDA guidelines both recommend a threshold of 105 CFU/mL. The question remains whether this is the optimal cutoff []; colony counts as low as 102 CFU/mL in midstream urine have been found in symptomatic premenopausal woman with Escherichia coli bacteriuria [, ].
Complicated UTI
Studies differed widely in their definition of complicated UTI. Since the majority of studies defined complicated UTI based on both complicating host factors and systemic involvement, different clinical phenotypes were included in each study. This not only contributes further to disparities between studies, it also affects the applicability of study results. Moreover, the aforementioned heterogeneity is compounded by the fact that host factors are very diverse in themselves and there is no consensus about which host factors should be included in the definition of complicated UTI. As astutely phrased by James Johnson [], “it may be time to find a different term than complicated UTI for UTIs that occur in patients with underlying predisposing factors, since this term seems hopelessly mired in ambiguity.” Johansen et al []. have proposed a UTI classification system for clinical and research purposes based on clinical phenotype, severity, host factors, and pathogen susceptibility. However, this classification system was not used by any of the included studies in our review. In the Netherlands, the primary care guidelines for UTI have already made a distinction between a UTI in a complicated host versus UTI with systemic involvement [].
Existing Research Guidelines
We found that few studies met symptom, pyuria, and urine culture criteria mentioned in FDA and EMA guidelines []. In addition, we identified that studies more frequently based UTI definitions on clinical practice guidelines. The use of clinical practice guidelines in the place of research guidelines seems inappropriate, as clinical guidelines are less stringent than research guidelines and base empirical treatment recommendations on limited diagnostic information. Taken together, our findings imply that a widely accepted, consensus-based gold standard for the diagnosis of UTI is lacking and is much needed in the field of UTI research.
Strengths and Limitations
Strengths of this systematic review include our comprehensive search strategy, including multiple electronic databases, and extracting data from supplemental material, as UTI definitions were frequently only mentioned in a supplemental protocol. Our study has several limitations. For some of the included therapeutic studies, eligibility criteria served as a proxy for the UTI definition, if a definition was not mentioned separately. This might have contributed to additional heterogeneity. For instance, prophylactic studies including patients with recurrent UTI had more frequently provided separate UTI definitions, since these often served as outcome measures. Also, some heterogeneity might be explained by the fact that we included studies that investigated different UTI phenotypes. However, this effect was mitigated by evaluating different UTI phenotypes separately. Another limitation is that we filtered our search strategy on publication date and study type. While expanding the time period would have provided more data, it would not reflect the most recent consensus and would likely have contributed to further heterogeneity, as these studies were published before the FDA and EMA guidance documents. Furthermore, including more observational studies most likely would not have reduced heterogeneity, as these are presumably less likely to follow FDA and EMA guidelines for drug approval. Since we did not find any recent studies that were conducted in LTCFs, and we excluded studies regarding CA-UTI and UTI in spinal cord injury patients, it is unclear how heterogeneous definitions are in these areas. Defining UTI might be even more challenging for these populations and settings.
CONCLUSIONS
UTI definitions differ widely across recent therapeutic and interventional studies. An international consensus-based reference standard is needed to reduce misclassification bias within studies and heterogeneity between studies. To avoid ambiguity, such a reference standard should veer away from the term “complicated UTI” and instead categorize UTI based on systemic involvement, as these are different entities with different treatments. Based on results of this systematic review, our group has initiated an international consensus study to construct a UTI reference standard for research purposes.
References
- 1. Gupta K, Grigoryan L, Trautner B. Urinary tract infection. Ann Intern Med2017; 167:ITC49–64.
- 2. Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am2014; 28:1–13.
- 3. European Medicines Agency. Evaluation of medicinal products indicated for treatment of bacterial infections. 2022. Available at: https://www.ema.europa.eu/en/evaluation-medicinal-products-indicated-treatment-bacterial-infections. Accessed 25 February 2023.
- 4. US Food and Drug Administration. Uncomplicated urinary tract infections: developing drugs for treatment guidance for industry. 2019. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/uncomplicated-urinary-tract-infections-developing-drugs-treatment-guidance-industry. Accessed 25 February 2023.
- 5. US Food and Drug Administration. Complicated urinary tract infections: developing drugs for treatment. 2018. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/complicated-urinary-tract-infections-developing-drugs-treatment. Accessed 25 February 2023.
- 6. Stone ND, Ashraf MS, Calder J, et al Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol2012; 33:965–77.
- 7. Gupta K, Hooton TM, Naber KG, et al International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis2011; 52:e103–20.
- 8. Bonkat G, Bartoletti F, Bruyere F, et al EAU guidelines on urological infections. Available at: https://uroweb.org/guidelines/urological-infections. Accessed 25 February 2023.
- 9. Page MJ, McKenzie JE, Bossuyt PM, et al The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ2021; 372:n71.
- 10. Aloush SM, Al-Awamreh K, Abu Sumaqa Y, Halabi M, Al Bashtawy M, Salama FB. Effectiveness of antibiotics versus ibuprofen in relieving symptoms of nosocomial urinary tract infection: a comparative study. J Am Assoc Nurse Pract2019; 31:60–4.
- 11. Arakawa S, Kawahara K, Kawahara M, et al The efficacy and safety of tazobactam/ceftolozane in Japanese patients with uncomplicated pyelonephritis and complicated urinary tract infection. J Infect Chemother2019; 25:104–10.
- 12. Babar A, Moore L, Leblanc V, et al High dose versus low dose standardized cranberry proanthocyanidin extract for the prevention of recurrent urinary tract infection in healthy women: a double-blind randomized controlled trial. BMC Urol2021; 21:44.
- 13. Boel JB, Antsupova V, Knudsen JD, Jarløv JO, Arpi M, Holzknecht BJ. Intravenous mecillinam compared with other β-lactams as targeted treatment for Escherichia coli or Klebsiella spp. bacteraemia with urinary tract focus. J Antimicrob Chemother2021; 76:206–11.
- 14. Botros C, Lozo S, Iyer S, et al Methenamine hippurate compared with trimethoprim for the prevention of recurrent urinary tract infections: a randomized clinical trial. Int Urogynecol J2022; 33:571–80.
- 15. Bruyère F, Azzouzi AR, Lavigne JP, et al A multicenter, randomized, placebo-controlled study evaluating the efficacy of a combination of propolis and cranberry (Vaccinium macrocarpon) (DUAB®) in preventing low urinary tract infection recurrence in women complaining of recurrent cystitis. Urol Int2019; 103:41–8.
- 16. Costache RC, Novac B, Bardan TR, Agapie DN, Edu A. Xyloglucan + gelose combination versus placebo as adjuvant therapy to first-line antimicrobials for uncomplicated urinary tract infection in adults. Urol Int2019; 102:468–75.
- 17. Diebold R, Schopf B, Stammer H, Mendling W. Vaginal treatment with lactic acid gel delays relapses in recurrent urinary tract infections: results from an open, multicentre observational study. Arch Gynecol Obstet2021; 304:409–17.
- 18. Drekonja DM, Trautner B, Amundson C, Kuskowski M, Johnson JR. Effect of 7 vs 14 days of antibiotic therapy on resolution of symptoms among afebrile men with urinary tract infection: a randomized clinical trial. JAMA2021; 326:324–31.
- 19. Eckburg PB, Muir L, Critchley IA, et al Oral tebipenem pivoxil hydrobromide in complicated urinary tract infection. N Engl J Med2022; 386:1327–38.
- 20. Edlund C, Ternhag A, Skoog Ståhlgren G, et al The clinical and microbiological efficacy of temocillin versus cefotaxime in adults with febrile urinary tract infection, and its effects on the intestinal microbiota: a randomised multicentre clinical trial in Sweden. Lancet Infect Dis2022; 22:390–400.
- 21. El Nekidy WS, Abdelsalam MM, Nusair AR, et al Is cefoxitin a carbapenem sparing agent in the management of urinary tract infections caused by ESBL producing Enterobacterales? Hosp Pharm 2021; 57:568–74..
- 22. Ferrante KL, Wasenda EJ, Jung CE, Adams-Piper ER, Lukacz ES. Vaginal estrogen for the prevention of recurrent urinary tract infection in postmenopausal women: a randomized clinical trial. Female Pelvic Med Reconstr Surg2021; 27:112–7.
- 23. Gama CRB, Pombo MAG, Nunes CP, et al Treatment of recurrent urinary tract infection symptoms with urinary antiseptics containing methenamine and methylene blue: analysis of etiology and treatment outcomes. Res Rep Urol2020; 12:639–49.
- 24. Gamble KC, Rose DT, Sapozhnikov J. Intravenous to oral antibiotics versus intravenous antibiotics: a step-up or a step-down for extended spectrum beta-lactamase (ESBL)-producing urinary tract infections without concomitant bacteraemia?Int J Antimicrob Agents2022; 59:106541.
- 25. Gágyor I, Hummers E, Schmiemann G, et al Herbal treatment with uva ursi extract versus fosfomycin in women with uncomplicated urinary tract infection in primary care: a randomized controlled trial. Clin Microbiol Infect2021; 27:1441–7.
- 26. Harding C, Chadwick T, Homer T, et al Methenamine hippurate compared with antibiotic prophylaxis to prevent recurrent urinary tract infections in women: the ALTAR non-inferiority RCT. Health Technol Assess2022; 26:1–172.
- 27. Jansaker F, Thonnings S, Hertz FB, et al Three versus five days of pivmecillinam for community-acquired uncomplicated lower urinary tract infection: a randomised, double-blind, placebo-controlled superiority trial. EClinicalMedicine2019; 12:62–9.
- 28. Kaye KS, Rice LB, Dane AL, et al Fosfomycin for injection (ZTI-01) versus piperacillin-tazobactam for the treatment of complicated urinary tract infection including acute pyelonephritis: ZEUS, A phase 2/3 randomized trial. Clin Infect Dis2019; 69:2045–56.
- 29. Kohno S, Bando H, Yoneyama F, et al The safety and efficacy of relebactam/imipenem/cilastatin in Japanese patients with complicated intra-abdominal infection or complicated urinary tract infection: a multicenter, open-label, noncomparative phase 3 study. J Infect Chemother2021; 27:262–70.
- 30. Koradia P, Kapadia S, Trivedi Y, Chanchu G, Harper A. Probiotic and cranberry supplementation for preventing recurrent uncomplicated urinary tract infections in premenopausal women: a controlled pilot study. Expert Rev Anti Infect Ther2019; 17:733–40.
- 31. Li Y, Yin Y, Peng X, et al A randomized, active-controlled, multicentre clinical trial to evaluate the efficacy and safety of oral sitafloxacin versus levofloxacin in Chinese adults with acute uncomplicated or complicated urinary tract infection. Ann Med2021; 53:217–26.
- 32. Lojanapiwat B, Nimitvilai S, Bamroongya M, et al Oral sitafloxacin vs intravenous ceftriaxone followed by oral cefdinir for acute pyelonephritis and complicated urinary tract infection: a randomized controlled trial. Infect Drug Resist2019; 12:173–81.
- 33. Mir MA, Chaudhary S, Payasi A, Sood R, Mavuduru RS, Shameem M. Ceftriaxone + sulbactam + isodium EDTA versus meropenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: PLEA, a double-blind, randomized noninferiority trial. Open Forum Infect Dis2019; 6:ofz373.
- 34. Mirzaei M, Daneshpajooh A, Farsinezhad A, et al The therapeutic effect of intravesical instillation of platelet rich plasma on recurrent bacterial cystitis in women: a randomized clinical trial. Urol J2019; 16:609–13.
- 35. Montelin H, Forsman KJ, Tängdén T. Retrospective evaluation of nitrofurantoin and pivmecillinam for the treatment of lower urinary tract infections in men. PLoS One2019; 14:e0211098.
- 36. Nestler S, Grune B, Schilchegger L, Suna A, Perez A, Neisius A. Efficacy of vaccination with StroVac for recurrent urinary tract infections in women: a comparative single-centre study. Int Urol Nephrol2021; 53:2267–72.
- 37. Overcash JS, Tiffany CA, Scangarella-Oman NE, et al Phase 2a pharmacokinetic, safety, and exploratory efficacy evaluation of oral gepotidacin (GSK2140944) in female participants with uncomplicated urinary tract infection (acute uncomplicated cystitis). Antimicrob Agents Chemother2020; 64:e00199–20.
- 38. Overcash JS, Bhiwandi P, Garrity-Ryan L, et al Pharmacokinetics, safety, and clinical outcomes of omadacycline in women with cystitis: results from a phase 1b study. Antimicrob Agents Chemother2019; 63:e02083–18.
- 39. Pierrotti LC, Pérez-Nadales E, Fernández-Ruiz M, et al Efficacy of beta-lactam/beta-lactamase inhibitors to treat extended-spectrum beta-lactamase-producing Enterobacterales bacteremia secondary to urinary tract infection in kidney transplant recipients (INCREMENT-SOT Project). Transpl Infect Dis2020; 23:e13520..
- 40. Rădulescu D, David C, Turcu FL, Spătaru DM, Popescu P, Văcăroiu IA. Combination of cranberry extract and D-mannose—possible enhancer of uropathogen sensitivity to antibiotics in acute therapy of urinary tract infections: results of a pilot study. Exp Ther Med2020; 20:3399–406.
- 41. Ryanto S, Wong M, Czarniak P, et al The use of initial dosing of gentamicin in the management of pyelonephritis/urosepsis: a retrospective study. PLoS One2019; 14:e0211094.
- 42. Safwat AS, Hasanain A, Shahat A, et al Cholecalciferol for the prophylaxis against recurrent urinary tract infection among patients with benign prostatic hyperplasia: a randomized, comparative study. World J Urol2019; 37:1347–52.
- 43. Sagan O, Yakubsevitch R, Yanev K, et al Pharmacokinetics and tolerability of intravenous sulbactam-durlobactam with imipenem-cilastatin in hospitalized adults with complicated urinary tract infections, including acute pyelonephritis. Antimicrob Agents Chemother2020; 64:e01506–19.
- 44. Sashidhar RB, Manoj KYM, Manisha S, Sree SRS, Kamala SH. Assessment of efficacy and safety of oral fosfomycin single dose in uncomplicated urinary tract infection at a tertiary care hospital in south India. Asian J Pharm Clin Res2022; 15:60–3.
- 45. Senard O, Lafaurie M, Lesprit P, et al Efficacy of cefoxitin versus carbapenem in febrile male urinary tract infections caused by extended spectrum beta-lactamase-producing Escherichia coli: a multicenter retrospective cohort study with propensity score analysis. Eur J Clin Microbiol Infect Dis2020; 39:121–9.
- 46. Sharara SL, Amoah J, Pana ZD, Simner PJ, Cosgrove SE, Tamma PD. Is piperacillin-tazobactam effective for the treatment of pyelonephritis caused by extended-spectrum beta-lactamase-producing organisms?Clin Infect Dis2020; 71:e331–7.
- 47. Sojo-Dorado J, López-Hernández I, Rosso-Fernandez C, et al Effectiveness of fosfomycin for the treatment of multidrug-resistant Escherichia coli bacteremic urinary tract infections: a randomized clinical trial. JAMA Netw Open2022; 5:e2137277.
- 48. Sorlí L, Luque S, Li J, et al Colistin for the treatment of urinary tract infections caused by extremely drug-resistant Pseudomonas aeruginosa: dose is critical. J Infect2019; 79:253–61.
- 49. Stalenhoef JE, van Nieuwkoop C, Menken PH, Bernards ST, Elzevier HW, van Dissel JT. Intravesical gentamicin treatment for recurrent urinary tract infections caused by multidrug resistant bacteria. J Urol2019; 201:549–55.
- 50. Tehrani S, Elyasi F, Abolghasemi S. Levofloxacin versus ceftriaxone for the treatment of acute pyelonephritis in Iranian adults. Infect Disord Drug Targets2021; 21:603–7.
- 51. Ten Doesschate T, van Werkhoven H, Meijvis S, et al Fosfomycin-trometamol for urinary tract infections in kidney transplant recipients. Transplantation2019; 103:1272–6.
- 52. Ten Doesschate T, Kuiper S, van Nieuwkoop C, et al Fosfomycin versus ciprofloxacin as oral step-down treatment for Escherichia coli febrile urinary tract infections in women: a randomised, placebo-controlled, double-blind, multicenter trial. Clin Infect Dis2021; 75:221–9..
- 53. Tseng CS, Chang SJ, Meng E, Chang HC, Lee YJ. The efficacy of pentosan polysulfate monotherapy for preventing recurrent urinary tract infections in women: a multicenter open-label randomized controlled trial. J Formos Med Assoc2020; 119:1314–9.
- 54. Tullos JB, Stoudenmire LL, Pouliot JD. Piperacillin-tazobactam versus carbapenems for the treatment of nonbacteremic urinary tract infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae. Hosp Pharm2020; 55:44–9.
- 55. Wagenlehner FME, Cloutier DJ, Komirenko AS, et al Once-daily plazomicin for complicated urinary tract infections. N Engl J Med2019; 380:729–40.
- 56. Wald-Dickler N, Lee TC, Tangpraphaphorn S, et al Fosfomycin vs ertapenem for outpatient treatment of complicated urinary tract infections: a multicenter, retrospective cohort study. Open Forum Infect Dis2022; 9:ofab620.
- 57. Nicolle LE, Bradley S, Colgan R, et al Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis2005; 40:643–54.
- 58. Rodhe N, Lofgren S, Matussek A, et al Asymptomatic bacteriuria in the elderly: high prevalence and high turnover of strains. Scand J Infect Dis2008; 40:804–10.
- 59. Ouslander JG, Schapira M, Fingold S, Schnelle J. Accuracy of rapid urine screening tests among incontinent nursing home residents with asymptomatic bacteriuria. J Am Geriatr Soc1995; 43:772–5.
- 60. Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection?JAMA2002; 287:2701–10.
- 61. Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med2013; 369:1883–91.
- 62. Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med1982; 307:463–8.
- 63. Klaassen IL, de Haas V, van Wijk JA, Kaspers GJ, Bijlsma M, Bokenkamp A. Pyuria is absent during urinary tract infections in neutropenic patients. Pediatr Blood Cancer2011; 56:868–70.
- 64. Bilsen MP, Aantjes MJ, van Andel E, et al Current pyuria cut-offs promote inappropriate UTI diagnosis in older women. Clin Infect Dis2023; 76:2070–6.
- 65. van den Broek D, Keularts IM, Wielders JP, Kraaijenhagen RJ. Benefits of the iQ200 automated urine microscopy analyser in routine urinalysis. Clin Chem Lab Med2008; 46:1635–40.
- 66. Wilson ML, Gaido L. Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis2004; 38:1150–8.
- 67. Johnson JR. Definition of complicated urinary tract infection. Clin Infect Dis2017; 64:529.
- 68. Johansen TE, Botto H, Cek M, et al Critical review of current definitions of urinary tract infections and proposal of an EAU/ESIU classification system. Int J Antimicrob Agents2011; 38(Suppl):64–70.
- 69. Bouma M, Geerlings SE, Klinkhamer S, et al NHG standaard: urineweginfecties [Dutch primary care guideline: UTI]. 2020. Available at: https://richtlijnen.nhg.org/standaarden/urineweginfecties. Accessed 15 March 2023.