With the availability of direct-acting antiviral (DAA) agents, hepatitis C virus (HCV) chronic infection has become a curable infection in nearly all cases and a potentially eliminable disease. However, liver-related complications may occur in individuals with HCV infection even after achieving sustained virological response (SVR), particularly among those with advanced liver disease [, ]. The main challenge remains to find markers that accurately predict clinical outcomes, allowing a more adequate surveillance after SVR. In this setting, liver stiffness (LS), measured by vibration-controlled transient elastography (VCTE), has proven to be a strong predictor of liver events, both during HCV active infection and after HCV cure []. Indeed, LS is the reflection of liver fibrosis and inflammation, as well as portal hypertension, so this procedure has a high predictive value for liver-related outcomes. Namely, a vale ≤14 kPa at the moment of SVR identifies patients with HCV infection, regardless of HIV coinfection, with low risk of developing liver complications, who may be candidates to discontinue surveillance measures []. However, the most important concern is that LS by itself is insufficient to detect individuals at high risk for these clinical outcomes, mainly due to a limited positive predictive value.
Steatotic liver disease (SLD) is becoming a leading cause of chronic liver disease. SLD is estimated to involve nearly one-third of the global population [], and it is mainly related to the obesity pandemic []. Among patients with HCV infection, the prevalence of concomitant SLD could be higher, up to 50% [, ], due to a direct lipogenic effect of the virus or metabolic dysfunction [, ] that may persist after HCV eradication [, ]. Concomitant SLD could play a role in the emergence of these liver-related events, enhancing fibrogenesis and hepatocarcinogenesis [, ]. The FibroScan-AST (FAST) score, which includes aspartate aminotransferase (AST), controlled attenuation parameter (CAP; measured by VCTE), and LS, is an indicator of steatohepatitis with greater risk of fibrosis progression [, ]. The FAST score reliably predicts clinical outcomes in patients with SLD [, ]. Consequently, it may also be a better predictor of clinical outcomes than simple LS in patients with HCV infection who achieve SVR. However, information on this is lacking.
Therefore, the aim of this study was to compare the predictive value of the FAST score vs LS for liver complications in patients with HCV infection and advanced fibrosis, with or without HIV coinfection, who achieve SVR.
METHODS
Study Design and Patients
This was a multicenter prospective study that included patients with HCV chronic infection, with or without HIV coinfection, from the GEHEP-011 Cohort (clinicaltrials.gov ID: NCT04460157). Individuals were followed at 17 infectious diseases units throughout Spain since October 2011. The inclusion criteria for this study were (1) an LS ≥9.5 kPa before starting treatment, (2) having achieved SVR with regimens containing ≥1 DAA, (3) having an LS available at the time of SVR, and (4) having a CAP measurement at the SVR time point. Patients with positive HBsAg were excluded.
Follow-up
The date of SVR was considered the baseline time point. Following a common protocol, individuals were clinically and analytically evaluated every 6 months. Participants were followed until the date of death, liver transplant, HCV reinfection, loss follow-up, or censoring date (November 30, 2022). Patients with cirrhosis were managed according to a specific protocol reported elsewhere []. In summary, screening of hepatocellular carcinoma (HCC) was performed biannually based on alpha-fetoprotein determination and liver ultrasound examination. In addition, in patients with LS ≥21 kPa, gastroesophageal varices surveillance was performed with serial upper gastrointestinal endoscopy.
Diagnosis Criteria
Liver events included hepatic decompensations (ascites, gastrointestinal bleeding due to portal hypertension, hepatic encephalopathy, spontaneous bacterial peritonitis, and hepatorenal syndrome) and HCC. Diagnosis of de novo HCC was established according to the American Association for the Study of Liver Diseases criteria []. Hepatic decompensations were diagnosed as reported previously [].
VCTE Examinations
LS and CAP were assessed by VCTE (FibroScan, Echosens, Paris, France) according to a standardized procedure. An M probe was used. At each center, examinations were performed by a trained operator. For determinations to be considered reliable, evaluations had to include at least 10 measurements, with a success rate ≥60% and an interquartile range <30% of the median LS.
End Point and Other Definitions
The primary end point of the study was the emergence of a liver-related event after SVR. SVR was defined as showing undetectable HCV RNA 12 weeks after the end of DAA-based therapy. In line with previous studies, a diagnosis of cirrhosis was established in individuals with LS ≥14 kPa [, , ]. SLD was defined as CAP ≥248 dB/m []. The FAST score [], which includes AST, CAP, and LS, was calculated. A FAST score ≥0.67 was interpreted as likely nonalcoholic steatohepatitis (NASH) with fibrosis stage ≥2, and a FAST score ≤0.35 was considered unlikely NASH with fibrosis ≥2.
Statistical Analysis
The cumulative incidence and incidence rate of liver-related complications were estimated. The time to the emergence of the main outcome was computed as the time elapsed from SVR to liver event occurrence. Life tables were built to calculate the survival estimates, expressed as the cumulative proportion of individuals who remained free from development of the end point. Survival curves were constructed using the Kaplan-Meier method, and the log-rank test was performed to compare the different categories. Variables associated with the main end point in the bivariate analysis with P < .05, along with age and sex at birth, were entered in a multivariable analysis, and Fine-Gray regression models for competing risks were created. Death by any cause was considered the competitive event. The performance of the models was assessed by comparing receiver operating characteristics (ROC) curves using the Hanley-McNeil test. The diagnostic accuracy of the FAST score and LS for the prediction of liver events after SVR was assessed by sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). We calculated the percentage of missed events and the proportion of individuals without hepatic complications identified. All estimates are provided, along with 95% CIs.
For the statistical analysis, the statistical package IBM SPSS 26 (SPSS Inc. IBM) and Stata, version 16.1 (StataCorp), were used.
Ethics
This study was conducted according to the Helsinki Declaration and was approved by the local ethics committee. All patients gave written informed consent before being recruited into the cohort.
RESULTS
Characteristics of the Patients
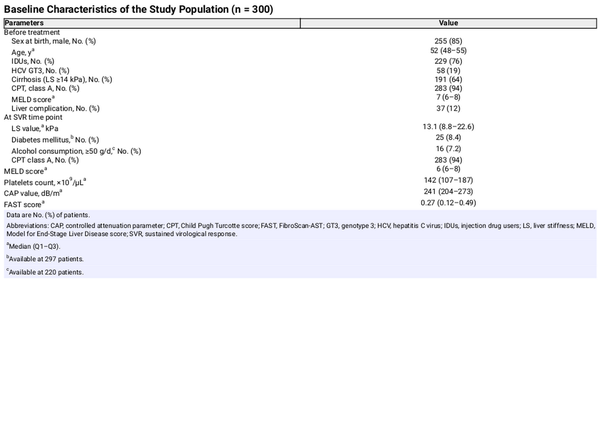
Three hundred patients were included, 213 (71%) of whom were people with HIV (PWH). One hundred fifty-four (51%) individuals showed compensated cirrhosis before starting DAA therapy. At the SVR time point, 142 (47%) had an LS ≥14.0 kPa. With respect to SLD, at the SVR time point, 131 (44%) had CAP values ≥248 dB/m. The FAST score was <0.35 in 182 (61%), 0.35–0.66 in 79 (27%), and ≥0.67 in 34 (12%) patients. Other relevant characteristics of the study population are listed in Table 1. All PWH were on antiretroviral therapy, and 161 (86%) of them had a plasma HIV-RNA <50 copies/mL. The median (Q1−Q3) CD4+ cell count was 489 (302–679) cells/mm3.
The median (Q1–Q3) follow-up was 73 (57–83) months. During this time, 44 (15%) patients died, 8 (3%) underwent a liver transplant, and 10 (3%) were lost to follow-up. The main causes of death were liver-related events (16 [36.4%]), non-HCC malignancies (12 [27.3%]), infectious diseases (5 [11%]), and other causes (11 [25.3%]).
Liver-Related Events Post-SVR
After HCV cure, 36 (12%) patients developed a liver complication. The liver complication rate was 1.9 (1.4–2.6) per 1000 person-years. The probability of remaining free from liver-related events at 1, 3, and 5 years after the SVR time point was 97% (94%–98%), 92% (88%–94%), and 89% (85%–92%), respectively. Specifically, 22 (7%) individuals had hepatic decompensation, and 15 (5%) patients developed HCC. With respect to liver decompensations, the most frequent one was ascites (12 [4%]), followed by portal hypertensive gastrointestinal bleeding (7 [2%]) and hepatic encephalopathy (3 [1%]).
Prediction of Clinical Outcome After SVR
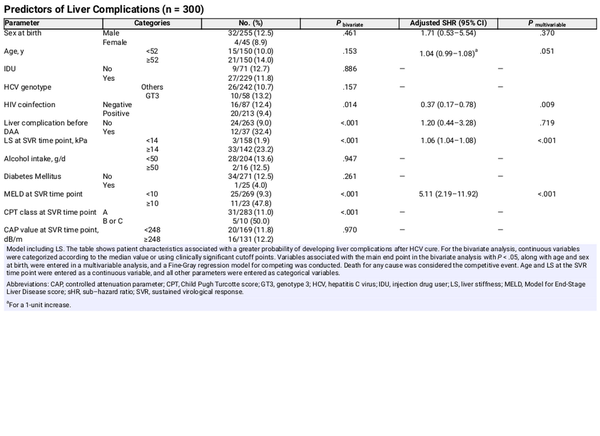
The presence of SLD was not associated with the emergence of liver complications after HCV cure (Table 2). The probability of developing the main outcome was greater for patients with LS ≥14.0 kPa and for those with higher FAST scores (Table 2, Figure 1). A first multivariable model was created, adjusted for sex at birth, age, HIV coinfection, emergence of liver events before SVR, and LS at the SVR time point. In this analysis, LS at the SVR time point was independently associated with an increased risk of developing liver complications (Table 2). Other predictors are shown in Table 2. In a second model, adjusted for sex at birth, age, HIV coinfection, emergence of liver events before SVR, FAST score at SVR, and MELD score at the SVR time point, FAST ≥0.35 was independently associated with greater risk of liver complications (Supplementary Table 1).
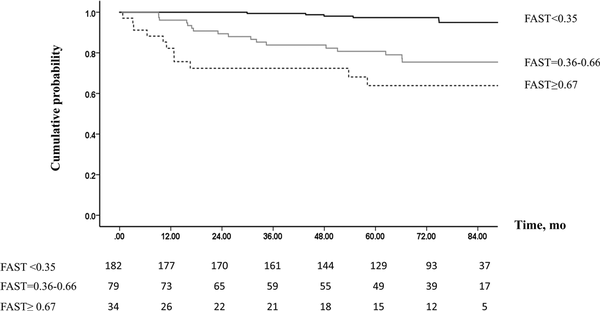
Figure 1
Probability of remaining free from liver-related outcomes after SVR, according to FAST score. Abbreviations: FAST, FibroScan-AST; SVR, sustained virological response.
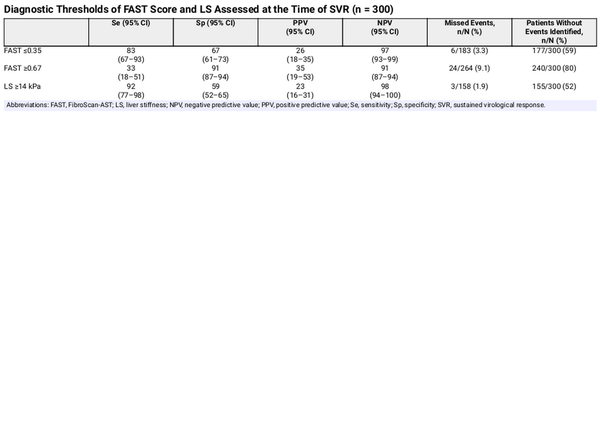
The diagnostic performance of the noninvasive tools studied for the emergence of liver complications after HCV cure is presented in Table 3. LS showed the highest NPV while missing the lowest proportion of clinical events. In addition, LS <14 kPa and FAST score ≤0.35 identified a similar percentage of individuals who did not develop liver complications after SVR. The PPV for the FAST cutoff, 0.67, and the one for LS were all low (Table 3). The AUROC of the model based on LS was 0.83 (95% CI, 0.76–0.91), and that of the model based on FAST 0.80 (95% CI, 0.72–0.88; P = .158) (Figure 2). The sensitivity analysis, including patients with SLD, yielded similar results (Supplementary Data). Sensitivity analyses according to HIV coinfection are shown in the Supplementary Data.
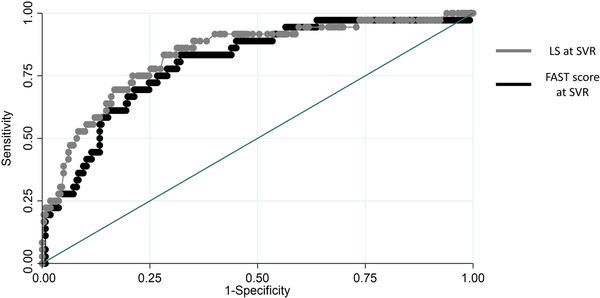
Figure 2
ROC curves of FAST score and LS for the prediction of liver-related events. Abbreviations: FAST, FibroScan-AST; LS, liver stiffness; ROC, receiver operating characteristics.
DISCUSSION
This study suggests that, after SVR, the FAST score is useful to identify individuals with HCV infection and advanced liver disease who are at low risk of developing liver-related outcomes. However, using the FAST score does not improve the predictive ability of simple LS for the emergence of these clinical events. Moreover, LS identifies a similar proportion of patients at low risk of liver complication occurrence while minimizing the number of missed events.
SLD is a growing concern because of its high prevalence, which is increased among individuals with HCV infection [, ]. In the present study, the proportion of individuals with SLD was greater than that estimated among the general population [, ], which is not surprising. In this setting, the interplay between HCV infection and SLD involves complex interactions that can influence the progression of liver disease. Understanding the contribution of factors such as fibrosis and SLD in this predictive model is crucial for tailoring follow-up care and interventions for individuals with HCV infection who achieve SVR. To date, information on this subject is still scarce. While attaining SVR addresses the direct effects of HCV on the liver, individuals with past HCV infection may still be at risk for metabolic issues, including SLD [, , ]. Indeed, insulin resistance, which is commonly associated with both HCV infection and SLD, might persist after SVR, contributing to metabolic disturbances []. In a recent small study, Chuaypen et al. demonstrated that the improvement observed in LS after HCV eradication was not associated with a decrease of hepatic steatosis in a high proportion of the study population []. Thus, after SVR, concomitant SLD might put patients with HCV chronic infection at a greater risk of developing liver-related complications, especially among those with advanced liver disease. In that regard, steatohepatitis-related biomarkers, specifically the FAST score, have proven to be useful for predicting the development of liver-related outcomes in different settings []. In the specific context of HCV infection, the presence of steatohepatitis, as indicated by a high FAST score, may contribute to an increased risk of HCC after SVR []. The inclusion of AST, CAP, and LS in the FAST score was intended to provide a comprehensive assessment of liver disease, considering both fibrosis and steatohepatitis. This combination aims to capture a broader spectrum of liver conditions that may influence the development of liver complications even after successful HCV treatment.
The findings of this study reveal that FAST score was independently associated with a higher risk of developing liver-related events, aligning with previous studies demonstrating the utility of the FAST score in predicting outcomes in individuals with SLD []. However, the comparison of the predictive capacities of LS and the FAST score did not yield a statistically significant difference. Moreover, LS showed a better diagnostic performance than FAST score with a greater NPV, maximizing the proportion of patients without clinical events detected while minimizing the number of missed complications. Finally, the PPV of the FAST score was similar to that of LS for occurrence of liver-related events, suggesting that neither LS nor FAST by itself is useful to accurately identify at-risk individuals. The similar AUROC values between the 2 models indicate that both are similarly effective in identifying individuals at low risk of developing liver complications. This implies that the additional information provided by CAP and AST in the FAST score did not significantly enhance the prognostic accuracy over LS alone. That suggests that fibrosis, whatever the origin is, either residual to prior HCV infection or to coexisting SLD, drives the emergence of complications after HCV cure. This observation raises important clinical implications, suggesting that focusing on fibrosis assessment by means of a simple measurement might be sufficient in this particular post-SVR setting. Consequently, devices measuring SLD in addition to LS are not strictly required to establish the prognosis in this setting. Likewise, simple blood markers of liver fibrosis, such as FIB-4 score, could be useful in settings where LS measurement devices are not available []. Definitively, there is a need for markers that, while maintaining the sensitivity and NPV of LS, increase specificity. However, FAST score, even at its upper cutoff value, fails to meet this requirement. Research in this area is imperative.
This study may have some limitations. First, the absence of a significant difference in predictive capacity prompts further exploration into the dynamic changes of LS and FAST over longer follow-up periods. It is plausible that the impact of SLD on outcomes may evolve over time, influencing the prognostic value of the FAST score in later stages post-SVR. Second, concomitant comorbidities and factors related to lifestyle have not been analyzed in this study, particularly alcohol intake after SVR. Nonetheless, this is the first study comparing the performance of FAST score and LS for the prediction of liver events after SVR. Moreover, long-term data from a large sample of patients with hepatitis C and advanced liver disease in which individuals are prospectively followed in real-life clinical practice settings are provided. Those are the strengths of this work.
In conclusion, the FAST score is independently associated with a higher risk of liver-related events after HCV cure. However, it does not surpass the predictive capacity of LS alone. Focusing on fibrosis assessment through LS or other biomarkers may suffice in this post-SVR setting, rendering additional devices measuring SLD alongside LS not strictly required for prognosis determination. This may be particularly relevant for resource-limited centers that rely on older devices or other types of elastography. Future studies should address SLD dynamic changes after HCV treatment and their impact on outcomes over time, potentially influencing the prognostic value of the FAST score in later stages post-SVR.
Acknowledgments
Author contributions. Conceptualization: A.C.G., J.M., J.A.P., L.M.R.; methodology: A.C.G., J.M., J.A.P.; formal analysis: A.C.G., J.M., J.A.P.; investigation: all authors; resources: all authors; data curation: all authors; writing—original draft: A.C.G., J.M., J.A.P.; writing—review & editing: all authors; visualization: all authors; supervision: L.M.R., J.M., A.C.G., J.A.P.; project administration: A.C.G.; funding acquisition: A.C.G., J.A.P. A.C.G. and J.M. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Data availability. The data that support the findings of this study are available from the corresponding author (A.C.G.) upon reasonable request.
Financial support . This study was partially funded by the Instituto de Salud Carlos III (Project PI19/01443), integrated in the Nacional I + D + i 2013–2016, and cofunded by the European Union (ERDF/ESF, “Investing in your future”), Gilead Biomedical Research Fellowship Program (GLD21_00096), and by GEHEP-SEIMC (GEHEP-011 project). Anaïs Corma-Gómez received a research extension grant, Acción B, Acción para el Refuerzo de la Actividad Investigadora en las Unidades Clínicas del Servicio Andaluz de Salud 2021, Clínicos Investigadores (grant number B-0061-2021). She has also received a Juan Rodès grant from the Instituto de Salud Carlos III (grant number JR23/00066). Juan Macías has received a research extensión grant, Acción A, Acción para el Refuerzo de la Actividad Investigadora en las Unidades Clínicas del Servicio Andaluz de Salud 2021, Intensificación anual (grant number A1-0060-2021). Jésica Martín Carmona is the recipient of a Rio Hortega grant by Instituto de Salud Carlos III-ISCIII (CM23/00255). Diana Corona Mata is the recipient of a Rio Hortega grant by Instituto de Salud Carlos III-ISCIII (CM22/00176). Ángela Carrasco Dorado is the recipient of an “INVESTIGO” research program grant funded by the European Union NextGenerationEU Plan. Antonio River-Juárez is supported by a contract from the Spanish Junta de Andalucía (Nicolas Monardes program: C1-0001-2023). The funders did not play any role in the design, conclusions, or interpretation of the study.
References
- 1. Kanwal F, Kramer JR, Asch SM, Cao Y, Li L, El-Serag HB. Long-term risk of hepatocellular carcinoma in HCV patients treated with direct acting antiviral agents. Hepatology2020; 71:44–55.
- 2. Corma-Gómez A, Macías J, Téllez F, et al Kinetics of emergence of liver complications in hepatitis C virus infected patients and advanced fibrosis, with and without HIV-coinfection, after sustained virological response. AIDS2021; 35:2119–27.
- 3. Anstee QM, Castera L, Loomba R. Impact of non-invasive biomarkers on hepatology practice: past, present and future. J Hepatol2022; 76:1362–78.
- 4. Corma-Gómez A, Macías J, Téllez F, et al Liver stiffness at the time of sustained virological response predicts the clinical outcome in HIV/HCV-coinfected patients with advanced fibrosis treated with direct-acting antivirals. Clin Infect Dis2019; 71:2354–62.
- 5. Fouda S, Jeeyavudeen MS, Pappachan JM, Jayanthi V. Pathobiology of metabolic-associated fatty liver disease. Endocrinol Metab Clin North Am2023; 52:405–16.
- 6. Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology2023; 77:1335–47.
- 7. Adinolfi LE, Rinaldi L, Guerrera B, et al NAFLD and NASH in HCV infection: prevalence and significance in hepatic and extrahepatic manifestations. Int J Mol Sci2016; 17:803.
- 8. Asselah T, Rubbia-Brandt L, Marcellin P, Negro F. Steatosis in chronic hepatitis C: why does it really matter?Gut2006; 55:123.
- 9. Negro F. Facts and fictions of HCV and comorbidities: steatosis, diabetes mellitus, and cardiovascular diseases. J Hepatol2014; 61:S69–78.
- 10. Moucari R, Asselah T, Cazals-Hatem D, et al Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology2008; 134:416–23.
- 11. Chuaypen N, Siripongsakun S, Hiranrat P, Tanpowpong N, Avihingsanon A, Tangkijvanich P. Improvement of liver fibrosis, but not steatosis, after HCV eradication as assessment by MR-based imaging: role of metabolic derangement and host genetic variants. PLoS One2022; 17:e0269641.
- 12. Leslie J, Geh D, Elsharkawy AM, Mann DA, Vacca M. Metabolic dysfunction and cancer in HCV: shared pathways and mutual interactions. J Hepatol2022; 77:219–36.
- 13. Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol2019; 16:411–28.
- 14. Newsome PN, Sasso M, Deeks JJ, et al FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol2020; 5:362–73.
- 15. Ravaioli F, Dajti E, Mantovani A, Newsome PN, Targher G, Colecchia A. Diagnostic accuracy of FibroScan-AST (FAST) score for the non-invasive identification of patients with fibrotic non-alcoholic steatohepatitis: a systematic review and meta-analysis. Gut2023; 72:1399–409.
- 16. Sariyar N, Kani HT, Celikel CA, Yilmaz Y. Predicting fibrosis progression in non-alcoholic fatty liver disease patients using the FAST score: a paired biopsy study. Hepatol Forum2024; 5:33–6.
- 17. Ogawa E, Takayama K, Hiramine S, Hayashi T, Toyoda K. Association between steatohepatitis biomarkers and hepatocellular carcinoma after hepatitis C elimination. Aliment Pharmacol Ther2020; 52:866–76.
- 18. Merchante N, Rivero-Juárez A, Téllez F, et al Liver stiffness predicts variceal bleeding in HIV/HCV-coinfected patients with compensated cirrhosis. AIDS2017; 31:493–500.
- 19. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology2018; 68:723–50.
- 20. Pineda JA, García-García JA, Aguilar-Guisado M, et al Clinical progression of hepatitis C virus-related chronic liver disease in human immunodeficiency virus-infected patients undergoing highly active antiretroviral therapy. Hepatology2007; 46:622–30.
- 21. Perez-Latorre L, Sanchez-Conde M, Rincon D, et al Prediction of liver complications in patients with hepatitis C virus-related cirrhosis with and without HIV coinfection: comparison of hepatic venous pressure gradient and transient elastography. Clin Infect Dis2014; 58:713–8.
- 22. Merchante N, Rivero-Juárez A, Téllez F, et al Liver stiffness predicts clinical outcome in human immunodeficiency virus/hepatitis C virus-coinfected patients with compensated liver cirrhosis. Hepatology2012; 56:228–38.
- 23. Karlas T, Petroff D, Sasso M, et al Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol2017; 66:1022–30.
- 24. Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol2023; 79:516–37.
- 25. Rout G, Nayak B, Patel AH, et al Therapy with oral directly acting agents in hepatitis C infection is associated with reduction in fibrosis and increase in hepatic steatosis on transient elastography. J Clin Exp Hepatol2019; 9:207–14.
- 26. Welsch C, Efinger M, Wagner V, et al Ongoing liver inflammation in patients with chronic hepatitis C and sustained virological response. PLoS One2017; 12:e0171755.
- 27. Shengir M, Elgara M, Sebastiani G. Metabolic and cardiovascular complications after virological cure in hepatitis C: what awaits beyond. World J Gastroenterol2021; 27:1959–72.
- 28. Younossi Z, Anstee QM, Marietti M, et al Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol2018; 15:11–20.
- 29. Ideno N, Nozaki A, Chuma M, et al Fib-4 index predicts prognosis after achievement of sustained virologic response following direct-acting antiviral treatment in patients with hepatitis C virus infection. Eur J Gastroenterol Hepatol2023; 35:219–26.